Abstract
Adulteration of extra virgin olive oil (EVOO) by addition of other vegetable oils or lower-grade olive oils is a common problem of the oil market worldwide. Therefore, we developed a fast protocol for detection of EVOO adulteration by mass spectrometry fingerprinting of triacylglycerol (TAG) profiles based on MALDI-TOF/MS. For that purpose, EVOO TAG profiles were compared with those of edible sunflower oil and olive oil composed of refined olive oil and virgin olive oils. Adulteration of EVOO was simulated by addition of sunflower and mixture of refined olive oil and virgin olive oils at 1, 10 and 20% w/w. Results of mass spectrometry TAG profiling were compared with routinely assessed K values for identification of adulteration. MALDI-TOF/MS technology coupled with statistical analysis was proven as useful for detection of adulteration in EVOO at a rate down to 1%. In contrast, standard spectrophotometric methods failed to identify minor adulterations. In addition, the ability of MALDI-TOF/MS in detection of adulteration was tested on EVOO samples from different geographical regions. Results demonstrated that MALDI-TOF/MS technology coupled with statistical analysis is able to distinguish adulterated oils from other EVOO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The quality of olive oil is important for customers’ trust towards manufacturers of olive oils. Still, this product is among the most adulterated food products in the world [1,2,3]. Limited amounts of produced olive oil, relatively high production costs, as well as unique properties in comparison with other vegetable oils and seed oils, are factors that contribute to a relatively high market price [2, 4]. A number of adulterations of extra virgin olive oil (EVOO) is due to addition of cheaper vegetable oils such as edible sunflower oil or refined olive pomace oil [2, 4, 5]. Indeed, addition of a cheaper vegetable oil to the EVOO at a rate of 20% might increase illegal profit about 10% [4]. Therefore, standardized control of olive oil is routinely performed within existing legal frameworks by authorized and certified laboratories which determine the quality, durability, and overall features of oil [6]. Still, adulteration often remains difficult to prove as results obtained by use of only one method may not be sufficient for deriving relevant conclusions. The most potent quality control routine method in adulteration detection is spectrophotometric determination of K values: K 232, K 270 and ∆K. K values are calculations based on ultraviolet light absorbance measured at several specific wavelengths. At 232 nm, absorbency is caused by hydroperoxides and conjugated dienes, whereas at 270 nm, absorbency is caused by carbonylic compounds and conjugated trienes. Lower absorbance values between 200 and 300 nm demonstrate a high-quality EVOO, whereas a higher level of absorption in this area indicates oxidation of olive oil and/or addition of refined oils. ∆K detects presence of refined or pomace oil by measuring the difference between absorbance at 270 nm and 266–274 nm [5]. Quality examination and authenticity test results are usually compared with standard values that are set by the International Olive Council (IOC) and European Community (EC) regulations for detection of potential abnormalities [7, 8]. However, current standard analytical methods that are routinely used for quality assessment of EVOO (free fatty acid FFA, peroxide value PV and K values determinations) cannot be used for detection of adulteration if less than 5% vegetable oils are added to the EVOO. Hence, certain limitations in the standard protocols and methods used for assessment of oil quality [2, 5] should be overcome by use of more sensitive and powerful technological platforms.
