Abstract
In Kawasaki disease (KD), giant coronary aneurysms in the proximal segments of the coronary arteries have long been among the serious complications associated with acute myocardial infarction. To treat myocardial ischemia in children, Kitamura et al. first performed coronary artery bypass grafting in a pediatric patient using an autologous saphenous vein. In the early 1980s, they began to use the internal thoracic artery (ITA) as a bypass graft to the left anterior descending artery, which later was proven to improve long-term life expectancy with its favorable long-term patency, as well as growth potential. Thus, the excellent characteristics of the ITA have come to be widely known among pediatric cardiac surgeons, and a growing number of coronary bypass surgery procedures using the ITA are now being performed worldwide. Although a longer follow-up with more patients is necessary, downsizing reconstructive procedure may be a treatment of choice for giant aneurysms of non-LAD territories to improve coronary circulation. The efficacy of surgical treatment for giant coronary aneurysms in pediatric patients with Kawasaki disease is now well established. Pediatric coronary artery bypass grafting using the ITA, either single or bilateral, can be safe not only for patients with Kawasaki coronary disease but also for infants with congenital coronary lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since Kawasaki disease (KD) was first described as an “acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children” [1] in 1967, its etiology has remained undetermined. At that time, postmortem pathological examinations of the victims of the disease suggested that the cause of death was acute myocardial infarction resulting from inflammatory coronary aneurysms [2]. These findings led quite a few pediatricians to have great concern about the disease.
With selective coronary angiography and ultrasonic two-dimensional echocardiography adopted in daily clinical practice in the 1980s, great efforts were made to investigate the clinical and histopathological nature of the coronary aneurysms in KD.
In this review article, the author presents a historical overview of the surgical treatment of Kawasaki coronary aneurysms causing myocardial ischemia, the indications for treatment, the characteristics of the graft, i.e., internal thoracic artery (ITA), and other issues. Furthermore, the author discusses the “downsizing operation” for a giant coronary aneurysm of non-left anterior descending artery (LAD) territories, which is usually difficult to manage with bypass surgery.
Histopathological characteristics of Kawasaki vasculitis
KD is a clinical disorder that belongs to “the systemic vasculitis syndrome” characterized by systemic vascular inflammation, in which all clinical manifestations originate from vasculitis. The main histopathological finding of KD vasculitis is proliferative inflammation consisting of marked accumulation of monocytes/macrophages [2,3,4]. Characteristically, medium-sized muscular arteries outside the solid organs (e.g., pancreas, kidney, spleen, etc.) are most frequently affected. In addition to coronary arteries, which are almost 100% affected, arteries outside the solid organs, such as the carotid, subclavian, renal, iliac, hepatic, and mesenteric arteries, are commonly affected. In contrast, inflammatory reactions are rarely seen in the arteries inside solid organs; if there is any, occasional perivascular cellular infiltration can only be seen [3,4,5].
The vascular inflammation of KD is also characterized by a monophasic inflammatory process, in which inflammatory reactions occur synchronously in all affected arteries. The phases of the inflammatory process, such as the acute phase, healing phase, and cicatricial phase, are not mixed in a single patient [2, 4].
Inflammation rapidly worsens and becomes panvasculitis involving all layers of the vessel wall. If the internal and external elastic lamina and smooth muscle cells in the media are damaged by severe inflammation, the artery becomes unable to withstand the blood pressure and dilates; thus, aneurysm formation occurs by about the 12th day of illness [4].
Following the acute phase of inflammation, if the healing process is delayed or incomplete for some reason, a coronary aneurysm may enlarge into a gigantic one.
Low shear stress due to slowed flow velocity in the aneurysm decreases endothelial function. The larger the size of the aneurysm, the more decreased is the flow velocity in the aneurysm. This results in a change of the blood flow waveform from laminar pulsatile to turbulent, leading to a further decrease in the shear stress. Furthermore, the aggregating function of platelets accelerates, along with an increase in their number. Thus, endothelial dysfunction due both to the inflammation and to decreased shear stress can facilitate thrombus formation [6,7,8].
Giant coronary aneurysm
A giant coronary aneurysm (GCA) is defined as: (1) an aneurysm with an internal diameter ≥ 8 mm; or, (2) in children ≥ 5 years of age, the internal diameter of a segment measures over four times that of an adjacent segment [9, Fig. 1].
