Abstract
The present article reports eco-friendly multi-functional polyurethane–ZnO hybrid nanocomposite coatings obtained from Thevetia peruviana seed oil (TPSO). Initially, the polyols were prepared by treating TPSO with glycerol and the formation was supported by Fourier transform infrared (FT-IR) and 1H-NMR studies. In the next stage, siloxane functionalized ZnO nanoparticles were added to the polyol mixture in different weight percentages (0, 1 and 2 %) and then treated with excess 4,4′-diisocyanatodicyclohexylmethane (H12MDI) in order to synthesize isocyanate terminated polyurethane nanocomposites. The polyurethane hybrids were then casted as thin films and cured under atmospheric moisture. After complete curing they were characterized by using FT-IR, 1H-NMR, 13C-NMR, X-ray diffraction, scanning electron microscopy, thermogravimetric analysis, and dynamic mechanical thermal analysis techniques. The hybrid nanocomposites showed superior thermo-mechanical and anti-corrosive properties compared to pristine polyurethane. Also, due to the presence of nano ZnO in the polyurethane matrix, the composite coatings are showing excellent resistance towards various bacterial and fungal stains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the years, petrochemical based polymers have been predominantly used in various industries for the development of a wide range of products like coatings, foams, elastomers, fibers, etc. These chemicals are non-biodegradable and are known to cause life-threatening environmental concerns such as pollution of water and air sources, erosion of top soil, increase in world temperature and the rise of sea levels (a result sparked by global warming). However, it is imperative that a sustainable and biodegradable raw material such as plant oils, proteins, natural rubber, wood, polysaccharides (basically starch and cellulose) and others be fully harnessed as substitutes to chemical feedstock generated from fossil fuels, which have limited world reserves and are unstable in pricing [1, 2]. In today’s chemical industry, plant oils play a pivotal role as renewable raw materials for the generation of useful products such as plastics, cosmetics, biodiesel, paints, lubricants, flooring materials and coatings [1]. The quest by researchers to prepare different types of polymeric coating formulations from plant oil-based monomers is unending. However, the modification of the seed oils is vital with regard to the emerging properties of the polymer obtained from it. Modifications such as epoxidation, transesterification, aminolysis, hydroformylation, boiled (blown oil) and partial glyceride (PG) formation are possible ways of creating functionalities (i.e., reactive sites), necessary for synthesizing organic coatings such as polyether amides, styrenation of esterified polyols, polyesteramide and polyesteramide-urethanes [3–5]. Among these, the formation of PG polyols has potential applications in the development of functional coatings due to their easy synthetic procedure, ease of isolation and the availability of multiple hydroxyl functional groups. These PG polyols have been synthesized from a number of drying and semidrying oils and used as base monomers in the synthesis of organic polymers [6]. However, a limited number of formulations have been carried out using non-drying seed oils. In this study, an underutilized non-drying seed oil of the Thevetia peruviana plant, having about 63 % seed oil content (of which 69.1 % is unsaturated and 30.1 % is saturated fatty acids) was used as a PG polyol source for the synthesis of polymeric composites. The 69.1 % unsaturated fatty acids composition of Thevetia peruviana seed oil (TPSO) is responsible for the 74.9 g IC l/100 g iodine value. The TPSO consists of oleic acid with the highest percentage composition of 48.1 % while palmitoleic and erucic acid both have <1 % of the composition [5].
The use of nanoparticles especially as fillers in coating formulations has been undergoing a steady increase over the years. These hybrid materials offer combinational properties of both organic and inorganic constituents. These ultrafine particles have the capability of orienting themselves within the polymer matrix thereby improving the polymeric properties (such as strength, stiffness, hardness, thermal stability, flame retardant properties, and conductivity, anti-fouling and adhesive properties) of the polymer [7–9]. Apart from the highlighted properties of hybrid nanoparticles, it could also serve as an inhibitive material towards the growth of microbes that may cause disbonding and blistering of protective surface coatings when exposed to various environmental conditions [10, 11].