The composition of triacylglycerol (TAG) is unique for each type of oil and it may be used for detection of EVOO adulteration [5, 6]. Even though TAG is directly connected to and depends on the fatty acyl residue composition [9], it was shown that TAG composition is plant-specific due to the genetically stereospecific plant acylglycerol synthethases [10]. Additionally, TAG composition is well-preserved during the analysis and yields a larger volume of information than fatty acid analysis. TAG can, indeed, provide evidence on addition of lower-quality olive oils (refined olive pomace oil), or other vegetable oils to the EVOO [4]. Still, a challenge in TAG separation and identification exists which is due to large variations between TAG species that have similar physico-chemical properties. Nowadays, methods of choice in TAG profiling are based on mass spectrometry (MS). The most commonly used ionization methods in MS are electrospray ionization (ESI) [11], atmospheric pressure chemical ionization (APCI) [3, 11, 12], and matrix-assisted laser desorption ionization (MALDI) techniques [9, 13, 14]. ESI provides information about exact molecular mass and identification of the individual fatty acyl residues and, therefore, liquid chromatography (LC) coupled with ESI-MS has become the widespread method for such analyses [11]. The advantage of APCI is in yielding less complex mass spectra where identification of positional isomers is also possible [13]. APCI proved to be a powerful tool in combination with ion trap analysers in detection of adulteration of olive oil with soybean oil [3]. Despite these advantages of LC-MS-based methods, in this paper, sensitive MS-based method, i.e. MALDI-TOF (time of flight)/MS was evaluated, due to its high throughput and resolution in TAG analyses in oils [6, 13, 15]. Indeed, it has been shown that TAG mixtures from olive oils, for example, acyl residue composition, can be easily characterized by MALDI-TOF/MS without a need for a time-consuming work-up [16]. Furthermore, TAG ions are exclusively detected as [M + Na]+ adduct ions by MALDI-TOF/MS. In comparison to MALDI-TOF/MS, measurements of vegetable oils with ESI-MS yield significantly more complex and more difficult mass spectra. Indeed, TAG Na+- and NH4+- adduct ions are formed during ESI in different ratios due to the sample and solvent composition [17]. Furthermore, collision-induced dissociation (CID) at high energies provides complete structural TAG characterization including structural isomers [18]. Although this approach was optimized to provide a more precise structural analysis, determination of exact double bond positions in a fatty acyl residue still remains a challenge [6].
In this research, we focused on evaluation of a rapid protocol for determination of adulterated oils by fingerprinting of a full TAG profile without further CID fragmentation. We thus evaluated MALDI-TOF/MS performance for assessment of Croatian EVOO TAG as well as adulteration of the same oil simulated by addition of sunflower oil or olive oil composed of refined olive oil and virgin olive oils. The MALDI-TOF/MS results were analysed and compared with the standard analytical spectrophotometric method.
Materials and Methods
Samples and Chemicals
EVOO from northwest Istria (EVOO sample denoted as 'sample E') was purchased directly from the producer. It was obtained exclusively by the mechanical process of cold pressing of mixed olive varieties, of which the most represented was the Leccino variety. Olive oil, composed from refined olive oil and virgin olive oils (R) was made in Croatian oil factory Trenton d.o.o., Gizdavac, Croatia. Its composition is 50% refined olive oils and 50% virgin olive oils. Sunflower oil (S) was obtained by a refining process in Croatian oil factory Čepin d.o.o., Čepin, Croatia. For simulation of adulteration, 1, 10 and 20% refined sunflower oil and olive oil mixtures were added to E (Table 1). Additional nine EVOO samples that were used for testing the ability of MALDI-TOF/MS adulteration detection were from northwest Istria, three from Dalmatia, two from Italy and one from Bosnia and Herzegovina. All of them were obtained from registered local olive oil producers. Starting test samples are blends of distinctive varieties of each selected region. All samples were stored within glass bottles at 20 °C in the dark. All reagents were analytically pure, except for isooctane purchased from BDH Prolabo (Lutterworth, UK), which was spectrophotometrically pure. Phenolphthalein, potassium hydroxide, sodium thiosulphate pentahydrate, potassium iodide, diethyl ether and acetic acid were also obtained from BDH Prolabo. Starch and chloroform were purchased from Kemika (Zagreb, Croatia), whereas sodium trifluoroacetate, 2,5-dihydroxybenzoic acid (DHB), tetrahydrofuran (THF) and standards bradykinin protein, angiotensin II, P14 R and triolein were purchased from Sigma-Aldrich (St. Louis, MO, USA). 96% ethanol was obtained from Gram-mol (Zagreb, Croatia).
Free Fatty Acids Determination
FFA content is the indicator of oil acidity and was measured according to the official methods described in regulation EC 2568/91 and its further modifications [8]. Analysis was conducted only on EVOO samples. Samples were measured in triplicate. FFA share is expressed as oleic acid percentage.