Giant coronary aneurysm (GA). a GA of the LAD: The distal segment of the LAD is not visualized yet, but is seen with a marked delay. b Sausage-like GA of the RCA. c The waveform pattern in the intact coronary artery and in the GA: In the GA, the blood flow waveform changes from pulsatile and laminar flow to vortex or turbulent flow, facilitating thrombus formation
Nearly 50% of coronary aneurysms remaining more than 30 days after onset may decrease in size during the convalescent phase. In cases of small- or medium-sized aneurysms, regression of the aneurysms often occurs within a couple of years after onset. In contrast, GCAs are less likely to regress and may persist or develop into stenosis [9,10,11,12,13]. The proximal segment of bilateral coronary arteries is usually involved in GCAs. Simultaneous development of GCAs both in the left and right coronary systems should be regarded as a serious condition [14, 15]. Patients with GCAs have a higher risk of fatal myocardial ischemia due to thrombotic occlusion of the aneurysms [9, 10, 12,13,14]. Acute myocardial infarction in most cases occurs within a year after onset of KD with a high mortality rate or with significant deterioration of LV function after survival [14].
GCAs located in the proximal RCA may sometimes develop luminal thrombotic occlusion with partial recanalization or with collateral circulation from the left coronary system. This pathological feature usually does not show an ischemic response [12, 14].
Studies using a Doppler flow guidewire and a pressure wire showed that average peak velocity and shear stress decreased significantly as the size of the aneurysm increased [6, 7]. Decreased shear stress and retarded blood flow increase platelet aggregation and coagulation, leading to thrombus formation. In addition, myocardial fractional flow reserve was significantly lower in patients with a GCA with no stenosis than in those without an aneurysm.
Antiplatelet and antithrombotic therapy for GCAs
Anticoagulant therapy is important to prevent thrombosis in the aneurysm [9, 15, 16].
There has been no randomized controlled trial comparing treatment options. Some studies found that treatment with aspirin plus warfarin was more effective than other regimens [16, 17]. In patients receiving warfarin plus aspirin, the incidence of coronary occlusion was significantly lower in those receiving warfarin alone or no medication during a mean follow-up of 9 years [17]. In another study, the incidence of myocardial infarction was significantly lower in patients receiving warfarin plus aspirin than in those receiving antiplatelet medications without warfarin (5.2 vs. 32.7%, p < 0.05) [16]. A multicenter study of 87 patients treated with warfarin plus aspirin showed that 91% were free from cardiac events at 10 years [15]. A meta-analysis of several observational studies showed that warfarin plus aspirin reduced the incidence of coronary artery occlusion, myocardial infarction, and death [18, 19]. Thus, in the guidelines, warfarin and antiplatelet therapy is classified as a class I treatment for GCA [9, 20].
The maintenance dose of warfarin should be 0.05–0.12 mg/kg/day, once daily, to achieve an international normalized ratio (INR) of 2.0–2.5, and it should be adjusted carefully to prevent bleeding due to excessive warfarin [6, 9]. Patients should be evaluated for symptoms of bleeding during warfarin therapy. While it is still investigative, new drugs such as factor FXa inhibitor may be better to obtain stable anticoagulant activity and minimize the risk of bleeding complications.
In patients who do not respond to anticoagulation treatment, coronary artery bypass grafting (CABG) using pedicled internal thoracic artery (ITA) grafts should be considered in a timely manner [9].
Overview of CABG for Kawasaki coronary aneurysm
Kitamura et al. took the first step in the surgical treatment of Kawasaki coronary disease by performing coronary revascularization for a 4-year-old boy who had an anteroseptal myocardial infarction after acute KD in 1974, and they published this case in 1976 [21]. The patient underwent double-bypass surgery to the left anterior descending artery (LAD) and the right coronary artery (RCA) using autologous vein grafts. Postoperative angiograms showed excellent graft patency with an improved left ventricular ejection fraction of 0.61. Unfortunately, both grafts occluded within 1 year.
