Abstract
Objective
The aim of this study was to identify appropriate method of diagnosis and treatment of spontaneous pneumomediastinum (SPM) based on our experience.
Methods
The medical records of patients who were diagnosed with SPM and treated at our hospital between April 2006 and July 2015 were, retrospectively, analyzed. The data included characteristics of the patients, method of diagnosis, treatment and clinical course.
Results
Forty-five patients were diagnosed with SPM and treated at our hospital. The mean age of patients was 18.96 ± 4.65 years and 35 patients were male. The main symptoms expressed by these patients were chest pain, throat pain or discomfort, and dyspnea. Nine patients had a precipitating event leading to SPM. Twelve patients had normal chest X-ray findings but were subsequently diagnosed with SPM on chest computed tomography (CT). Additional procedures including esophagogram (n = 36), bronchoscopy (n = 14) and endoscopy (n = 1) were done but none of patients were found to have organ damage. All patients received oxygen inhalation therapy. Oral intake was restricted in 36 patients and 43 patients received prophylactic antibiotics. The mean time taken for symptomatic improvement was 1.73 ± 0.85 days from diagnosis. The mean hospital stay was 3.93 ± 1.44 days and no patient developed recurrence of SPM during the follow-up period.
Conclusions
In addition to chest X-ray, chest CT is recommended for accurate diagnosis of SPM. However, further invasive investigations, restriction of oral intake and the use of prophylactic antibiotics have minimal role in the diagnosis and treatment of SPM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
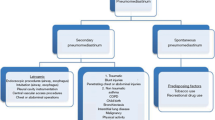
Spontaneous pneumomediastinum (SPM) is a condition where free air arises in the mediastinum. It is generally believed to be caused by rupture of the alveolar due to rapid increase in intrathoracic pressure [1, 2]. However, SPM can also arise without a clear cause of increased intrathoracic pressure, such as severe cough, strenuous exercise and vomiting [3–6]. SPM is an uncommon condition with a benign nature that responds well to conservative treatment [3, 7–9].
There is no clear guidelines for SPM diagnosis and treatment [6, 9, 10]. Many patients with SPM undergo invasive investigations such as esophageal endoscopy and/or bronchoscopy to rule out esophageal or tracheal damage, and are admitted to hospital for observation as there is a risk of developing subsequent tension pneumomediastinum and other complications [3, 6, 9, 11, 12]. Therefore, the aim of this study was to identify appropriate methods for diagnosis and treatment of SPM based on our clinical experience.
Patients and methods
Patients
The medical records of patients who were diagnosed with pneumomediastinum and treated at our hospital between April 2006 and July 2015 were retrospectively analyzed. The diagnosis of pneumomediastinum was based on the finding of free air in mediastinum on chest X-ray or chest computed tomography (CT) images.
Patients who had traumatic pneumomediastinum or iatrogenic pneumomediastinum following invasive investigations, had spontaneous pneumomediastinum but were not admitted, or who were transferred to other hospitals were excluded from this study. Therefore, only those who were diagnosed with spontaneous pneumomediastinum and were admitted to our hospital for treatment were included in this study. Fever was defined as axillary temperature of 37.2 °C or greater, leukocytosis was defined as white cell count of 10,800/mm3 or greater, and elevated C-reactive protein was defined as 3.0 mg/dL or greater. The demographics of the patients, symptoms of complaint, precipitating events, investigation findings, the management and clinical course were investigated. This study was approved by the Institutional Review Board of our institution (SCHCA 2016-07-046-001).
Statistical analysis
SPSS 14.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Continuous variables were displayed as mean and standard deviation and categorical or dichotomous variables were displayed as the frequency and percentage (%).
Results
A total of 65 patients were diagnosed and treated for pneumomediastinum at the department of thoracic and cardiovascular surgery of our hospital. Of these patients, the following were excluded from this study: 12 who had developed pneumomediastinum following trauma such as motor vehicle accident, fall, direct trauma and stab injury; 2 who had developed iatrogenic pneumomediastinum following invasive procedures or investigations; and 6 who were treated as outpatients or transferred to other hospitals for treatment of spontaneous pneumomediastinum. The remaining 45 patients were enrolled in this study.
The mean age of these patients was 18.96 ± 4.65 years, and 35 (77.8%) of these patients were male. The mean body mass index (BMI) was 20.40 ± 3.08 kg/m2. Five patients (11.1%) had previous history of respiratory conditions, two of whom had pneumothorax, two had tuberculosis, and one patient had asthma. Eight patients (17.8%) were current smokers. The most common symptom was chest pain (40.7%), followed by throat pain or discomfort (38.3%), and dyspnea (18.5%).
