Abstract
Lipases from Geotrichum candidum were produced in two different medium: A = 12 % (w/v) clarified corn steep liquor (CCSL) + 0.6 % (w/v) soybean oil (SO) and B = 3.5 % (w/v) yeast hydrolysate (YH) + 0.7 % (w/v) SO. Lipases were partially purified from both media by hydrophobic interaction chromatography using 3.0 mol L−1 of NaCl as mobile phase, and they were characterized in the crude and partially purified forms. The recovery of lipase activity from CCSL and YH via HIC were 96 and 94.3 %, and the purification factors were 44.3 and 86.7-fold, respectively. All evaluated lipases had similar optimum pH (7.0–7.7), but, for the CCSL crude lipase, optimum temperature (47 °C) was 10 °C higher than others lipases evaluated. CCSL crude lipase possessed a higher thermo stability than YH crude lipase, e.g., at 37 °C (pH 7.0) the half-life of CCSL crude lipase was 19.25 h and at pH 8.0 (30 °C) the half-life was 48 h, which are five and ten times higher than with YH crude lipase, respectively. On the other hand, the YH crude lipase possessed a higher catalytic constant (k cat = 2.3 min−1) but with almost the same catalytic efficiency (K m/k cat = 32.12 mg mL min−1) in relation to CCSL crude lipase. The lipases differ in biocatalytic properties between substrates, suggesting that the two lipases can be employed for different applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipases (E.C.3.1.1.3), commonly encountered in many animals, plants and microorganisms, are responsible for catalyzing the hydrolysis of ester bonds at the lipid/water interface and they can be used in the several applications in the food, beverage, chemical, leather, medical, waste-water-treatment and detergent industries [1, 2]. Therefore, studies focusing upon production, purification and characterization of lipases are relevant to demonstrate their potential utility.
Among other downstream purification methods, hydrophobic interaction chromatography (HIC) is a suitable technique for lipase purification due to the high degree of hydrophobicity that occurs on the surface of these enzymes. Mendieta-Taboata et al. applied this technique as a single step for the purification of lipase from Geotrichum sp. with a butyl Sepharose column and it was observed that the kinetics parameters of the adsorption isotherms were very dependent of NaCl concentration [3]. The maximum column efficiency of 71 % was obtained in the cited study using 2.0 mol L−1 of NaCl and 0.76 cm min−1 of superficial velocity [3].
Another important aspect that controls the purification of lipases and their resultant biocatalytic properties is the medium composition for their production via fermentation. Use of alternative nitrogen sources (such as agro-industrial residues) is an important consideration for the fermentation process since it is possible to reduce costs. Due to the complex composition of these sources, upstream purification steps (such as clarification) may be necessary to facilitate the downstream steps, for example, to remove contaminants that can inactivate chromatographic columns [1, 4–6].
Therefore, to complement published studies on lipases from G. candidum and Geotrichum sp. [7–18], the goal of this study is to investigate the effect of alternative substrates clarified corn steep liquor (CCSL) [11] and yeast hydrolysate (YH) [8], for G. candidum cultivation on the biocatalytic properties of the lipases produced. Lipases were partially purified from the fermentation broths in one step by hydrophobic interaction chromatography and then, crude and partial purified lipases from the two substrates were evaluated for optimal pH and temperature. Thermal and freezing stability; activation and denaturation energies and kinetic constants were also evaluated only for crude lipases.
Materials and Methods
Microorganism and Inoculum
Geotrichum candidum NRRLY-552, kindly provided by USDA Agricultural Research Service Collection (Washington DC, USA), was maintained in yeast malt agar (% w/v: 0.3 % yeast extract, 0.3 % malt extract, 0.5 % peptone, 1.0 % glucose and 3.0 % agar) at 4 °C. The inoculum was prepared from spores obtained from yeast malt agar using 1.0 mL of distilled water. These spores were incubated on yeast malt agar in petri dish for 48 h at 30 °C. A circular disk was then cut out and added to a 500 mL Erlenmeyer flask containing 100 mL of the inoculum medium (% w/v: 5.0 % peptone, 0.1 % NaNO3, 0.1 % MgSO4 and 1.0 % soybean oil). This medium was incubated for 15 h at 30 °C and 250 rpm. Subsequently, 10 % (v/v) of inoculum mediumwas transferred to the fermentation medium [7–12, 15].