Suma et al. in 1982 [22] and Kitamura et al. in [23] both reported low early and late postoperative patency rates of autologous vein grafts. Unfortunately, the long-term patency of autologous vein grafts proved unsatisfactory, particularly in small children. Since patients with Kawasaki coronary disease are often small children, the ideal graft must have a patency that would last their lifetime. Since the ITA had been gradually recognized as a quite reliable graft with excellent long-term patency, Kitamura et al. began to use the ITA for children and reported two successful cases in 1985 [24]. They subsequently reported 12 LITA-LAD bypass cases with 100% patency in 1988 [25], and, furthermore, they reported cases in which bilateral ITAs were used in 1990 [26].
Another important issue was how grafts would respond to the physical growth of children. Kitamura et al. reported a very important fact [25]. In their study, graft length was measured using the 3-dimensional Pythagorean rule applied to bilateral angiograms. These measurements showed that the pedicled ITA graft could grow longitudinally and circumferentially along with a child’s physical growth. The growth potential of the ITA combined with the excellent long-term patency clearly indicated the ability of the ITA to stay as a “live” conduit that works throughout the patients’ life.
On the other hand, another study showed that the autologous vein graft did not have growth potential, causing distal flow disturbance by distorting the coronary artery just like “pitching a tent” at the anastomotic site [27, 28]. Because of the lack of growth potential and worse early- and long-term patency rates when compared to ITA, the autologous vein has proven to be inadequate as a graft.
Takeuchi et al. reported a patient with KD in which a right gastroepiploic artery (GEA) graft was used to revascularize the RCA [29]. The GEA is now widely used in adult CABG as a pedicled arterial graft to revascularize the RCA. Since the GEA is the fourth branch of the abdominal aorta, it carries a greater risk of flow competition than the ITA in a small child with an underdeveloped body. In addition, the size of the artery varies individually. Therefore, the GEA may be used safely only for limited patients with a larger body size (Fig. 2). Thus, the ITA graft became the single gold standard for pediatric coronary bypass surgery.
In regard to the long-term outcome of pediatric CABG using ITA grafts in KD, a multicenter cooperative study in 1994 showed a survival rate of 98.7% at 90 months [30], demonstrating the benefit of ITA grafts for the long-term survival of children with KD. Yoshikawa et al. reported excellent long-term patency of the ITAs in 2000 [31], adding that 83 of 100 children enjoyed their daily life with no restriction. In their study group, there was one sudden death, and another one received heart transplantation, both of which had severe left ventricular dysfunction due to old myocardial infarction in the LAD territory [31]. In a 25-year observational study including 114 surgical patients by Kitamura et al. [32], the patency rates of the ITA and the saphenous vein graft (SVG) were 87 and 42%, respectively, while surgical mortality was 0%, and the overall survival rate was 95%. Considering that approximately 30% of patients had a history of myocardial infarction, surgery is likely to improve the survival of patients with severe Kawasaki coronary disease.
When cardiac events were defined as (1) events related to the graft of any cause, (2) late progression of coronary disease, (3) impaired left ventricular function, and (4) ventricular tachyarrhythmia, the cardiac event-free rate was as low as 70% at 20 years and 62% at 25 years, indicating that various problems continued as the postoperative period lengthened [32]. However, among children with well-functioning grafts, 84% could take part in athletic programs at school, although all of these patients had been strictly excluded from school programs because of the fear of sudden death [32].
The number of reports of ITA usage in Kawasaki coronary disease in European countries has recently increased [33,34,35,36,37]. With the prominent nature of the ITA as a graft being widely recognized, the artery has come to be used not only in Kawasaki coronary disease but also in congenital anomalies requiring coronary revascularization [35]. Furthermore, in light of the widely recognized effectiveness of pediatric coronary bypass surgery, some emphasize that CABG should be adopted as part of fundamental training in the field of pediatric cardiac surgery [38].
Indications for surgical revascularization in pediatric patients with KD
Indications for LITA-LAD grafting
In patients with GCAs involving the left main trunk (LMT)-LAD who do not respond to anticoagulant treatment and are at high risk of myocardial ischemia, LITA-LAD grafting should be considered. Without GCAs in the LMT-LAD territories, surgical treatment is not indicated. Single vessel obstruction of the RCA is often accompanied by marked development of collateral vessels without symptoms [39, 40]. Therefore, a patient with GCAs confined to the RCA territory is not a surgical candidate.