This present study addresses the preparation of amine functionalized ZnO nanoparticles by their reaction with a 0.1 weight percentage of 3-aminopropyltrimethoxysilane (APTMS) coupling agent according to a reported procedure [7, 9]. The surface amine groups helps in the formation of a chemical linkage between ZnO and the polyurethane matrix (synthesized by treating PG polyols with H12MDI) by forming urea linkages with free-NCO units present in the polyurethane matrix. The resulting Polyurethane/APTMS-ZnO nanocomposite coatings were characterized by Fourier transform infrared spectroscopy (FT-IR), 1H NMR, 13C NMR, thermogravimetric analysis (TGA), dynamic mechanical thermal analysis (DMTA), X-ray diffraction (XRD) analysis, scanning electron microscopy (SEM) and the fog test.
Experimental Section
Materials and Methods
Air-dried seedlings of Thevetia peruviana obtained from the compound of Alhaji and Alhaja R.A. Amodu at Emure-Ekiti, south-western Nigeria were subjected to an oil extraction process using a Soxhlet apparatus (with n-hexane as solvent). The glycerol, calcium oxide, methanol, carbon tetrachloride, potassium hydroxide, and iodine trichloride were obtained from S.D. Fine Chemicals (Mumbai, India). 4,4′-diisocyanatodicyclohexylmethane (H12MDI), 3-aminopropyltrimethoxysilane (APTMS) was procured from Alfa Aesar Chemicals UK. The zinc oxide nano powder (particle size 50–70 nm) was purchased from Aldrich Chemicals (Milwaukee, WI).
The FT-IR, 1H NMR and 13C NMR were used to confirm the chemical structure of partial glyceride polyols, surface modified ZnO nanoparticles and the synthesized hybrid polyurethane composites. FT-IR spectra of all samples were taken over KBr on a Perkin Elmer spectrum 100 spectrometer (PerkinElmer Inc. USA) by scanning 8 times. 1H NMR and 13C NMR were respectively recorded on a Varian VXR-Unity 200 MHz spectrometer and a Bruker UXNMR 400 MHz spectrometer by using CDCl3 or DMSO-d6 as a solvent and tetramethyl silane (TMS) as an internal standard. The acid and hydroxyl values of the resin samples were determined by using the ASTM D1639-89 and ASTM D4274-94 procedures. The thermal stability of the hybrid resins and curing behavior were studied by thermogravimetric analysis (Perkin Elmer TGA 7, TA Instrument, and USA) and differential scanning calorimetry (Perkin Elmer TA DSC Q100 USA) at a constant heating rate of 10 °C min−1 in a nitrogen environment. The viscoelastic behavior of the synthesized polyurethane hybrid coating films (with dimensions 15 × 10 × 0.15 mm3) was analyzed with a DMTA IV instrument (Rheometric Scientific, USA) in tensile mode at a frequency of 1 Hz and a heating rate of 3 °C min−1 under a nitrogen atmosphere. The morphological studies were carried out on an SEM Hitachi-S520 (Oxford link ISIS-SEM model), Japan. Elcometer was used to obtain the thickness of the coatings on metal steel panels. A refractometer RFM 800 instrument was used to analyze the refractive indices of all the resin samples.
In vitro antimicrobial activity of the polymers was tested against Gram-positive organisms viz. Bacillus subtilis (MTCC 441), Staphylococcus aureus (MTCC96) and Gram-negative organisms viz. Escherichia coli (MTCC 443), and Klebsiella pneumoniae (MTCC 618) by the agar diffusion method [12]. The ready-made nutrient agar was suspended in double distilled water (1,000 ml) and heated to boiling for 25 min until it dissolved completely; the medium and Petri dishes were autoclaved at pressure of 15 lb/in2. The medium was transferred into sterile Petri dishes under aseptic conditions in a laminar air flow chamber. When the medium in the plates had solidified, 0.5 ml (approx. 106 CFU/ml) of culture of test organism was inoculated and evenly spread over the agar surface with a sterile L-shaped rod. Embedded polymer samples with 2 cm × 2 cm (approx.) samples were cleaned with double distilled water and placed on the medium and incubated at 37 °C for 24 h. The antibacterial activity was carried out based on the formation of an inhibition zone and loss of growth of organisms beneath and surrounding the films placed on the agar medium. Three replicates were used for each treatment. MTCC is the Microbial Type Culture Collection in IMTECH (CSIR LAB) Chandigarh, India.