Medium Composition and Growth Conditions
Geotrichum. candidum cultivation was conducted in 500 mL conical shake flasks containing 100 mL of medium with an initial pH of 7.0 at 30 °C and 250 rpm for 48 h. Two different nitrogen sources were employed in this study:
-
medium A: 12.0 % (w/v) clarified corn steep liquor (CCSL) and 0.6 % (w/v) soybean oil [11], which was clarified via activated carbon per the method of Treichel et al. [5].
-
medium B: 3.5 % (w/v) yeast hydrolysate and 0.7 % (w/v) soybean oil [8]. No clarification method was applied.
Clarified corn steep liquor (CCSL) and YH (Prodex Lac®) were donated by Ingredion™ (São Paulo, Brazil) and Prodesa (São Paulo, Brazil), respectively.
Partial Purification of Lipase
Partial purification of lipases obtained from media A and B was carried out through a single hydrophobic interaction chromatographic (HIC) step [3]. Fermentation broth containing crude lipases was treated with vacuum filtration to remove cellular debris and major impurities prior to HIC. The separation was performed using a GE Amersham Bioscience AKTA fast protein liquid chromatography M-925 INV-907 FPLC system (Anaheim, USA) with a column loaded with butyl sepharose stationary phase. Phosphate buffer (0.01 mol L−1, pH 7.0) with different NaCl concentrations (2, 3 and 4 mol L−1), served as mobile phase in the adsorption, delivered at 0.5 mL min−1 and filtered prior to use. In the elution, the same buffer without NaCl was used. In preliminary tests, an analytical-scale column (1 cm × 1 cm ID) was used with buffer at three different NaCl concentrations (2, 3 and 4 mol L−1) and fractions (8.0 mL) were collected. The percentage of recovery (R) was calculated by the relationship:
where U purified and U crude refer to the total lipase activity (U) for the purified and crude lipase, respectively. The concentration of 3.0 mol L−1 of NaCl was then selected as the mobile phase for preparative-scale separations, which employed a 10 cm × 1 cm ID column and fractions of 20 mL. The purification factor (P) was calculated at the ratio of the specific activity (U mg−1) for purified lipase to that of crude lipase. Protein content, determined by Lowry’s method, may reflect the concentration of both lipases and impurities not removed by HIC. The samples with the highest lipase activity were selected for analysis of optimal pH and temperature. For sample identification, crude and (partially) purified lipases are identified by C and P; respectively, while the medium used to produce each lipase are identified by A and B for CCSL and YH, respectively.
Characterization of the Biocatalytic Activity of Lipases
The hydrolytic activity of lipases was measured in triplicate for each condition using a tritimetric assay. The reaction mixture consisted of 19 mL of an oil-in-water emulsion containing olive oil (5 % w/v) and gum Arabic (5 % w/v) emulsified in 100 mmol L−1 potassium phosphate buffer, pH 7.0. This mixture was homogenized in a blender for 3 min and the lipolytic reaction started by adding 1 mL of lipase solution. The assay was carried out at 37 °C and 200 rpm (r = 2.5 cm) for 30 min. The reaction was then stopped by adding 20 mL of acetone–ethanol 1:1 (v/v). The amount of free fatty acid produced was determined via titration with 0.05 mol L−1 NaOH until reaching an endpoint of pH 11.0, via an automatic titration apparatus (DL21 Mettler Toledo, Barueri, Brazil). However to determine optimum pH and temperature, the conditions of temperature and pH used in the analysis of lipase activity were adjusted accordingly. One unit of lipase activity (U) was defined as the amount of enzyme that liberated 1 μmol of fatty acid per minute under the assay conditions [7–13, 20, 21].
The optimum pH and temperature conditions were determined for crude and partially purified lipases from media A and B, while crude lipases from A and B were also characterized for stability in relation to pH (6–8), temperature (25–60 °C), and freezer storage (−18 °C), and kinetic parameters. The enzymatic solutions used for characterization contained 13.00 and 9.90 U mL−1 (medium A) and 28.16 and 9.43 U mL−1 (medium B) for the crude and partially purified lipases, respectively.