According to the guideline on adult coronary revascularization, i.e.,“2014ESC/EACTS Guidelines on Myocardial Revascularization” [41], CABG is recommended for a patient with LMT disease and/or triple-vessel disease without a proximal LAD lesion. When significant stenosis exists in the proximal LAD, even a patient with single-vessel or double-vessel disease is a surgical candidate. This decision-making is supported by the fact that ITA-LAD grafting is superior to a percutaneous coronary intervention/stent in long-term outcomes.
In the “Guidelines for Diagnosis and Management of Cardiovascular Sequelae in Kawasaki Disease (JCS 2014)”, a similar decision-making process is addressed for pediatric patients [9]. In addition, these guidelines strongly recommend surgical treatment for children with left ventricular dysfunction due to myocardial infarction.
Inasmuch as children have a life expectancy of well over 50 years or more, special care should be taken to avoid the occurrence of myocardial infarction that leads to poor ventricular function. Even in an infant, when there is a threat of myocardial ischemia, LITA-LAD grafting should be carried out without delay [9, 10, 13, 14]. According to our surgical data, there were no cases of myocardial infarction preoperatively if the patients were referred to us appropriately after the signs of myocardial ischemia were recognized. Consequently, these patients showed excellent long-term outcomes with a low incidence of cardiac events [42]. Not a few reports have emphasized the importance of prompt management of thrombus formation in the aneurysm causing myocardial ischemia [43, 44].
To avoid flow competition, some argue that the indication for LITA-LAD grafting should be carefully evaluated when an apparent stenosis (> 75%) is not visualized on the angiogram [32, 45, 46]. Flow competition can cause a string phenomenon in ITA grafts. In pediatric coronary bypass surgery, however, the incidence of ITA graft recanalization after the string phenomenon classified as occluded or nonfunctioning was 20–25% (31). When flow competition between the graft and the recipient coronary artery diminishes due to progression of coronary artery obstructive lesions, the ITA regains its flow and function as a graft unless the lumen is completely thrombosed [47].
In reality, however, we sometimes encounter a patient with signs of myocardial ischemia without apparent stenosis around the aneurysm on the angiogram. To evaluate the functional severity of coronary artery lesions, it is useful to determine average peak flow velocity (APV), coronary flow reserve (CFR), and myocardial fractional flow reserve (FFRmyo) using a 0.014-inch guide wire equipped with an ultrasonic probe and a high-sensitivity pressure sensor (Doppler wires or pressure wires). CFR and FFRmyo are obtained as follows:
CFR = [stress APV]/[APV at rest], where APV is the value at peak dilatation after infusion of papaverine hydrochloride
FFRmyo = [Mean pressure at a site distal to the coronary lesion of interest]-{[mean right atrial pressure]/[mean pressure at the coronary ostium]}-[mean right atrial pressure]
The reference values in children are 2.0 for CFR and 0.75 for FFRmyo.
These measurements are suitable to evaluate the presence/absence and severity of myocardial ischemia, and in selecting appropriate treatment strategies and postoperative evaluation [9, 48]. Some aneurysms with an internal diameter > 8 mm have normal blood flow waveform, APV, and CFR. Because giant aneurysms with normal hemodynamics may be present, functional assessment of aneurysms should be performed to identify aneurysms at risk.
Indication for RCA revascularization
GCAs often develop at the proximal segment of the LAD and RCA concomitantly. As mentioned above, a LITA-LAD graft is the gold standard for obstructive lesions in the LAD territory. In regard to coexisting aneurysms in the RCA, some issues have to be noticed.
In teenage or adolescent patients with large body size, obstructive lesions in association with aneurysms may be treated in the same manner as in the adult. Right ITA and GEA, for the proximal and the distal part of the RCA, respectively are the graft of choice. Although age distribution is not available, in a reported series by Kitamura et al., they used 32 RITA and 13 GEA for revascularization in the RCA territory with an excellent long-term patency [32].
On the other hand, however, infants and young children cannot be managed in the same way. Lack of suitable arterial grafts is a major concern. Their body size is so small that the length of RITA or GEA is limiting. GCAs extend to the middle segment of RCA (Fig. 1b) in most instances. On such occasion, RITA cannot be anastomosed beyond the aneurysm. Underdeveloped GEA may also be unavailable in small children.
As mentioned previously, however, GCAs in the proximal RCA may often develop thrombotic occlusion with recanalization or with collateral circulation from the left coronary system. This pathological feature usually is not associated with an ischemic response [12,13,14, 39, 40], which means collateral circulation may be sufficient enough. Therefore, once the LAD is protected by a LITA-LAD grafting, some GCAs in the RCA may well be managed conservatively.