Nine patients (20%) had history of precipitating events leading to pneumomediastinum, which were cough (n = 4, 8.9%), exercise (n = 3, 6.7%), and vomiting (n = 2, 4.4%). The mean time taken from the first onset of symptoms to hospital admission was 0.82 ± 1.13 days. The oxygen saturation at hospital presentation was 98.04 ± 1.19. Forty-one patients (91.1%) had fever, but none of the patients had high fevers of 38.5 °C or higher. Sixteen patients were found to have leukocytosis (35.6%) and 7 patients had elevated CRP (15.6%) (Table 1).
Thirty-three patients (73.3%) had pneumomediastinum seen in the initial chest X-rays. Of these patients, 20 (44.4%) had pneumomediastinum alone, and 12 (26.7%) patients had subcutaneous emphysema as well as pneumomediastinum. One patient (2.2%) had pneumomediastinum with pneumothorax. The remaining 12 patients (26.7%) did not have clear sign of pneumomediastinum in the initial chest X-rays.
All patients underwent chest CT to investigate for pneumomediastinum as well as other causes of their symptoms. Pneumomediastinum was confirmed in all patients. Of these patients, 17 (37.8%) had subcutaneous emphysema and 2 (4.4%) had pneumothorax as well as pneumomediastinum in their CT. Thirty-six (80%) patients underwent esophagogram which showed that none of these patients had esophageal rupture or leakage. None of the 14 patients (31.1%) who underwent bronchoscopy had abnormal bronchoscopy findings. One patient who had melena (2.2%) subsequently underwent esophagram and endoscopy, which showed chronic gastritis but no other significant conditions (Table 2).
All patients received oxygen inhalation therapy (n = 45, 100%) and all except two patients received prophylactic intravenous antibiotics (n = 43, 95.6%). Oral intake was restricted in 36 patients (80%). All patients improved without further complications. The mean time taken from the onset of symptoms to symptomatic improvement was 2.5 ± 1.36 days, and the mean time taken for symptomatic improvement from hospital admission was 1.73 ± 0.85 days. There were no cases of recurrence of pneumomediastinum during the follow-up period (Table 3).
Discussion
This study investigated the medical records of patients who were diagnosed with pneumomediastinum (with exclusion of patients with traumatic pneumomediastinum, iatrogenic pneumomediastinum, and SPM patients who were transferred to other hospitals after diagnosis) to define appropriate method of diagnosis and treatment of SPM. SPM is an uncommon condition, with a reported incidence of 1 in 30,000 of emergency department presentations by Newcomb et al. [3], 1 in 41,600 of emergency department presentations reported by Esayag et al. [5], and 1 in 10,470 reported by Takada et al. [6].
The exact pathogenesis of SPM is unclear, but Macklin et al. [1] have suggested that it is caused by alveolar rupture due to an increase in intrathoracic pressure, leading to air leakage that dissects the interstitial sheath and moves to the mediastinum. Sakai et al. [2] have suggested that more than 95% of cases of pneumomediastinum are caused by alveolar rupture, and that it is more common than esophageal or tracheo-bronchial damage.
Although SPM can be preceded by events that increase intrathoracic pressure such as coughing, severe vomiting, strenuous exercise and asthma attacks, some patients do not have precipitating events prior to developing SPM. Caceres et al. [4] reported that 21% of their patients with SPM did not have identifiable precipitants, while Bakhos et al. [3] ,Takada et al. [6], Kim et al. [10], Esayag et al. [5] reported that of 41, 44, 53, and 54%, respectively.
A study of Medline database for 22 years showed that 34% of patients with SPM did not have specific precipitating factors [7]. In this current study, the precipitating events leading to SPM included cough (n = 4, 8.9%), physical activity and exercise (n = 3, 6.7%), and vomiting (n = 2, 4.4%), while 36 patients (80%) did not have a clear precipitating event (Table 1).
Previous studies have reported that SPM is more common in thin patients, as it is for spontaneous pneumothorax [4, 13–15]. However, other studies have reported mean BMI of 20.2 ± 4.0 and 21.1 ± 2.0 kg/m2 in patients with SPM [6, 16]. In this study, the mean BMI of SPM patients was 20.40 ± 3.08 kg/m2, which is also in the normal range. Most of the patients in this study were young males, whose main complaints were chest pain, throat pain or discomfort, and dyspnea, which is similar to previous studies [3, 4, 6, 7, 9, 10].
As discussed earlier, SPM can develop without a precipitating event, and can also occur in patients of normal BMI. Therefore, although the incidence is low, SPM should be considered as a differential diagnosis when a young male patient presents with chest pain, throat pain or dyspnea.
There is no consensus on the method of diagnosis of SPM [9]. While some authors report that simple chest X-ray alone is diagnostic [17, 18], other authors state that chest X-ray is sufficient for the diagnosis of SPM if a patient did not have preceding trauma, vomiting or swallowing history, and that further investigation is not required if the patient is well [5]. On the other hand, some authors suggested that all patients should undergo chest CT to diagnose SPM and investigate for other underlying conditions [14], while other recommend that chest X-ray is the initial method of diagnosis, but chest CT and other investigations should be performed if SPM and other conditions are suspected [6, 13].