Optimum Temperature and pH
Central composite rotatable designs (four factorial trials, four axial points and three central points) [19] were applied to evaluate the effect of the independent variables: temperature (27–47 °C) and pH (6.0–8.0), in the activity of the crude and partial purified lipases obtained from media A and B. The evaluated ranges of temperature and pH were defined based in previous studies [7–15]. The design matrix and the results obtained are presented in Table 2. For validation of optimum pH and temperature, the activity of C–A lipase was investigated at pH 7.0 vs. temperature, from 27 to 55 °C.
Thermal Stability
Thermal stability of crude lipases from media A and B were determined through incubation of the enzyme in phosphate buffer (100 mmol L−1/pH = 7.0) in different temperatures (25–60 °C). Samples of each condition were collected in different times and lipase activity was measured. The results obtained were used to calculate the deactivation constant (k d; min−1) and the half-life (t 1/2; min) for each temperature evaluated according to Eqs. 2 and 3, where v is lipase activity at incubation time t (μmol min−1) and v 0 is the lipase activity at time zero.
In order to evaluate the crude lipase stability regarding a freezing storage (−18 °C and pH 7.0 during 45 days), the samples were defrosted and their activities measured as described above. The stability of crude lipases at several different pH values (6–8) was determined as described above, with incubation occurring at 30 °C [20].
Activation (E a) and Denaturation (E d) Energies
The activation energy (E a) was calculated for the crude lipases using the data obtained at 27–55 °C, pH 7.0 according to Arrhenius equation (Eq. 4)
where R is the ideal gas law constant and T is the absolute temperature (K). The denaturation energy (E d) was calculated from k d constants through an Arrhenius relationship (Eq. 5):
where k is the denaturation constant of the enzymatic reaction at the temperature of reference.
Determination of Kinetic Parameters k cat and K m/k cat
The Michaelis–Menten constants K m (mg mL−1) and V max(U mL−1) were estimated via a Lineweaver–Burke plot: (1/v) versus (1/S), where S refers to the concentration of substrate (olive oil), ranging from 8.0 to 100 mg mL−1. The catalytic constant k cat (min−1) was obtained by the expression (V max/[E]), where [E] is the enzyme concentration (U mL−1). The catalytic efficiency, represented by the ratio K m/k cat (mg min mL−1), was also obtained for each crude enzyme. Each data point of the Lineweaver–Burk plot represents a separate experiment.
Results and Discussion
Partial Purification of Lipase
Preliminary assays were carried out to select the best NaCl concentration for the HIC mobile phase. The recovery values (%R) obtained for the lipases produced with CCSL and YH are presented in Table 1. According to these results, %R values for CCSL lipase were not affected by NaCl concentration, and for YH lipase, the 4.0 mol L−1 NaCl maximized %R. However, the condition with the lowest recovery factors (3.0 mol L−1 of NaCl) was selected, for both media, because it allowed the recovery of lipase in just one fraction, which facilitates the subsequent steps of purification and also the characterization study of the enzyme. In subsequent experiments, the height of resin in the column was increased to 10 cm, the quantity of crude lipase injected was also increased to 20 mL and samples of 10 mL were collected. Under these conditions, partially purified CCSL- and YH-lipases were obtained with activities of 9.90 and 9.43 U mL−1, respectively. The pooled fractions were the second and the third fraction for CCSL- and YH-lipase, respectively. Desorption occurred within a short time after the removal of salt from the buffer solution (Fig. 1). The recovery factor (R) were 96.0 and 94.3 % for CCSL- and YH-lipases, respectively, when employing HIC using the 10 cm column, representing a 2.1- and 2.4-fold higher value than obtained using a column with 1 cm of height.
Hydrophobic interaction chromatography for lipases from G. candidum NRRLY-552 cultivated in: a clarified corn steep liquor and b yeast hydrolysate. Experiments were conducted with a 10 cm high butyl Sheparose® column and a mobile phase (phosphate buffer 0.01 mol L−1, pH 7.0) with 3.0 mol L−1 of NaCl and a flow rate of 0.5 mL min−1
The purification factors (P) obtained were 44.3 and 86.7 for the CCSL- and the YH-lipases, respectively, using a column with 10 cm of height. HIC performed well for recovery of lipases from both media. Perhaps P was lower for CCSL-lipase because of the clarification step employed, which facilitates the downstream HIC step.