Coexisting GCAs in the RCA without obstructive lesions may often be encountered in the clinical setting. Although bypass grafting is not indicated in such an instance, fear of distal thrombotic emboli causing myocardial ischemia is a concern. Close observation with administration of warfarin and aspirin or other antiplatelet agents is the management of choice [9, 20].
Special issues in pediatric CABG with ITA grafts
In small body-sized pediatric patients, coronary bypass surgery using ITA is technically demanding. According to reports on the long-term outcomes of surgical treatment, the patency rate of ITA grafts of younger children was significantly low [45]. A recent report by Kitamura et al., however, showed no age difference in graft patency among their children, indicating possible technical problems [32].
The ITA of small body-sized children is a short and thin-walled artery with a diameter of 1 mm or less. Pediatric CABG requires meticulous and precise dissection and anastomosis under high-magnification (4–6 power) surgical glasses with fine and small needles and sutures. A microscope may be useful in pediatric CABG [32, 38]. Use of cardiopulmonary bypass with cardioplegic arrest is recommended as the best approach.
As mentioned previously, Kitamura et al. showed that the ITA graft in children could grow in accordance with somatic growth [25]. In contrast, the autologous SVG had poor long-axis growth potential in children [27]. The author would like to emphasize again that the growth potential of the ITA graft in children is a great advantage (Fig. 3).
A GEA-RCA graft of a 17-year-old male patient. His height was 172 cm. He had undergone LITA-LAD grafting at the age of 10 years. 7 years later, the RCA became totally occluded. After confirming the size of the GEA on angiogram, GEA-RCA grafting was performed off-pump via the subxiphoid approach. This is only a single case of our series in which a GEA graft was used
Medium-sized muscular arteries located outside solid organs are most frequently affected by the inflammatory reaction in KD. The ITA is not in the same histological category; its wall has a well-developed internal elastic lamina with a scanty smooth muscle layer, and an inflammatory reaction has rarely been seen in it. Therefore, the ITA can almost always be used as a bypass graft.
Generally, the ITA graft has such flow capacity as to be able to respond to the flow demand of the anastomosed coronary artery depending on the severity of the stenosis. In small children, however, an underdeveloped ITA sometimes falls short of our expectations. Thus, great care should be taken to avoid flow competition or possible technical errors when considering sequential grafting (Fig. 4) or making a composite graft (Y-graft) in children.
Sequential LITA-diagonal-LAD graft. Note that the ITA graft is widely patent. Both the LAD and the diagonal branch are clearly visualized through the graft, indicating that there is no flow competition between the ITA and coronary arteries. In carefully selected patients, sequential grafting of the LITA is technically feasible
Reconstructive downsizing for aneurysms of non-LAD territories
As mentioned above, coexisting GCAs of non-LAD territories without obstructive lesions are managed by administration of warfarin and antiplatelet agents to prevent distal thromboembolism. However, anticoagulant therapy must be carefully monitored because warfarin can cause serious hemorrhagic complications. In addition, thrombogenesis often cannot be controlled despite adequate anticoagulation [6].
Since the size of the aneurysm correlates ***well with the extent of flow disturbance, we have adopted a procedure as a concomitant one with LITA-LAD grafting by which the aneurysm is reconstructed to make a cylinder-shaped form (Fig. 5). By doing so, the waveform of the blood flow inside the aneurysm changes from turbulent to pulsatile. Consequently, the APV is expected to increase significantly to prevent thrombus formation. Details of the operative procedure have been described elsewhere [49].
The “new” internal diameter of the aneurysm should be no smaller than 5 mm because of the possibility of further postoperative-negative remodeling. Care should be taken to avoid stenosis around the transition between the aneurysm and the intact RCA. A small pericardial patch may be preferable at the transitional site. To obtain a smooth, round-shaped lumen, the wall of the aneurysm should preserve elasticity without calcification.
When postoperative measurement by a Doppler flow wire confirms the improved values of APV (≥ 15 cm/sec) and CFR (≥ 2.0) along with a change in the waveform pattern (turbulent to pulsatile), anticoagulation therapy can be discontinued [48].