However, some studies have reported that approximately 30% of patients with SPM have normal chest X-ray findings [11, 19, 20]. A study of 11 adolescent patients with SPM reported that only 3 of these patients had X-ray findings of SPM in their initial chest X-rays, and after re-examination of the chest X-ray 5 patents were diagnosed with SPM [8]. In this study all patients had both chest X-ray and chest CT for the diagnosis of SPM and other underlying conditions and it was found that 26.7% (n = 12) of these patients had normal chest X-rays but had SPM diagnosed on chest CT. Therefore, it is suggested that the initial chest X-ray is carefully interpreted for accurate diagnosis, and that patients with symptoms suggestive of SPM should undergo chest CT even if they have normal chest X-ray findings.
In addition to chest X-ray and CT, a number of patients in this study also underwent esophagogram (n = 36, 80%), bronchoscopy (n = 14, 31.1%) or endoscopy (n = 1, 2.2%) to rule out esophageal perforation or tracheo-bronchial injury, which were all normal except for one patient who was found to have chronic gastritis in endoscopy. This is consistent with previous studies [6, 8–10]. Based on these findings, we do not think it is necessary to perform invasive investigations such as esophagogram, bronchoscopy, and endoscopy in patients with suspected SPM without traumatic event.
Many authors have used conservative methods to treat SPM, such as bed rest, analgesia and oxygen inhalation therapy to absorb free air in the mediastinum [5, 7–10, 14, 21] and similarly, all patients in this study underwent oxygen inhalation therapy to promote free air absorption. The indication for the restriction of oral intake and using prophylactic antibiotics is still debated, with some authors recommending its use for the prevention of mediastinitis [10, 14, 16, 22, 23] and others reporting that it is not recommended as the clinical benefits are unclear [6, 8].
Although there were 16 patients (35.6%) with leukocytosis and 7 patients with elevated CRP (15.6%), most patients in this study were managed by restriction of oral intake (n = 36, 80%) and administration of prophylactic antibiotics (n = 43, 95.6%) to prevent mediastinitis and other infections (Table 3). However, as mentioned eariler, there were no patients with esophageal injuries, and the vast majority of patients improved immediately after conservative treatment. The results of this study suggest that restriction of oral intake and the use of prophylactic antibiotics are not required in patients with SPM.
The reported time taken for symptomatic improvement of SPM patients is within 24 h [5], after 1.8 days [6], after 1–3 days [24], or within 7 days [25] of developing symptoms, which is similar to the results in this study (Table 3). Radiological findings of improvement are reported to take 3–5 days [24], or up to 3 weeks [5].
The mean duration of hospital stay was longer than the duration of symptomatic improvement in this study (Table 3) which is due to performing further investigations and tests to follow-up on the patient’s progress. Thus, as mentioned earlier, we believe that it would be possible to reduce the duration of hospital stay by avoiding unnecessary invasive investigations and restriction of oral intake.
There were no cases of recurrence of SPM in this study. As reported in previous studies, the risk of recurrence of SPM is uncommon [3, 7, 10], and patients generally do not require long term follow-up [6, 10]. We agree with this opinion. This study is limited in its retrospective nature, and also by the small number of enrolled patients.
Conclusions
This study showed that SPM is a self-limiting, benign condition that responds well to conservative treatment. SPM can occur without precipitating events, and can also occur in patients who are not thin. Therefore, SPM should be considered as a differential diagnosis in young male patients presenting with chest pain, throat pain or discomfort, or dyspnea. Chest X-ray as well as chest CT is recommended for accurate diagnosis, as SPM may not be visualized on chest X-ray alone. However, we believe that performing additional invasive investigations such as esophagogram, bronchoscopy and endoscopy does not have an important role in the diagnosis of SPM patients, and restriction of oral intake and the use of prophylactic antibiotics have minimal a role in the treatment of SPM patients.
References
Macklin MT, Macklin CC. Malignant interstitial emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions: interpretation of the clinical literature in the light of laboratory experiment. Medicine (Baltimore). 1944;23:281–358.
Sakai M, Murayama S, Gibo M, Akamine T, Nagata O. Frequent cause of the Macklin effect in spontaneous pneumomediastinum: demonstration by multidetector-row computed tomography. J Comput Assist Tomogr. 2006;30:92–4.
Bakhos CT, Pupovac SS, Ata A, Fantauzzi JP, Fabian T. Spontaneous pneumomediastinum: an extensive workup is not required. J Am Coll Surg. 2014;219:713–7.
Caceres M, Ali SZ, Braud R, Weiman D, Garrett HE Jr. Spontaneous pneumomediastinum: a comparative study and review of the literature. Ann Thorac Surg. 2008;86:962–6.