The partial purification of lipase from Geotrichum sp. cultivated in corn steep liquor (CSL) (not clarified) using the same technique presented lower recoveries (R) values from 60 to 68 % [3, 17, 18], which show that the clarification improved purification via HIC, similar to that described above for CCSL. Only a partial HIC-based purification was performed in the present study because a good lipase activity was achieved in a single HIC step, leading to lower purification cost. Purification of lipases has been achieved by several different bioseparations techniques, generally with more than one step, and a few of them are discussed here for comparison. Brabcová et al. obtained purification factors of 1.2–24.2, for three different extracellular lipases from G. candidum 4013 by cascade adsorption and desorption with Triton X-100 [22]. Cai et al., when working with lipases from Geotrichum sp. SYBC WU-3, were able to purify two different extracellular lipases with recovery factors of 9.2 and 11.2 % with ammonium sulfate precipitation followed by ion exchange and gel filtration chromatographies [23]. Huang et al. purified lipases from G. marinum (ATCC 20614) using affinity and gel-filtration chromatography and achieved a purification factor of 76 and a recovery factor of 46 % [24]. Even though in this present study the purification consisted of just one chromatographic step, the R and P factors obtained by us were comparable to or higher than the studies cited.
Characterization of Lipases
Optimum pH and Temperature
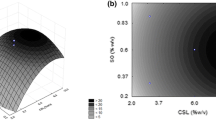
The effects of pH and temperature on the lipolytic activity of crude (C-A and C-B) and partially purified lipases (P-A and P-B) obtained using CCSL (medium A) and YH (medium B), were determined using central composite rotational designs (CCRD) to each situation. The results are shown in Table 2. Considering only the statistically significant coefficients (p < 0.10), models derived from the CCRD are depicted at Table 3. According to the analysis of variance (ANOVA), all four models were statistically significant at 90 % confidence (p < 0.10). However, by analyzing the coefficients of determination (R 2) and adjusted determination (\(R_{\text{adj}}^{ 2}\)), it is observable that the P-A model presented the lowest values and it is not reliable; but the other three models resulted in acceptable values (R 2 ≥ 0.75; \(R_{\text{adj}}^{ 2}\) ≥ 0.69). The surface responses and contour curves obtained can be seen in Figs. 2 and 3 and the discussion of each lipase (C-A, P-A, C-B and P-B) is described in the next paragraphs.
Lipase activity (U mL−1) as function of temperature and pH for the lipase from G. candidum NRRLY-552 cultivated in medium B [3.5 % of yeast hydrolysate and 0.7 % (v/v) of soybean oil] at 30 °C, 250 rpm, 48 h and initial pH of 7.0: a and c response surfaces for the crude (C-B) and partially purified (P-B) lipases, respectively; b and d contour curves for C-B and P-B, respectively
According to C-A model, the optimal conditions for lipase activity were 47 °C and pH 7.0, resulting in a predicted value of 15.88 ± 2.30 U mL−1 (90 % confidence), in agreement with the experimental value operated under the same conditions (17.52 ± 0.16 U mL−1; Table 2). The relative errors
where Exp and Pred refer to experimental and predict values, respectively, ranged, in general, from 1.0 to 10 % under all conditions evaluated, which reinforces the result that the C-A model is in agreement with the experimental values. According to Fig. 2, the optimum pH is near the central point condition (pH = 7.0) and the optimum temperature within the parameter space investigated (47 °C) was the highest level evaluated. For that reason, complementary assays were carried out in triplicate at fixed pH value (7.0) in order to investigate higher temperatures. The results obtained (Fig. 4) indicates the highest values of activity are between 47 to 53 °C.
The model for P-A showed the lowest values of R 2 and \(R_{\text{adj}}^{ 2}\); however the best conditions predicted by the P-A model are also in agreement with the experimental values (Table 2). In addition, the highest relative errors were obtained with the axial points (i.e., the highest temperature and the lowest pH). According to Table 2, the central points and the trial four resulted in the highest values of activity for P-A lipase, which corresponds to optimum conditions of pH (7.0–7.7) and temperature (37–44 °C). Comparatively, the C-A lipase showed optimum temperature 10 °C above (Fig. 4) than P-A lipase, but P-A lipase had more efficient in higher values of pH (7.0–7.7; Table 2) than C-A lipase. These differences can permit different applications for the two partial purified lipases according to the medium of production.