Different grades of Silicon carbide papers were used for the preparation of mild steel strips; which were washed with water, ethanol and acetone. The degreased metal strips were dried under a vacuum for 3 h. The pristine and hybrid coatings were prepared by brush application of 60 wt% resin in xylene on the mild steel strips. For the chemical resistance test in water, acid (5 wt% HCl), alkali (5 wt% NaOH) standard sizes of mild steel strips of 30 mm × 10 mm × 1 mm were placed in 3 in. diameter porcelain dishes. The salt spray tests were conducted in a salt mist chamber following ASTM B 117-11.
Synthesis of Polyols from Thevetia peruviana Seed Oil (PG Polyols)
100 g of oil (TPSO) and 8.5 g glycerol were placed in a 250-ml four-necked round-bottom flask equipped with a mechanical stirrer, a nitrogen inlet tube, a thermometer, and a condenser and heated and when the temperature reached to 218 °C, a catalytic amount of calcium oxide (CaO) was added. The temperature was maintained at 235–240 °C for 45 min (Scheme 1). The acid and hydroxyl values of the resulting polyols (Table 1) were used to monitor the progress of product formation.
Synthesis of Amine Functionalized ZnO Nanoparticles
In a 500-ml round-bottom flask, 10 gm of ZnO (0.1228 mol) nanopowder was dispersed in 50 g of toluene solvent and stirred for 10 min using a magnetic stirrer followed by 30 min in an ultrasonic bath in order to obtain a uniform suspension. Then, 1 g APTMS was added to the suspension and stirring of the mixture was continued for 15 min at room temperature (Scheme 1). A slightly yellow coloration was obtained. The reaction mixture was then refluxed for 24 h. A rotary evaporator was used to remove the solvent from the APTMS modified ZnO (APTMS-ZnO). Unreacted APTMS was removed by washing the compound with ethanol. The powder was dried at 50 °C for 1 h under vacuum. The modified powder was ground and further dried at 100 °C for 2 h.
Synthesis of Polyurethane Nanocomposites
PG polyols (6.0 g) were dissolved in 4-methyl pentan-2-one in a three-necked round-bottom flask and the calculated amount (0, 1, 2 wt% with respect to PG) of APTMS modified ZnO was dispersed by using an ultrasonic bath. To the reaction mixture, H12MDI (2.024 ml) dissolved in 1 ml of 4-methyl pentan-2-one solvent was added drop wise (here NCO: OH ratio was maintained as 2:1). The resulting reaction mixture was stirred at 75 °C for 3 h under a nitrogen atmosphere (Scheme 1). The progress of the reaction was monitored by TLC and hydroxyl value determination. Finally, the pristine and hybrid nanocomposite films were achieved by casting the solutions of polyurethane hybrids on a tin foil supported over a glass plate using a manually driven square shaped applicator. They were cured under atmospheric moisture at ambient temperature for 1 week. After complete curing, the tin foil was removed by amalgamation with mercury to get polyurethane hybrid free films. Cured films were coded as PGU (partial glyceride urethane), PGU-1 % APTMS-ZnO, and PGU-2 % APTMS-ZnO respectively. The polyurethane hybrids were also coated onto mild steel panels for the evaluation of the anticorrosive properties.