Esayag Y, Furer V, Izbicki G. Spontaneous pneumomediastinum: is a chest X-ray enough? A single-center case series. Isr Med Assoc J. 2008;10:575–8.
Takada K, Matsumoto S, Hiramatsu T, Kojima E, Watanabe H, Sizu M, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med. 2008;102:1329–34.
Dajer-Fadel WL, Arguero-Sanchez R, Ibarra-Perez C, Navarro-Reynoso FP. Systematic review of spontaneous pneumomediastinum: a survey of 22 years’ data. Asian Cardiovasc Thorac Ann. 2014;22:997–1002.
Kim SH, Huh J, Song J, Kang IS. Spontaneous Pneumomediastinum: A Rare Disease Associated with Chest Pain in Adolescents. Yonsei Med J. 2015;56:1437–42.
Lee SC, Lee DH, Kim GJ. Is primary spontaneous pneumomediastinum a truly benign entity? Emerg Med Australas. 2014;26:573–8.
Kim KS, Jeon HW, Moon Y, Kim YD, Ahn MI, Park JK, et al. Clinical experience of spontaneous pneumomediastinum: diagnosis and treatment. J Thorac Dis. 2015;7:1817–24.
Sahni S, Verma S, Grullon J, Esquire A, Patel P, Talwar A. Spontaneous pneumomediastinum: time for consensus. N Am J Med Sci. 2013;5:460–4.
Takada K, Matsumoto S, Hiramatsu T, Kojima E, Shizu M, Okachi S, et al. Spontaneous pneumomediastinum: an algorithm for diagnosis and management. Ther Adv Respir Dis. 2009;3:301–7.
Newcomb AE, Clarke CP. Spontaneous pneumomediastinum: a benign curiosity or a significant problem? Chest. 2005;128:3298–302.
Koullias GJ, Korkolis DP, Wang XJ, Hammond GL. Current assessment and management of spontaneous pneumomediastinum: experience in 24 adult patients. Eur J Cardiothorac Surg. 2004;25:852–5.
Al-Mufarrej F, Badar J, Gharagozloo F, Tempesta B, Strother E, Margolis M. Spontaneous pneumomediastinum: diagnostic and therapeutic interventions. J Cardiothorac Surg. 2008;3:59.
Miura H, Taira O, Hiraguri S, Ohtani K, Kato H. Clinical features of medical pneumomediastinum. Ann Thorac Cardiovasc Surg. 2003;9:188–91.
Campillo-Soto A, Coll-Salinas A, Soria-Aledo V, Blanco-Barrio A, Flores-Pastor B, Candel-arenas M, et al. Spontaneous pneumomediastinum: descriptive study of our experience with 36 cases. Arch Bronconeumol. 2005;41:528–31.
Freixinet J, Garcia F, Rodriguez PM, Santana NB, Quintero CO, Hussein M. Spontaneous pneumomediastinum long-term follow-up. Respir Med. 2005;99:1160–3.
Kaneki T, Kubo K, Kawashima A, Koizumi T, Sekiguchi M, Sone S. Spontaneous pneumomediastinum in 33 patients: yield of chest computed tomography for the diagnosis of the mild type. Respiration. 2000;67:408–11.
Ho AS, Ahmed A, Huang JS, Menias CO, Bhalla S. Multidetector computed tomography of spontaneous versus secondary pneumomediastinum in 89 patients: can multidetector computed tomography be used to reliably distinguish between the 2 entities? J Thorac Imaging. 2012;27:85–92.
Chapdelaine J, Beaunoyer M, Daigneault P, Berube D, Butter A, Ouimet A, et al. Spontaneous pneumomediastinum: are we overinvestigating? J Pediatr Surg. 2004;39:681–4.
Langwieler TE, Steffani KD, Bogoevski DP, Mann O, Izbicki JR. Spontaneous pneumomediastinum. Ann Thorac Surg. 2004;78:711–3.
Jougon JB, Ballester M, Delcambre F, Mac Bride T, Dromer CE, Velly JF. Assessment of spontaneous pneumomediastinum: experience with 12 patients. Ann Thorac Surg. 2003;75:1711–4.
Halperin AK, Deichmann RE. Spontaneous pneumomediastinum: a report of 10 cases and review of the literature. N C Med J. 1985;46:21–3.
Abolnik I, Lossos IS, Breuer R. Spontaneous pneumomediastinum. A report of 25 cases. Chest. 1991;100:93–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Song, IH., Lee, S.Y., Lee, S.J. et al. Diagnosis and treatment of spontaneous pneumomediastinum: experience at a single institution for 10 years. Gen Thorac Cardiovasc Surg 65, 280–284 (2017). https://doi.org/10.1007/s11748-017-0755-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-017-0755-3