For the lipases produced in the YH medium, C-B and P-B, the best conditions were in the central points (37 °C and pH 7.0), which resulted in lipase activities of 27.0 and 9.0 U mL−1, respectively. The C-B and P-B models presented were able to predict values in the optimal region with low relative errors in relation to experimental values (4.2 and 3.6 %, respectively). It was possible to observe that the substrate used in medium B did not modify the optimal pH significantly in comparison to the use of CCSL, although, the activities for both lipases (C-B and P-B) were higher when compared to CCSL-lipase (C-A and P-A). Since the substrates, CCSL and YH, have almost the same amount of total protein in its composition (43 and 44 %, respectively), it is possible to evaluate the specific protein composition that may have a direct impact in lipase production by G. candidum.
The most significant difference was that the optimum temperature for C-A lipase which was 10 °C higher than the other lipases (P-A, C-B and P-B). The clarification step applied in medium A may protect the crude lipase at higher temperature which did not occur for medium B (not clarified). The purification step may remove this protection, which can explain the same optimum temperature for P-A and P-B lipase. The clarification step resulted in a lipase (C-A) with highest optimal temperature in this study, which is a great advantage for several industrial applications. This procedure could be applied in other further studies using alternative substrates in order to obtain other lipases with highest optimal temperature as C-A lipase.
From the literature, it is possible to cite some optimum points observed with different lipases, such as: pH 7.0/37 and 50 °C for the partially purified lipase from Geotrichum-like strain R59 [25]; pH 7.0/40 °C for the purified lipase from G. candidum [26]; and pH 9.0/35 and pH 8.0/35 °C for the cell-bond and the extracellular purified lipases from G. candidum 4013, respectively [27]. The optimal pH and temperature results observed here in agree with the literature.
Thermal Stability
Crude lipases produced with two nitrogen sources (CCSL and YH) were incubated at different temperatures to determine their thermal stabilities. The results obtained for the denaturation constant (k d) and half-life (t 1/2) at several different temperatures are presented in Table 4. At 37 °C, C-A possessed a t 1/2 of 19.25 h, which is 5.0-fold higher than C-B. At 50 °C the estimated t 1/2 for lipases obtained from both media were very low, with C-A having a slightly higher stability. Goldbeck and Maugeri Filho obtained three lipases from yeast strains with similar t 1/2 values: at 30 °C, 16–20 h and at 37 °C, 2–3 h [28].
To determine the thermal stability under freezer conditions, both crude lipases were frozen and maintained at −18 °C and evaluated from 0 to 45 days. At the end of this period, 98 % of the lipase activity was retained for C-A and 63 % for C-B (data not presented), demonstrating that CCSL exhibited superior stability as occurred for other temperatures, perhaps due to the removal of certain impurities or ions that are inactivate via the clarification step. Loo et al. obtained a similar result but with a mycelium-bound lipase from G. candidum IMI 387428, stored at 4 °C for 1 month, with a 4.3 % reduction on its activity [29].
Determination of the Activation (E a) and the Denaturation (E d) Energies
The k d values were used for the determination of E a and E d for lipases recovered from both media and the results are presented in Table 4. Lower values of E a and E d indicate that the reaction will more readily occur. Based on the estimated energy values for C-A, the energy required to initiate denaturation is 16-fold higher than for activation; for C-B, denaturation is only twofold higher. This suggests that C-A is more stable.
The energies determined in this study are similar to values reported in the literature; for example, lipases from different yeast strains presented E d values between 45–50 kcal mol−1 [28]. A lipase from Staphylococcus epidermidis has an E a value estimated to be 6.25 kcal mol−1 [30]. E a and E d for Candida antartica lipase are 4.13 and11.94 kJ mol−1, respectively [31]. Lipase from Bacillus subtilis NS 8 had an E a value of 17.43 kJ mol−1 [32].
pH Stability
Three important pH values were selected to evaluate the stability of the lipases produced using alternative nitrogen substrates (CCSL and YH) and the k d and t 1/2 values obtained are presented in Table 4. The literature demonstrates that pH 8.0 is harmful for the lipase activity, indicating the end of fermentation; pH 7.0 is usually determined as an optimum condition and pH 6.0 is a favorable condition for reactions such as the activity determination [7–12, 15].