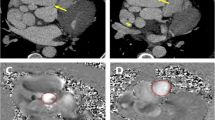
Nine patients underwent this downsizing reconstruction for giant aneurysms of non-LAD territories concomitant with LITA-LAD grafting at our center (Nippon Medical School Hospital). The size of the GCAs was reduced sufficiently in five patients to discontinue warfarin (Fig. 6). Occlusion of the RCA occurred in 2 patients following the procedure because endothelial function within the aneurysm might have remained impaired. In 2 patients, the procedure could not be completed sufficiently due to severe calcification.
Preoperative and postoperative angiograms of a patient undergoing downsizing reconstruction of the RCA. In this patient, the procedure was performed concomitantly with LITA-LAD grafting for a GA in the LAD. a Sausage-like GA of the proximal RCA. b One year after operation, the patient could discontinue anticoagulants
Since we do not think that this procedure is the alternative to LITA-LAD grafting, we have never elected to perform the downsizing procedure on the GCA of LAD. Our patients were referred to us for surgery with clearly documented signs of myocardial ischemia due to GCA in the LMT-LAD territories, a LITA-LAD grafting was indicated in all patients. At present, our surgical approach to KD is that without indication of LITA-LAD grafting, there is no indication for surgery.
Although a longer follow-up with more patients is necessary, if appropriate indications for this procedure can be established, downsizing reconstruction may be a treatment of choice for GCAs of non-LAD territories to improve coronary circulation and allow warfarin discontinuation [9].
The development of surgical treatment of Kawasaki coronary disease using pedicled ITAs established by Kitamura et al. [21, 23,24,25,26, 30, 32] has contributed to the standardization of pediatric coronary surgery. The procedure using a single ITA or bilateral ITAs has been accepted as the treatment of choice for infants and small children with coronary artery lesions due to not only KD but also other congenital heart diseases. Pediatric coronary artery bypass surgery carries a low risk of mortality and provides long-term benefits for small children.
References
Kawasaki T, Kosakai F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54:271–6.
Fujiwara H, Hamashima Y. Pathology of the heart in Kawasaki disease. Pediatrics. 1978;61:100–7.
Landing BH, Larson E. Pathological features of Kawasaki disease (mucocutaneous lymph node syndrome). Am J Cardiovasc Pathol. 1987;1:218–29.
Naoe S, Takahashi K, Masuda H, Tanaka N. Kawasaki disease. With parcicular emphasis on arterial lesions. Acta Pathol Jpn. 1991;41:785–97.
Amano S, Hazama F, Hamashima Y. Pathology of Kawasaki disease: II. Distribution and incidence of the vascular lesions. Jpn Circ J. 1979;43:741–8.
Ohkubo T, Fukazawa R, Ikegami E, Ogawa S. Reduced shear stress and disturbed flow may lead to coronary aneurysm and thrombus formations. Pediat Int. 2007;49:1–7.
Kuramochi Y, Ohkubo T, Takechi N, Fukumi D, Uchikoba Y, Ogawa S. Hemodynamic factors of thrombus formation in coronary aneurysms associated with Kawasaki disease. Pediatr Int. 2000;42:470–5.
Hamaoka K, Onouchi S. Effects of coronary artery aneurysms on intracoronary flow velocity dynamics in Kawasaki disease. Am J Cardiol. 1996;77:873–5.
Subcommittee of cardiovascular sequelae SoST, Kawasaki Disease Research Committee. Guidelines for treatment and management of cardiovascular sequelae in Kawasaki disease. Heart Vessels. 1987; 3:50–4.
Kato H, Sugimura T, Akagi T, Sato N, Hashino K, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94(6):1379–85.
JCS Joint Working Group. Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2013). Circ J Jpn Circu Soc, 2014;78:2521–2562.
Nakano H, Ueda K, Saito A, Nojima K. Repeated quantitative angiograms in coronary arterial aneurysm in Kawasaki disease. Am J Cardiol. 1985;56:846–51.
Suzuki A, Kamiya T, Arakaki Y, Kinoshita Y, Kimura K. Fate of coronary arterial aneurysms in Kawasaki disease. Am J Cardiol. 1994;74:822–4.
Kato H, Ichinose E, Kawasaki T. Myocardial infarction in Kawasaki disease: clinical analyses in 195 cases. J Pediatr. 1986;108:923–7.