Results and Discussions
Physico-Chemical Characteristics of Polyol and the PU Resin
The physico-chemical characterization data of PG (partial glyceride) polyol and PGU (resin) obtained from TPSO is presented in Table 1. It was observed that conversion of PG to PGU led to a decrease in hydroxyl values and the NCO value, while simultaneously increasing the refractive index. The present observation is in agreement with the reports of Zafar et al. [13] which involves the polymerization of N,N-bis(2-hydroxyethyl) linseed oil fatty amide (HELA) resin. The solubility property of PG and PGU resins were observed in various solvents like chloroform, acetone, toluene, diethyl ether, methanol, ethanol, dimethylformamide (DMF), MIBK, DMSO and xylene (The test was performed by taking the 0.1–0.2 g resin sample in 10 ml of solvent and stirring well for up to 15 min and heating if necessary). PG and PGU were found to have excellent solubility in all solvents by forming a light yellow colored transparent solution.
Spectroscopic Characterization of PG Polyols
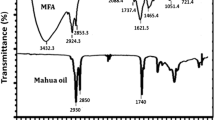
The formation of PG from TPSO was confirmed from FT-IR spectral analysis. The presence of the broad peak observed around 3,400 cm−1 in the FT-IR spectrum of PG, corresponds to the characteristic absorption of alcoholic groups. The peaks at 2,925 and 2,854 cm−1 corresponds to the –CH2 asymmetric and symmetric stretching. The carbonyl ester stretching vibrations are observed around 1,740 cm−1. The alkane and alkene C–H bending vibrations were observed around 1,460 and 720 cm−1, respectively. The overlay FT-IR spectra of TPSO and PG was given in Fig. 1a.
Additionally, the 1H-NMR studies also proves the formation of PG polyols (Fig. 1b).The signals at δ 0.87–0.89 ppm attributed to terminal methyl protons of the fatty acid chain on the PG structure. The peaks at δ 1.27–2.31 ppm correspond to the protons of all the internal CH2 on the fatty acids chains, but the carbon double bond protons on the fatty acid chain resonated at 5.36 ppm. The hydroxyl proton on the PG polyol was observed at δ 3.70 ppm. The glycerol CH2 back bone protons of the PG were observed at δ 4.30 ppm: 4.17 ppm, δ 4.35 ppm, and δ 3.91 ppm: δ 3.65 ppm.
Spectroscopic Investigation of PGU Hybrid Polymers
The FT-IR absorption spectra of PGU and hybrid polymer films (PGU-1 % APTMS-ZnO, PGU-2 % APTMS-ZnO) are illustrated in Fig. 2a. The formation of the polyurethane is confirmed by the presence of characteristic peaks around 3,320 cm−1 corresponding to N–H stretching, peaks around 2,900 and 2,850 cm−1 corresponding to C–H asymmetric and symmetric stretching. The C=O stretching of the urethane and ester linkages were observed around 1,750 cm−1. The C–N stretching of the urethane linkage observed at 1,230 cm−1. The disappearance of the –NCO stretching around 2,200 cm−1 and the increment in the intensity of N–H and C=O stretching with loading of the modified ZnO nanoparticles (which is due to the reaction between amine group on ZnO nanoparticles and isocyanate) suggests the formation of the polyurethane nanocomposites.
Additionally, the formation of the polyurethane was supported by 1H-NMR and 13C-NMR analysis. From 1H-NMR studies, the presence of peak corresponding to urethane N–H at δ 8.20 ppm and the presence of peaks related to cyclohexane ring in range 1.3–2.0 ppm confirms the formation of polyurethane reaction. The detailed spectrum was given in Fig. 2b. The PGU formation was further corroborated from the 13C-NMR spectra in Fig. 2c. From 13C-NMR studies, the presence of a peak corresponding to urethane N–H at δ 155.47 ppm and the presence of peaks related to cyclohexane ring around 30–35 ppm is evidence of the formation of the polyurethane. Further, the peaks corresponding to the terminal methyl group carbon and the internal methylene carbons (–CH2) present in the fatty acid chains were observed at δ 14.08 ppm and δ 24.90–34.45 ppm respectively. The olefin carbons on the fatty acid chains appeared at δ 130.54 ppm.