As the results show for C-B, the estimated t 1/2 at pH 8.0 is 11-fold lower than at pH 7.0. The same was not observed for C-A; moreover, t1/2 did not differ significantly between pH 8.0 and 7.0. The ability to work in alkaline pHs is a great advantage for lipase to allow its use in detergent products. Perhaps the clarification step for C-A prevented its denaturation at pH 8. In addition, C-B possessed a very similar t 1/2 value at pH 6.0 and 7.0, in contrast to C-A. Our results for C-B agree with those of Burkert et al., who observed that lipase from Geotrichum candidum cultivated with peptone had a t 1/2 of 20 h at 30 °C and pH 7.0, but when the pH was increased to 8.0 at the end of the fermentation, a rapid decrease in lipase activity was observed.
Determination of the Kinetic Parameters k cat and K m/k cat
From the kinetic data (using Lineweaver–Burke plot) the kinetic constants V max and K m were estimated (R 2 ≈ 0.97—Supplementary File 1) and from these parameters the turnover number (k cat) and the catalytic efficiency (K m/k cat), were calculated. k cat represents the time required by one enzymatic unit to “turn over” one milligram of substrate and the K m/k cat serves as a measure of the “preference” of substrates. According to the results (Fig. 5), lipolysis by C-B lipase was almost 74 % faster than C-A lipase, and the former presented a preference for the substrate almost 14 % higher than the latter. By applying the same approach, Brabcová et al. obtained a k cat equivalent to 1.5 min−1 and a K m/k cat of almost 0.27 mM−1 min−1 with p-nitrophenylpalmitate as the substrate for an extracellular lipase from G. candidum 4013 [33]; and Huang et al. determined, for a purified extracellular lipase from G. marinum, a k cat value of 0.13 min−1 and a K m/k cat of 88.5 mM−1 min−1 using olive oil emulsified with taurocholic acid as the substrate [24]. As it can be seen, although values can vary depending on lipase and substrate types, values are generally similar.
Determination of the kinetic parameters for crude lipases from G. candidum NRRLY-552 cultivated in different nitrogen substrates: clarified corn steep liquor (C-A; dark circles) and yeast hydrolysate (C-B; gray circles). The kinetic substrate was olive oil emulsified with gum Arabic in phosphate buffer (100 mM, pH = 7.0) and the reaction occurred at 30 °C. In detail are present the estimated values for k cat (min−1) and K m/k cat (mg min mL−1), based on Lineweaver–Burke plot (Supplementary File 1). Figure was obtained with the software Origin® 8; dotted straight lines were used to connect the points and to guide the eyes; bars represent the deviations
Conclusions
It is known that the medium composition can directly affect (in different aspects) the morphology of microbial cells and therefore the performance of their enzymes. From this study, differences between the lipases from G. candidum cultivated in different medium compositions were observed. When comparing CCSL and YH as substrates, the CCSL lipase is more stable in different temperatures (half-life of 1.5-, 5.0- and 2.2-fold at 30, 37 and 40 °C, respectively), under freezer conditions (residual relative activity 56 % higher) and in higher pH (half-life tenfold higher at pH = 8.0); has reduced activation energy (2.93 kcal mol−1, 83.5 % lower) and increased deactivation energy (47.16 kcal mol−1, 19.3 % higher); optimum temperature 10 °C higher (47–53 °C) in the crude form than the YH lipase. On the other hand, the YH resulted in a faster lipase (higher k cat, 2.30 min−1); presented a higher substrate affinity (higher K m/k cat, 32.12 mg min mL−1) and higher lipase activities (27.0 and 9.0 U mL−1, for C-B and P-B, respectively) than the CCSL lipase. In general, the CCSL lipase was more stable in relation to temperature and pH and the YH lipase showed more affinity for its substrate. For the purification using the hydrophobic interaction chromatography (HIC) the recovery factors were very high (above 90 %) for both lipases. In addition, the HIC was very efficient to purify both enzymes presenting high purification factors (44.3- and 86.7-fold for CCSL- and YH-lipase, respectively). It is important to emphasize that each enzyme has different characteristics which can be utilized in different situations. The greatest value of this study is shown that different substrates can produce lipases with several different characteristic using the same microorganism.