Suda K, Kudo Y, Higaki T, Nomura Y, Miura M, et al. Multicenter and retrospective case study of warfarin and aspirin combination therapy in patients with giant coronary aneurysms caused by Kawasaki disease. Circ J. 2009;73:1319–23.
Sugahara Y, Ishii M, Muta H, Iemura M, Matsuishi T, Kato H. Warfarin therapy for giant aneurysm prevents myocardial infarction in Kawasaki disease. Pediatr Cardiol. 2008;29:398–401.
Onouchi Z, Hamaoka K, Sakata K, Ozawa S, Shiraishi I, et al. Long-term changes in coronary artery aneurysms in patients with Kawasaki disease: comparison of therapeutic regimens. Circ J. 2005;69:265–72
Levy DM, Silverman ED, Massicotte MP, McCrindle BW, Yeung RS. Longterm outcomes in patients with giant aneurysms secondary to Kawasaki disease. J Rheumatol. 2005;32:928–34
Su D, Wang K, Qin S, Pang Y. Safety and efficacy of warfarin plus aspirin combination for giant coronary aneurysm secondary to Kawasaki disease: a meta-analysis. Cardiology 2014;129:55–64
Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, et al. American academy of pediatrics. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–71
Kitamura S, Kawashima Y, Fujita T, Mori T, Oyama C. Aortocoronary bypass grafting in a child with coronary artery obstruction due to mucocutaneous lymphnode syndrome: report of a case. Circulation. 1976;53:1035–40.
Suma K, Takeuchi Y, Shiroma K, et al. Early and late postoperative studies in coronary arterial lesions resulting from Kawasaki’s disease in children. J Thorac Cardiovasc Surg 1982;84:224–9
Kitamura S, Kawachi K, Harima R, Sakakibara T, Hirose H, Kasashima Y. Surgery for coronary heart disease due to mucocutaneous lymph node syndrome (Kawasaki disease). Report of 6 patients. Am J Cardiol 1983;51:444–8.
Kitamura S, Kawachi K, Oyama C, Miyagi Y, Morita R, Koh Y, et al. Severe Kawasaki heart disease treated with an internal mammary artery graft in pediatric patients. A first successful report. J Thorac Cardiovasc Surg. 1985;89:860–6.
Kitamura S, Seki T, Kawachi K, Morita R, Kawata T, Mizuguchi K, et al. Excellent patency and growth potential of internal mammary artery grafts in pediatric coronary artery bypass surgery. New evidence for a “live” conduit. Circulation 1988;78(1):I 129-I 139.
Kitamura S, Kawachi K, Seki T, Morita R, Nishii T, Mizuguchi K, et al. Bilateral internal mammary artery grafts for coronary artery bypass operations in children. J Thorac Cardiovasc Surg 1990;99:708–15.
Kameda Y, Kitamura S, Taniguchi S, Kawata T, Mizuguchi K, et al. Differences in adaptation to growth of children between internal thoracic artery and saphenous vein coronary bypass grafts. J Cardiovasc Surg (Torino). 2001;42:9–16.
Wakisaka Y, Tsuda E, Yamada O, Yagihara T, Kitamura S. Long-term results of saphenous vein graft for coronary stenosis caused by Kawasaki disease. Cir J. 2009;73:73–7.
Kitamura S, Kameda Y, Seki T, Kawachi K, Endo M, Takeuchi Y, et al. Long-term outcome of myocardial revascularization in patients with Kawasaki coronary artery disease. A multicenter cooperative study. J Thorac Cardiovasc Surg 1994;107:663–73.
Yoshikawa Y, Yagihara T, Kameda Y, Taniguchi S, Tsuda E, Kawahira Y, et al. Result of surgical treatments in patients with coronary-arteial obstructive disease after Kawasaki disease. Eur J Cardiothorac Surg. 2000;17:515–9.
Kitamura S, Tsuda E, Kobayashi J, Yoshikawa Y, Yagihara T, et al. Twenty-five-year outcome of pediatric coronary artery bypass surgery for Kawasaki disease. Circulation. 2009;120:60–8.
Takeuchi Y, Gomi A, Okamura Y, Mori H, Nagashima M. Coronary revascularization in a child with Kawasaki disease: use of right gastroepiploic artery. Ann Thorac Surg. 1990;50:294–6.