Morphology of the Hybrid Composites
The SEM images of pristine ZnO nanoparticles, APTMS modified ZnO, PGU, PGU-1 % APTMS-ZnO, PGU-1.5 % APTMS-ZnO, and PGU-2 % APTMS-ZnO are shown in Fig. 3. It can be observed that the pristine ZnO nanoparticles are aggregated (Fig. 3a), but on modifying the peripheral of the ZnO with APTMS (APTMS-ZnO) the agglomeration is reduced. A noticeable uniform distribution can be observed in Fig. 3b, which is established as being due to the steric repulsions on the part of the grafted organic groups attached to the periphery of ZnO particles. The micrographs of the polyurethane–ZnO hybrid films (PGU, PGU-1 % APTMS-ZnO, PGU-1.5 % APTMS-ZnO, and PGU-2 % APTMS-ZnO) shown in Fig. 3c–f, respectively. From these results, it is observed that there is no agglomeration of nanoparticles in all the micrographs. These impressive and non-agglomerated hybrid composite films may be due to the uniform dispersion of the modified nanoparticles within the matrix of PGU.
X-ray Diffraction (XRD) Analysis
The powder X-ray diffraction patterns of pristine ZnO nanoparticles and APTMS modified ZnO nanoparticles are shown in Fig. 4a. The diffraction peaks of the two spectra show resemblance with typical of wurtzite structure of bulk ZnO. The modified nanoparticles show distinctive peaks at 29°–30°, 36°–37° and 45° which are in contrast to that of the pristine ZnO nanoparticles (represented in Fig. 4a as X, Y and Z peaks of APTMS-ZnO XRD spectra). The presence of these new peaks may be due to the formation of Zn–O–Si linkages in the modified nanoparticles [14]. The average particle sizes of pristine and modified ZnO nanoparticles were determined from the highly intense peak at 2θ value at 36.5° using Scherrer equation (Eq. 1). The average crystallite size of ZnO was calculated to be about 79.41 nm, while that of APTMS-ZnO was estimated at 75.63 nm. This may be due to the decrease in the aggregation of the modified nanoparticles.
Where K is a constant related to crystallite shape (normally taken as 0.9), λ is wave length of X-rays used (usually 1.54 Å for copper Kα1 source), β is the peak width of the peak at half maximum height (in radians) and θ correspond to Bragg’s angle (in radians).
The overlay XRD spectra of both pristine and its hybrid polyurethane films are given in Fig. 4b. All the polyurethane films are showing broad and intense peak between 15° and 23°. It is observed that the intensity of the peak increases and the broadness of the peak decreases with loading of modified ZnO. The heterogeneous arrangements formed by the nanoparticles as a result of high cross-link density are the reason for the decrease in the broadening of peaks [14].
TGA and DMTA Analysis
The thermogravimetric (TGA), differential thermogravimetric (DTG) and DMTA analyses of PGU and their hybrids (such as PGU-1 % APTMS-ZnO, PGU-2 % APTMS-ZnO) are presented in Figs. 5a, b and 6 respectively. The thermograms under investigation show a three-step degradation profile (with respect to first derivative of TG thermogram) of which the second step is the most prominent. At the onset of degradation, entrapped moisture and solvent were observed to evaporate. The first step corresponds to the degradation of urethane linkages. This decomposition stage (first step) is probably due to dissociation of isocyanate and alcohol which led to the formation of primary amines, olefins and the formation of secondary amines. The second decomposition step is due to ester bond dissociation while the decomposition of hydrocarbon chains and siloxane components are observed at the third degradation step. Similar types of decomposition stages have been reported [12, 13]. It is observed that the thermal stability was improved with loading of ZnO nanoparticles. For instance, the onset decomposition temperatures of PGU, PGU-1 % APTMS-ZnO and PGU-2 % APTMS-ZnO are 252.83, 256.15, and 278.85 °C, respectively. The results are correlated with the increase in the storage modulus value of the composite films (determined from DMTA analysis). For instance, the storage modulus (at −55 °C) of PGU, PGU-1 % APTMS-ZnO and PGU-2 % APTMS-ZnO are 9 × 107, 1.4 × 108 and 1.6 × 108 Pa, respectively. This increase of thermal stability and storage modulus profiles is due to the formation of strong network structures by the urea groups and surface hydroxyl groups of the modified ZnO nanoparticles with the polyurethane matrix through hydrogen bonding, which act as a thermal insulator and mass transport barrier to the volatile products generated during decomposition and thus increases the degradation temperature [15].