References
Treichel H, Oliveira D, Mazutti MA, Di Luccio M, Oliveira JV (2010) A review on microbial lipases production. Food Bioprocess Technol 3:182–196
Chaibakhsh N, Basri M, Rahman MBA, Adnani A, Salleh AB (2012) Lipase-catalyzed synthesis of ergosterol ester. Biocatal Agric Biotechnol 1:51–56
Mendieta-Taboata O, Kamimura ES, Maugeri F (2001) Modelling and simulation of the adsorption of the lipase from Geotrichum sp. on hydrophobic interaction columns. Biotechnol Lett 23:781–786
Manera AP, Ores JC, Ribeiro V, Rodrigues MI, Kalil SJ, Maugeri Filho F (2011) Utilização de resíduos agro-industriais em processo biotecnológico para produção de beta-galactosidase de Kluyveromyces marxianus CCT 7082 (in Portuguese). Acta Sci Technol 33:155–161
Treichel H, Mazutii MA, Maugeri Filho F, Rodrigues MI (2009) Technical viability of the production, partial purification and characterization of inulinase using pretreated agroindustrial residues. Bioprocess Biosyst Eng 32:425–433
Sguarezi C, Longo C, Ceni G, Boni G, Silva MF, Di Luccio M, Mazutti MA, Maugeri F, Rodrigues MI, Treichel H (2009) Inulinase production by agro-industrial residues: optimization of pretreatment of substrates and production medium. Food Bioprocess Technol 2:409–414
Maldonado RR, Aguiar-Oliveira E, Fogaça FM, Ramos GG, Macedo GA, Rodrigues MI (2015) Evaluation of partial purification and immobilization of lipase from Geotrichum candidum. Biocatal Agric Biotechnol 4:321–326
Maldonado RR, Aguiar-Oliveira E, Pozza EL, Costa FAA, Mazutti MA, Maugeri F, Rodrigues MI (2015) Application of yeast hydrolysate in extracellular lipase production by Geotrichum candidum in shaken flasks, stirred tank and airlift reactors. Can J Chem Eng 93:1524–1530
Resende-Maldonado R, Burkert JFM, Aguiar-Oliveira E, Durrant L, Mazutti MA, Maugeri Filho F, Rodrigues MI (2014) Elucidation of the effects of inoculum size and age on lipase production by Geotrichum candidum. Biotecnol Appl 31:216–221
Maldonado RR, Macedo GA, Rodrigues MI (2014) Lipase production using microorganisms from different agro-industrial by-products. Int J Appl Sci Technol 4:108–115
Maldonado RR, Aguiar-Oliveira E, Pozza EL, Costa FAA, Maugeri Filho F, Rodrigues MI (2014) Production of lipase from Geotrichum candidum using corn steep liquor in different bioreactors. J Am Oil Chem Soc 91:1999–2009
Maldonado RR, Burkert JFM, Mazutti MA, Maugeri F, Rodrigues MI (2012) Evaluation of lipase production by Geotrichum candidum in shaken flasks and bench-scale stirred bioreactor using different impellers. Biocatal Agric Biotechnol 1:147–151
Maldonado RR, Panciera AL, Macedo GA, Mazutti MA, Maugeri F, Rodrigues MI (2012) Improvement of lipase production from Geotrichum sp. in shaken flasks. Chem Biochem Eng Q 18:459–464
Asses N, Ayed L, Bouallagui H, Rejeb IB, Gargouri M, Hamdi M (2009) Use of Geotrichum candidum for olive mill wastewater treatment in submerged and static culture. Bioresour Technol 100:2182–2188
Burkert JFM, Maldonado RR, Maugeri F, Rodrigues MI (2005) Comparison of lipase production by Geotrichum candidum in stirring and airlift fermenters. J Chem Technol Biotechnol 80:61–67
Burkert JFM, Maugeri F, Rodrigues MI (2004) Optimization of extracellular lipase production by Geotrichum sp. using factorial design. Bioresour Technol 91:77–84
Kamimura ES, Medieta-Taboata O, Rodrigues MI, Maugeri F (2001) Studies of lipase-affinity adsorption using response-surface analysis. Biotechnol Appl Biochem 33:153–159
Kamimura ES, Mendieta-Taboata O, Sato HH, Pastore G, Maugeri F (1999) Production of lipase from Geotrichum sp. and adsorption studies on affinity resin. Braz J Chem Eng 16:103–112