Peroxide Value Determination
PV is an indicator of hydroperoxide presence in oil samples and was determined according to the official methods described in regulation EC 2568/91 and its further modifications [8]. The method was used only for analysis of EVOO samples. Samples were measured in triplicate and PV is expressed in mmol O2/kg.
UV Spectrophotometer Analysis—Assessment of K 232, K 270 and ΔK values
K values are indicators of oil oxidation. K 232, K 270 and ΔK values were according to the official methods described in regulation EC 2568/91 and its further modifications [8] on a UV-spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). The method was conducted on all tested oil samples dissolved in isooctane. All samples were measured in triplicate. Specific extinctions at 232, 266, 270 and 274 nm were measured, and then K 232, K 270 and ∆K values were calculated.
TAG Analysis by MALDI-TOF/MS Method
The method was tested on all oil samples. Matrix DHB, sodium trifluoroactetate, and oil samples were dissolved in THF to 40 mg/mL, 1 mg/mL and 10 µL/mL, respectively. The final solution was prepared by mixing DHB, sodium trifluoroacetate and sample solution in the ratios of 1:1:2 (v/v/v). A drop of prepared solution was applied on the MALDI ground steel plate in duplicate. The device was calibrated using prepared solution of a standard mixture of bradykinin protein, angiotensin II and P14 R. The samples were analysed using a Bruker Ultraflextreme TOF mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany) equipped with 355-nm smartbeam II laser (Nd:YAG laser) technology. Spectra were recorded in the positive ion reflection mode (laser repetition rate, 2000 Hz; ion source 1 voltage, 20 kV; ion source 2 voltage, 17.9 kV; lens voltage, 7.3 kV; mass range 240–2400 Da), using the FlexControl software package (version 3.4 Bruker Daltonics). To form a single spectrum for each oil sample, 6 independent subspectra (500 shots per subspectrum) from different positions within a sample spot were manually collected. Subsequently, spectra were baseline-subtracted and analysed. Monoisotopic masses were determined using FlexAnalysis (version 3.4 Bruker Daltonics) with the SNAP peak picking algorithm. Peak intensities were corrected for the isotope distribution. The MALDI-TOF/MS method was also tested with TAG standard triolein.
Statistical Analysis
For statistical analysis of obtained analytical data, principal component analysis (PCA) was used. PCA is a statistical tool for identification of similarities and differences among analysed samples. It reduces the data dimensionality, but retains most of its variations. Principal components (PCs) are identified directions along which the data have the largest variations. Due to their orthogonal calculation to each other, each PC can be described separately. PCA plots visualise variances between samples and provide information about sample grouping possibilities [19]. Obtained results were analysed in Statistica 12 (Dell, STATISTICA 12, 2015.).
Results
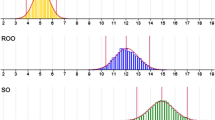
The criteria used to determine EVOO category included (a) assessment of free fatty acids, (b) assessment of peroxide number expressed in mmol O2/kg of oil and (c) assessment of K values. All tested olive oil samples (Table 2): EVOO sample E used for protocol development and additional EVOO samples from the northern Istria, Dalmatia, Italy and Bosnia and Herzegovina (EVOO samples IS, DA, IT and BH) were assigned to the EVOO category according to maximal allowed values [8]. For R and S samples as well as their mixtures, only K values were measured. Comparison of obtained K values was performed according to standard EVOO limit values. Results for K 232 values are presented in Fig. 1, where it can be observed that S and S20 samples were beyond limit values. Hence, they cannot be classified into the EVOO category. Obtained K 270 values show that samples R, R10, R20, S, S10 and S20 were not classified within the EVOO category. ∆K values results confirmed only adulteration by addition of 20% R in EVOO E, while lower amounts of added vegetable oils remained unrecognized.