Antimicrobial Examinations of PGU and Its Hybrid Films
The antimicrobial activity of pristine PGU and the ZnO loaded PGU nanocomposite films were executed on gram positive (Staphylococcus aureus and Bacillus subtilis), gram negative (Escherichia coli and Klebsiella pneumonia) bacterial strains and a fungal stain (Aspergillus niger). All the bacterial and fungal stains used for the study were grown on a Czapek–Dox medium. In these grown stains, the coating films were embedded and incubated for 24 h at an ambient temperature of 28 ± 4 °C. The antimicrobial activities were determined based on the formation of an inhibition zone on the bacterial or fungal growth beneath and in the surroundings of the films that was placed on the Luria–Bertani agar medium. All the antimicrobial test results are given in Table 2, which shows that PGU-2 % APTMS-ZnO is highly active on Staphylococcus aureus and Escherichia coli while PGU-1.5 % APTMS-ZnO shows moderate activity. This level of activity shown by the hybrid films may be due to the presence of nano ZnO in the nanomaterial. The pristine PGU film and other hybrid films display zero activity for Bacillus subtilis. Chao et al. [16] have shown how the ZnO nanoparticles show the inhibitive properties on gram negative bacteria, E. coli. The main reason for the nano ZnO inhibitive properties against bacterial growth is the generation of hydrogen peroxide (H2O2) from the surface of ZnO [15]. Similar results were also observed in an antifungal activity test. The pristine polymer film did not show any form of inhibition on the fungus but the growth of the fungus did not mask/cover the polymer film. PGU-2 % APTMS-ZnO hybrid film showed a gray area around and beneath the film, which means that it has inhibitive properties on the fungus. These results confirm the antibacterial and antifungal properties of the hybrid polymer composite.
Fog Test Results
The pure PGU and the nanocomposites were coated on mild steel panels (3 × 4 cm) by maintaining the thickness at around 100 microns to evaluate the anticorrosion properties in 5 % NaCl salt fog. The photographs of the coated panels before and after 350 h of salt spray test are shown in Fig. 7. It can be clearly observed that with the loading of modified ZnO nanoparticles the corrosion resistance of the coating is improved (i.e., PGU-2 % APTMS-ZnO showed the least corrosion). This is mainly due to the formation of more cross-linked structures with increasing ZnO content, which restricts the penetration of corrosive species by acting as a strong physical barrier. The observed result suggests that the modified ZnO nano filler included uniformly in the polyurethane matrix and improves the coating resistance of the hybrid formulations in salt water (marine) environments.
Conclusions
The present work shows a feasible path for developing eco-friendly antimicrobial and anticorrosive polyurethane–ZnO nanocomposite coatings from polyols obtained from previously uninvestigated Thevetia peruviana seed oil. The excellent anti microbial activity of these nanocomposites against Escherichia coli, Staphylococcus aureus, Klebsiella pneumonia, and Aspergillus niger is mainly due to the presence of nano ZnO present in the coating formulation. Additionally, the nanoparticle-loaded polyurethanes show superior thermal, mechanical and crystalline properties to those of the pristine polyurethane. Thus the present polyurethane–ZnO hybrid nanocomposites could represent a new exciting direction that may open up opportunities for the use of Thevetia peruviana seed oil in various scientific fields.