Rodrigues MI, Iemma AF (2014) Experimental design and process optimization. CRC Press, Boca Raton
Freire DM, Teles EMF, Bom EPS, Lippel Sant’anna G (1997) Lipase production by Penicillium restrictum in a bench-scale fermenter: effect of carbon and nitrogen nutrition, agitation, and aeration. Appl Biochem Biotechnol 63:409–421
Sacristán N, González L, Castro JM, Fresno JM, Tornadijo ME (2012) Technological characterization of Geotrichum candidum strains isolated from a traditional Spanish goats’ milk cheese. Food Microbiol 30:260–266
Brabcová J, Demianová Z, Vondrášek J, Jágr M, Zarevúcka M, Palomo JM (2013) Highly selective purification of three lipases from Geotrichum candidum 4013 and their characterization and biotechnological applications. J Mol Catal B Enzym 98:62–72
Cai Y, Wang L, Liao X, Ding Y, Sun J (2009) Purification and partial characterization of two new cold-adapted lipases from mesophilic Geotrichum sp. SYBC WU-3. Process Biochem 44:786–790
Huang Y, Locy R, Weete JD (2004) Purification and characterization of an extracellular lipase from Geotrichum marinum. Lipids 39:251–258
Ginalska G, Bancerz R, Kornillowicz-Kowalska T (2004) A thermostable lipase produced by a newly isolated Geotrichum-like strain, R59. J Ind Microbiol Biotechnol 31:177–182
Gopinath SCB, Hilda A, Lakshmi priya T, Annadurai G, Anbu P (2003) Purification of lipase from Geotrichum candidum: conditions optimized for enzyme production using Box-Behnken design. World J Microbiol Biotechnol 19:681–689
Hlavsová K, Zarevúcka M, Wimmer Z, Macková M, Sovová H (2009) Geotrichum candidum 4013: extracellular lipase versus cell-bound lipase from the single strain. J Mol Catal B Enzym 61:188–193
Goldbeck R, Maugeri Filho F (2013) Screening, characterization, and biocatalytic capacity of lipases producing wild yeasts from brazil biomes. Food Sci Biotechnol 22:79–87
Loo JL, Khoramnia A, Lai OM, Long K, Ghazali HM (2014) Mycelium-bound lipase from a locally isolated strain of Geotrichum candidum. Molecules 19:8556–8570
Winny X, Khosasih V, Suwanto A, Kim HK (2012) Characterization of lipases from Staphylococcus aureus and Staphylococcus epidermidis isolated from human facial sebaceous skin. J Microbiol Biotechnol 22:84–91
Chesterfield DM, Rogers PL, Al-Zaini EO, Adesina AA (2012) Production of biodiesel via ethanolysis of waste cooking oil using immobilised lipase. Chem Eng J 207:701–710
Olusesan AT, Azura LK, Forghani B, Bakar FA, Mohamed AKS, Radu S, Manap MYA, Saari N (2011) Purification, characterization and thermal inactivation kinetics of a non-regioselective thermostable lipase from a genotypically identified extremophilic Bacillus subtilis NS 8. New Biotechnol 26:738–745
Brabcová J, Zarevúcka M, Macková M (2010) Differences in hydrolytic abilities of two crude lipases from Geotrichum candidum 4013. Yeast 27:1029–1038
Acknowledgments
The authors would like to thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Brazil), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) for their financial support and also to thank Ingredion (Brazil) and Prodesa (Brazil) for supplying the industrial residues used as substrates in this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Maldonado, R.R., Pozza, E.L., Aguiar-Oliveira, E. et al. Characterization of Crude and Partially Purified Lipase from Geotrichum candidum Obtained with Different Nitrogen Sources. J Am Oil Chem Soc 93, 1355–1364 (2016). https://doi.org/10.1007/s11746-016-2875-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-016-2875-9