Comparison of analytically determined K values for E, S and R oils and their mixtures: a K 232, b K 270, c ∆K. The results are presented as averages of three measurements ± standard deviations. Standard EVOO K values limits are K 232 ≤ 2.5; K 270 ≤ 0.22; ∆K ≤ 0.01 (denoted as the horizontal line on the graphs)
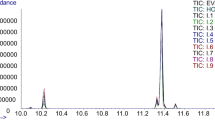
Evaluation of the olive oil TAG profiling in detection of foreign oils addition into the EVOO was performed by use of MALDI-TOF/MS (Table 3). TAG profiles were identified in the mass spectrum region between 850 and 920 m/z (Fig. 2), and the general annotation was done by comparison of experimentally detected m/z values with theoretical m/z values (Table 4) calculated by freely accessible LIPID MAPS Online Tools [20]. For final TAG annotation, previously reported scientific data on TAG composition in olive and sunflower oils were also taken into account [6, 15, 21]. TAG profiles were compared between tested samples E, R and their mixtures (Fig. 3), as well as between E sample used for method development, S and their mixtures (Fig. 4). Changes in the E TAG MS profiles upon addition of foreign oils were visible and are presented in Figs. 3 and 4. and were further analysed by a statistical approach. Specifically, the power of statistical discrimination between EVOO (represented by sample E) and mixtures was tested by use of PCA (Fig. 5) and separation of E from oil mixtures, R and S has been the main objective of this analysis. Principal component 1 (PC1) and principal component 2 (PC2) represented main components that contributed to the highest cumulative variance. The strength of the model was evaluated on additional nine EVOO samples from the same region as E sample and 6 EVOO samples from other regions (Table 2). Results proved that presented model based on TAG profiling by MALDI-TOF/MS in combination with PCA was sufficient to distinguish EVOO oils from R and S oils and their mixtures.
Comparison of relative amounts of the most abundant TAG (m/z): 881.6, 901.7, 903.7, 905.8 and 907.8 for Istrian extra virgin olive oil (E) and olive oil made of refined olive oil and virgin olive oils (R) and its 1% (R1), 10% (R10) and 20% (R20) mixtures. Addition of more R–E gradually changes the TAG composition and increases the contribution of minor TAG whilst decreasing the contribution of most abundant TAG
Comparison of relative amounts of the most abundant TAG (m/z): 881.6, 901.7, 903.7, 905.8 and 907.8 in Istrian extra virgin olive oil (E) and sunflower oil (S) and corresponding 1% (S1), 10% (S10) and 20% (S20) mixtures. Addition of more S to E gradually changes the TAG composition and increases the contribution of minor TAG while decreasing the contribution of most abundant TAG
Discussion
K values are important parameters that provide information on the olive oil quality. This is due to the olive oil undergoing oxidation that causes the formation of conjugated dienes and trienes that absorb ultraviolet light at specific wavelengths. Furthermore, K values may be used for identification of foreign oils in EVOO which may not be performed by other routine analysis, e.g. fatty acid determination or determination of peroxide values [5]. Therefore, in this paper, absorbance at 232 nm and 270 nm was measured for a set of different oils comprising EVOO, sunflower oil, olive oil and their mixtures. Sunflower oil sample S was easily recognized as non-EVOO oil by assessment of its K values. Olive oil sample R composed of refined olive oil and virgin olive oils had higher K 270 and ∆K absorbance values than those set for the EVOO category. Indeed, S and R samples were obtained by a refining process, and the presence of conjugated dienes and trienes was expected as these are indicators of such a production method [1] and EVOO quality is known to be ruined by addition of lower-quality oils. Although assessment of K values was not sufficient for detection of S and R addition at the level of 1% to the EVOO E sample used for method development, determination of conjugated triene (K 270) successfully detected at least 10% addition of R, and 10% addition of S oils. In comparison to Škevin et al. [4], where the K 232 value did not prove successful in detection of 20% sunflower oil or refined olive pomace oil addition to the EVOO, in this research, 20% of S was successfully detected in E by measurement of K 232. However, in both studies, K 