References
Lu Y, Larock RC (2009) Novel polymeric materials from vegetable oils and vinyl monomers: preparation, properties, and applications. ChemSusChem 2:136–147
Siyanbola TO, Ajanaku KO, James OO, Olugbuyiro JAO, Adekoya JA (2011) Physico-chemical characteristics of industrial effluents in Lagos state, Nigeria. Glob J Pure Appl Sci Tech 1:49–54
Meier MAR, Metzgerb JO, Schubert US (2007) Plant oil renewable resources as green alternatives in polymer science. Chem Soc Rev 36:1788–1802
Sharmin E, Ashraf SM, Ahmad S (2007) Epoxidation, hydroxylation, acrylation and urethanation of Linum usitatissimum seed oil and its derivatives. Eur J Lipid Sci Technol 109:134–146
Siyanbola TO, Sasidhar K, Anjaneyulu B, Kumar KP, Rao BVSK, Narayan R, Olaofe O, Akintayo ET, Raju KVSN (2013) Anti-microbial and anti-corrosive poly(ester amide urethane) siloxane modified ZnO hybrid coatings from Thevetia peruviana seed oil. J Mater Sci 48:8215–8227
Cocks LV (1966) Rede VC laboratory handbook for oil and fat analyst. American Press, London and New York
Jena Kishore K, Raju KVSN (2007) Synthesis and characterization of hyperbranched polyurethane–urea/silica based hybrid coatings. Ind Eng Chem Res 46:6408–6416
Chattopadhyay DK, Raju KVSN (2007) Structural engineering of polyurethane coatings for high performance applications. Prog Polym Sci 32:352–418
Jena KK, Narayan R, Raju KVSN (2012) Investigation of the effect of ZnO nanoparticles on the thermomechanical and microbial properties of hyperbranched polyurethane-urea hybrid composites. Polym Int 61:1309–1317
Jayakumar R, Nanjundan S, Rajkumar M, Nagendran R (2001) Studies on metal-containing polyurethanes based on divalent metal salts of mono(hydroxyethoxyethyl)phthalate. J Macromol Sci Pure Appl Chem A 38:869–888
Zafar F, Ashraf SM, Ahmad S (2007) Studies on zinc-containing linseed oil based polyesteramide. React Funct Polym 67:928–935
Linday ME (1962) Practical introduction to microbiology. E and F.N. Spon, London, p 177
Zafar F, Ashraf SM, Ahmad S (2004) Air drying polyesteramide from a sustainable resource. Prog Org Coat 51:250–256
Grasset F, Saito N, Li D, Park D, Sakaguchi I, Ohashi N, Duguet E (2003) Surface modification of zinc oxide nanoparticles by aminopropyltriethoxysilane. J Alloys Comp 360:298–311
Mishra AK, Mishra RS, Narayan R, Raju KVSN (2010) Effect of nano ZnO on the phase mixing of polyurethane hybrid dispersions. Prog Org Coat 67:405–413
Chao W, Lian-Long L, Ai-Ting Z, Peng X, Jian-Jun L, Xiao-Ting Z (2012) Antibacterial effects of zinc oxide nanoparticles on Escherichia coli K88. Afri J Biotech 11:10248–10254
Acknowledgments
The author, Dr. Tolutope O. Siyanbola is grateful to TWAS and CSIR (India) for the TWAS-CSIR 2010 Postgraduate Fellowship Award. The Indian Institute of Chemical Technology and CSIR Intel-Coat Project (CSC-0114) are appreciated for providing the enabling environment needed for my bench work. Covenant University Ota, Nigeria is well appreciated for creating the platform leading to the award of my Ph.D. Dr. Tolutope O. Siyanbola is also thankful to Prof. K.O. Okonjo, Dr. Oladele O. James, Adeogo Adedayo, Oluwatoni Siyanbola, Toluwase Siyanbola and Mrs Tunmike S. Siyanbola for their support.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Siyanbola, T.O., Sasidhar, K., Rao, B.V.S.K. et al. Development of Functional Polyurethane–ZnO Hybrid Nanocomposite Coatings from Thevetia peruviana Seed Oil. J Am Oil Chem Soc 92, 267–275 (2015). https://doi.org/10.1007/s11746-014-2587-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-014-2587-y