Mavroudis C, Backer CL, Duffy CE, Pahl E, Wax DF. Pediatric coronary artery bypass for Kawasaki congenital, post arterial switch, and iatrogenic lesions. Ann Thorac Surg. 1999; 68:506–12.
Coskun KO, Coskun ST, El Arousy M, Aminparsa M, Hornik L, et al. Pediatric patients with Kawasaki disease and a case report of Kitamura operation. ASAIO J. 2006;52:e43–e7.
Newburger JW, Fulton DR. Coronary revascularization in patients with Kawasaki disease. J Pediatr. 2010;157:8–10.
Viola N, Alghamdi AA, Al-Radi OO, Coles JG, Van Arsdell GS, Caldarone CA. Midterm outcomes of myocardial revascularization in children. J Thorac Cardiovasc Surg. 2010;139:333–8.
Legendre A, Chantepie A, Belli E, Vouhe PR, Neville P, Dulac Y, et al. Outcome of coronary artery bypass grafting performed in young children. J Thorac Cardiovasc Surg. 2010;139:349–53.
Vida VL, Torregrossa G, De Franceschi M, Padalino MA, Belli E, Berggren H, et al. European Congenital Heart Surgeons Association (ECHSA). Pediatric coronary artery revascularization: a European multicenter study. Ann Thorac Surg 2013;96:898–903.
Suzuki S, Kamiya T, Ono Y, Takahashi N, Naito Y, Kou Y. Indication of aorto-coronary bypass for coronary arterial obstruction due to Kawasaki disease. Heart Vessels 1985;1:94–100.
Suzuki A, Kamiya T, Ono Y, Kinoshita Y, Kawamura S, Kimura K. Clinical significance of morphologic classification of coronary arterial segmental stenosis due to Kawasaki disease. Am J Cardiol 1993;71:1169–73.
ESC/EACTS Guidelines on myocardial revascularization. The Task Force on Myocardial Revascularization of the European Society of Cardiology(ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2014;https://doi.org/10.1093/eurheartj/ehu278.
Yamauchi H, Ochi M, Fujii M, Hinokiyama K, Ohmori H, et al. Optimal time of surgical treatment of Kawasaki coronary artery disease. J Nippon Mde Sch. 2004;71:279–86.
Tsuda E, Hirata T, Matsuo O, Abe T, Sugiyama H, Yamada O. The 30-year outcome for patients after myocardial infarction due to coronary artery lesions caused by Kawasaki disease. Pediatr Cardiol. 2011;32:176–82.
Tsuda E, Hamaoka K, Suzuki H, Sakazaki H, Murakami Y, Nakagawa M, et al. A survey of the 3-decade outcome for patients with giant aneurysms caused by Kawasaki disease. Am Heart J. 2014;167:249–58.
Tsuda E, Kitamura S, Kimura K, Kobayashi J, Miyazaki S, Echigo S, et al. Long-term patency of internal thoracic artery grafts for coronary artery stenosis due to Kawasaki disease: comparison of early with recent results in small children. Am Heart J. 2007;153:995–1000.
Tsuda E, Fujita H, Yagihara T, Yamada O, Echigo S, Kitamura S. Competition between native flow and graft flow after coronary artery bypass grafting. Impact on indications for coronary artery bypass grafting for localized stenosis with giant aneurysms due to Kawasaki disease. Pediatr Cardiol. 2008;29:266–70.
Ogawa S, Ohkubo T, Fukazawa R, Kamisago M, Kuramochi Y, UchikobaY, et al. Estimation of myocardial hemodynamics before and after intervention in children with Kawasaki disease. J Am Coll Cardiol. 2004;43:653–61.
Kitamura S. Long-term graft patency and surgical outcomes of coronary artery bypass surgery in children with Kawasaki disease. Saji BT et al, Kawasaki diseaseeditors. Springer, Japan 2017:407–17.
Maruyama Y, Ochi M. Long-term outcomes of pediatric coronary artery bypass grafting and down-sizing operation for giant coronary aneurysm. Saji BT et al (eds) Kawasaki disease. Springer, Japan 2017:389–406.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ochi, M. Review: surgical treatment of giant coronary aneurysms in pediatric patients with Kawasaki disease. Gen Thorac Cardiovasc Surg 66, 121–129 (2018). https://doi.org/10.1007/s11748-017-0877-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-017-0877-7