270 values were able to detect addition of 10% of sunflower oil and refined olive pomace oil or olive oil to the EVOO. Škevin et al. [4] point out the great potential of ∆K values for determining EVOO adulteration detection at 1–10% sunflower and refined olive pomace oil addition, while Houshia et al. [5] mention a detection limit for this method above 5% addition of refined oils. In contrast, our results showed that the ∆K value was valuable for detection of only higher percentages of R oils (20%) to the E. Clearly, our rationale for testing a more sensitive analytical method for detection of EVOO adulteration by addition of refined oils was confirmed. Therefore, the MALDI-TOF/MS method was chosen for analysis of simulated adulteration in EVOO samples as it can be used for fast and accurate mapping of TAG profiles specific for each oil type [6, 10, 13, 15, 17, 20] and in particular, TAG "fingerprints” have been already tested as a possible parameter for detection of EVOO adulteration [3]. Upon MALDI-TOF/MS profiling of tested oil samples, we observed that identified TAGs (Table 4) were differently represented in oil samples (Table 3). For example, oleic fatty acid was the most common one in the olive oil [22], and it would be expected that TAG triolein (OOO) would be, by analogy, the most represented in E as well. The E sample contained mainly TAG at m/z 907.8 (43.21%) and 881.8 (32.19%; Table 3) whose structures most likely correspond to OOO and TAG composed by one palmitoyl residue and two oleoyl residues (POO), respectively (Table 4). These results were already well confirmed in literature [6, 23]. In the S sample, the most common TAG were at m/z 901.7 (22.83%), 903.7 (33.18%) and 905.8 (17.87%; Table 3). Sodiated TAG ions located at m/z 901.7 likely correspond to the TAG composed of three linoleoyl residues on the glycerol backbone (LLL), whereas m/z 903.7 likely correspond to the TAG with two linoleoyl residues and one oleoyl residue (LLO/LOL). Furthermore, it is assumed that at position m/z 905.8 TAG is composed of one linoleoyl and two oleoyl residues on the glycerol backbone (LOO). In the R sample, the most common TAGs were at m/z 901.7 (15.85%), 903.7 (22.02%), 905.8 (18.83%) and 907.7 (19.44%; Table 3). In conclusion, different TAG profiles were assessed for analysed vegetable oils (Fig. 2).
Individual comparison of TAGs in sunflower mixtures (Fig. 3) showed that a higher percentage of added S was accompanied by increased occurrence of LLL (m/z 901.7), LLO/LOL (m/z 903.7) and LOO (m/z 905.8) TAG, that indicates changes in the structure of TAG in E. Since the natural sunflower oil contains 41–77% of linoleic acid (C 18:2), it belongs to linoleic acid-rich oils [15]. Each change in this TAG profile may be indicative of possible adulteration [1, 3]. The S-specific TAGs, therefore, have little or no presence in the E sample, and an addition of 10 and 20% of S in EVOOs can be noticed as a change in the TAG profile. Furthermore, within the S1 oil mixture, there has been a decrease in TAG POO (m/z 881.8) quantity, which means that TAG OOO (m/z 907.8) is more frequent, regardless of its intensity in the mass spectrum, and is equal to that in E. Individual comparison of TAG profiles of E with TAG profiles for R oil and their mixtures (Fig. 4) showed that a higher percentage of added R was accompanied by increased LLL (m/z 901.7), LLO/LOL (m/z 903.7) and LOO (m/z 905.8). TAG ion intensities are indicative for TAG composition changes in EVOO. The results showed the power of MALDI-TOF/MS to identify 10 and 20% R addition to the E. Furthermore, even the addition of 1% R had a slight influence on the TAG profile, altering the abundance of some individual TAG molecular species, i.e. m/z 907.8. However, for a majority of individual molecular species, 10% R addition was required for significant alteration of individual TAG species. Therefore, the next step involved the statistical analysis of complete TAG profiles for separation of adulterated samples from the originals based on measured TAG spectra by MALDI-TOF/MS. Previous reports demonstrated that for adulteration recognition, a great potential is in complete TAG profiling [24]. Also, additional MS/MS fragmentation is crucial for differentiation of TAG positional isomers which may provide additional useful information for vegetable oil origin detection [18], but requires additional time and expertise.
PCA was used for MS data interpretation for detection of adulteration of EVOO. Therefore, in Fig. 5, projection of samples’ MS data is given in the PC areas (PC1 and PC2). The first two PC factors explain 77.59 and 10.62% of total variance in expression data, respectively. According to their authenticity upon simulated adulteration, a separation of oil samples can be observed. PC1 shows the largest variation in the analysed data and provides the largest portion of information. The E sample is, consequently, located at the negative side along the PC1 axis and the positive side along the PC2 axis and it is well-separated from S to R samples that are located in the highly positive area of PC1. Therefore, S and R samples have sizable roles in explaining the PC1 variation. When oil mixtures were grouped along the negative side of PC2 as a separate category in opposition to E, detection of mixtures of E with S or R at 1% was possible. Evaluation of the MALDI-TOF/MS in detection of olive oil adulteration with additional nine EVOO samples from the same region as the E sample, three samples from Dalmatia, two from Italy and one from Bosnia and Herzegovina by PCA (Fig. 6), showed a good classification power. PC1 explains 66.06% whereas PC2 15.29% of total variance in expression data. All Istrian samples were grouped in the restricted area of the principal component plot close to the E sample. Additionally, EVOO from other regions was mostly separated from Istrian samples, but also from altered ones (Fig. 6). In conclusion, all EVOO samples were distinguished from altered samples, so PCA was successful in separation of EVOO samples from altered ones regardless the oil's geographical origin.
However, considering the influence of climate and region on fatty acyl residues ratios in oils [6, 21], it would be necessary to test the impact of olive oil adulteration for each region separately.
Bearing in mind all these facts, it may be concluded that the MALDI-TOF/MS approach is generally useful for mapping of oil TAG profiles with high sensitivity in detection of even minor changes in TAG profile caused by adulteration.
Conclusions
MALDI-TOF/MS analysis proved useful in mapping of TAG profiles in different vegetable oils, and specific TAG "fingerprints“ were obtained for EVOO, S and R samples which proves this high-throughput, analytical method is reliable for olive type verification purposes. Obtained TAG profiles might be used for detection of EVOO adulteration by addition of sunflower oil and olive oil composed of refined olive oil and virgin olive oils as well. For the majority of individual TAG molecular species, 10% addition of foreign oils was required for confident detection of adulteration. However, complete TAG profile statistical analysis by use of PCA proved to be more efficient in separation of adulterated samples from the originals. Such a combined approach enabled separation of different oil categories and proved effective in detection of adulterated EVOO mixtures up to 1% addition of S and R. In comparison with the K values method, MALDI-TOF/MS proved better for detection of adulterated EVOO. Furthermore, our model was tested by addition of other EVOO and our approach successfully distinguished EVOO samples from adulterated test sample. Finally, additional analyses to determine the geographical impact on the final results are necessary in MALDI-TOF/MS-based adulteration studies of oils.
References
Garcia R, Martins N, Cabrita MJ (2013) Putative markers of adulteration of extra virgin olive oil with refined olive oil: prospects and limitations. Food Res Int 54:2039–2044
Dalmia A (2014) Rapid Measurement of olive oil adulteration with seed oils with minimal sample preparation using DSA/TOF. PerkinElmer Inc, Waltham
Fasciotti M, Pereira Netto AD (2010) Optimization and application of methods of triacylglycerol evaluation for characterization of olive oil adulteration by soybean oil with HPLC–APCI-MS–MS. Talanta 81:1116–1125
Škevin D, Kraljić K, Miletić L, Obranović M, Neđeral S, Petričević S (2011) Adulteration of Oblica virgin olive oil with edible sunflower and refined olive pomace oil. Hrvat. Časopis Za Prehrambenu Tehnol Biotehnol Nutr 6:117–122
Houshia OJ, Zaid O, Shqair H, Zaid M, Fashafsheh N, Bzoor R (2014) Effect of olive oil adulteration on peroxide value, delta-K and on the acidity Nabali-Baladi olive oil quality. Adv Life Sci 4:235–244
Peršurić Ž, Osuga J, Galinac Grbac T, Peter-Katalinić J, Kraljević Pavelić S (2016) MALDI-SpiralTOF technology for assessment of triacylglycerols in Croatian olive oils. Eur J Lipid Sci Technol. doi:10.1002/ejlt.201500375
International Olive Council (IOC) (2011) Trade standard applying to olive oils and olive-pomace oils. COI/T.15/NC No 3/Rev. 8
Commission European (1991) Characteristics of olive oil and olive-residue oil and relevant methods of analysis. commission regulation EEC/2568/91 as amended. Off J Eur Communities 310:1–83
Firestone D (1999) Physical and chemical characteristics of oils, fats and waxes. AOCS Press 81:99–101
Alves E, Melo T, Rey F, Moreira ASP, Domingues P, Domingues MR (2016) Polar lipid profiling of olive oils as a useful tool in helping to decipher their unique fingerprint. LWT-Food Sci Technol 74:371–377
Cozzolino R, De Giulio B (2011) Application of ESI and MALDI-TOF MS for triacylglycerols analysis in edible oils. Eur J Lipid Sci Technol 113:160–167
Holčapek M, Jandera P, Zderadička P, Hrubá L (2003) Characterization of triacylglycerol and diacylglycerol composition of plant oils using high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J Chromatogr A 1010:195–215
Smaniotto A, Montanari L, Flego C, Rizzi A, Ragazzi E, Seraglia R, Traldi P (2008) Can crude oils be distinguished by different component distribution obtained by laser desorption ionization mass spectrometry and evaluated by chemometrics? Rapid Commun Mass Spectrom 22:1597–1606
Asbury GR, Al-Saad K, Siems WF, Hannan RM, Hill HH (1999) Analysis of triacylglycerols and whole oils by matrix-assisted laser desorption/ionization time of flight mass spectrometry. J Am Soc Mass Spectrom 10:983–991
Krist S, Stuebiger G, Bail S, Unterweger H (2006) Detection of adulteration of poppy seed oil with sunflower oil based on volatiles and triacylglycerol composition. J Agric Food Chem 54:6385–6389
Hillenkamp F, Peter-Katalinic J (2013) MALDI MS: a practical guide to instrumentation, methods, and applications, 2nd edn. Wiley, United States
Weise C, Achstetter T, Kloeck G, Arrey T, Moehring T, Zeller M, Strupat K, Scientific TF (2012) Characterization of triacylglycerides in vegetable oils upon MALDI LTQ orbitrap XL. Thermo Fisher Scientific, United States
Pittenauer E, Allmaier G (2009) The renaissance of high-energy CID for structural elucidation of complex lipids: MALDI-TOF/RTOF-MS of alkali cationized triacylglycerols. J Am Soc Mass Spectrom 20:1037–1047
Jolliffe I (2002) Principal component analysis, 2nd edn. Springer, United States
Fahy E, Sud M, Cotter D, Subramaniam S (2007) LIPID MAPS online tools for lipid research. Nucleic Acids Res 35:606–612
Chapagain BP, Wiesman Z (2009) MALDI-TOF/MS Fingerprinting of triacylglycerols (TAG) in olive oils produced in the Israeli Negev Desert. J Agric Food Chem 57:1135–1142
Koprivnjak O, Vrhovnik I, Hladnik T, Prgomet Ž, Hlevnjak B, Majetić Germek V (2012) Characteristics of nutritive value of virgin olive oils from Buža, Istarska bjelica, Leccino and Rosulja cultivars. Hrvat Čas Prehrambenu Tehnol Biotehnol Nutr 7:172–178
Kubo A, Satoh T, Itoh Y, Hashimoto M, Tamura J, Cody RB (2013) Structural analysis of triacylglycerols by using a MALDI-TOF/TOF system with monoisotopic precursor selection. J Am Soc Mass Spectrom 24:684–689
Holčapek M, Lísa M (2009) Statistical evaluation of triacylglycerol composition by HPLC/APCI-MS. Lipid Technol 21:261–265
Acknowledgements
We greatly appreciate access to equipment in possession of University of Rijeka within the project “Research Infrastructure for Campus-based Laboratories at University of Rijeka”, financed by the European Regional Development Fund (ERDF). We also acknowledge University of Rijeka research support 13.11.1.1.11.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Jergović, AM., Peršurić, Ž., Saftić, L. et al. Evaluation of MALDI-TOF/MS Technology in Olive Oil Adulteration. J Am Oil Chem Soc 94, 749–757 (2017). https://doi.org/10.1007/s11746-017-2994-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-017-2994-y