Abstract
The Barth syndrome (BTHS) is caused by an inborn error of metabolism that manifests characteristic phenotypic features including altered mitochondrial membrane phospholipids, lactic acidosis, organic acid-uria, skeletal muscle weakness and cardiomyopathy. The underlying cause of BTHS has been definitively traced to mutations in the tafazzin (TAZ) gene locus on chromosome X. TAZ encodes a phospholipid transacylase that promotes cardiolipin acyl chain remodeling. Absence of tafazzin activity results in cardiolipin molecular species heterogeneity, increased levels of monolysocardiolipin and lower cardiolipin abundance. In skeletal muscle and cardiac tissue mitochondria these alterations in cardiolipin perturb the inner membrane, compromising electron transport chain function and aerobic respiration. Decreased electron flow from fuel metabolism via NADH ubiquinone oxidoreductase activity leads to a buildup of NADH in the matrix space and product inhibition of key TCA cycle enzymes. As TCA cycle activity slows pyruvate generated by glycolysis is diverted to lactic acid. In turn, Cori cycle activity increases to supply muscle with glucose for continued ATP production. Acetyl CoA that is unable to enter the TCA cycle is diverted to organic acid waste products that are excreted in urine. Overall, reduced ATP production efficiency in BTHS is exacerbated under conditions of increased energy demand. Prolonged deficiency in ATP production capacity underlies cell and tissue pathology that ultimately is manifest as dilated cardiomyopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Barth syndrome (BTHS) is a rare X-linked recessive disorder characterized by cardiolipin abnormalities, lactic acidosis, organic aciduria, skeletal muscle weakness, neutropenia and cardiomyopathy [1]. The underlying cause of BTHS has been definitively traced to mutations in the TAZ gene [2, 3]. This discovery launched a concerted research effort to decipher how mutations in TAZ lead to the BTHS phenotype. A key finding revealed that TAZ encodes a phospholipid transacylase, termed tafazzin [4, 5]. Defective tafazzin activity leads to alterations in the content and composition of cardiolipin and the appearance of monolysocardiolipin [6]. Experimental models of BTHS, including yeast [7], Drosophila [8], zebrafish [9] and mice [10] have validated a cause/effect relationship between TAZ mutations and cardiolipin abnormalities. Evidence of the impact of changes in cardiolipin content and composition can be seen by morphological changes to the inner mitochondrial membrane (IMM) associated with BTHS [11]. This review describes how mutations in TAZ manifest as skeletal muscle weakness and cardiomyopathy.
TAZ Mutations and Cardiolipin Remodeling
The tafazzin transacylase localizes to mitochondria and functions in remodeling cardiolipin fatty acyl chains. Cardiolipin is distinct from other glycerophospholipids in that two phosphatidate moieties share the same glycerol head group. This gives rise to an anionic phospholipid with four esterified fatty acyl chains and a cone-shaped structure. In eukaryotes, cardiolipin is mainly confined to the IMM [12]. Interestingly, unlike most tissues, up to 90% of cardiolipin in cardiac and skeletal muscle mitochondria exists as a single molecular species, tetralinoleoylcardiolipin [12]. Establishment and maintenance of this molecular species composition depends on phospholipid remodeling reactions that involve tafazzin transacylase activity [13]. An in vitro transacylation reaction catalyzed by tafazzin is shown in Fig. 1. Acyl chain transfer from the sn-2 position of phosphatidylcholine to the corresponding hydroxyl of monolysocardiolipin is depicted. Although phosphatidylcholine is the acyl chain donor in this reaction, glycerophospholipids with other head groups can also serve as acyl group donor [14, 15]. In addition to tafazzin-mediated transacylation, cardiolipin remodeling can occur via the endoplasmic reticulum (ER) localized enzyme, acyl CoA lysocardiolipin acyltransferase (ALCAT1). In this reaction a fatty acid from fatty acyl CoA is transferred to monolysocardiolipin to produce cardiolipin [15, 16]. Aside from ALCAT1, a mitochondrial monolysocardiolipin acyltransferase (MLCAT) has been reported that catalyzes acylation of monolysocardiolipin in a reaction that employs linoleoyl CoA as substrate [17, 18]. Whereas the relative contributions of these alternate routes to the pool of mature cardiolipin in the IMM is unclear at present, the fact that TAZ mutations correlate with characteristic changes in cardiolipin content and composition indicates ALCAT1- and MLCAT-mediated reactions are not able to fully compensate for loss of tafazzin.
Tafazzin-mediated transacylation activity. The tafazzin enzyme has been shown to catalyze transfer of an acyl group (red) from phosphatidylcholine (or other glycerophospholipids) to monolysocardiolipin. Through an iterative process that may involve additional enzymatic reactions, tafazzin functions in maturation of nascent cardiac and skeletal muscle cardiolipin, resulting in a predominantly uniform molecular species, tetralinoleoylcardiolipin (color figure online)
Individuals harboring mutations in TAZ (i.e. BTHS) manifest compromised or missing tafazzin enzymatic activity. This results in specific alterations in cardiolipin including increased molecular species heterogeneity, decreased levels of cardiolipin, and increased levels of monolysocardiolipin. If a given TAZ mutation gives rise to a tafazzin protein with residual enzyme activity, lesser changes in cardiolipin content and composition, and a milder phenotype, may be anticipated [19]. On the other hand, more severe mutations in TAZ that lead to complete loss of transacylase activity, will have a more deleterious effect in terms of both IMM structure and the function of proteins embedded therein. Despite this, TAZ mutations have been described [20] in which the correlation between genotype and phenotype is not apparent. Conceivably, individual differences in TAZ mRNA splicing, mRNA stability or differences in tafazzin protein turnover rate could affect residual enzyme activity. By the same token, one or more extraneous phenotypic modifiers, ranging from environmental to biochemical, could be responsible for differences in the degree of disease manifestation.
Altered Cardiolipin Composition Affects the Electron Transport Chain
Insofar as electron transport chain (ETC) proteins are embedded in the IMM, it is not surprising that alterations in the phospholipid component of this membrane have a measurable impact on aerobic respiration [21]. Indeed, the effect of perturbations in IMM lipid content and/or composition on the functional properties of ETC proteins are likely amplified by the fact that the IMM is one of the most protein-rich membranes in nature. Unlike an average bilayer membrane that contains 30–50% protein, the IMM is comprised of ~75% protein and 25% lipid. Given this exceptionally high protein content, changes in its phospholipid composition are likely to impact the structure, orientation and/or activity of IMM proteins. Indeed, studies have shown that cardiolipin directly interacts with each of the five protein complexes that comprise the ETC [12, 22, 23]. Moreover, cardiolipin is required for the optimal function of complexes I, III, and IV and this function cannot be substituted by other phospholipids, including monolysocardiolipin [22, 24, 25]. Consistent with its altered cardiolipin content, the activity of ETC proteins is decreased in BTHS [26, 27]. In addition to direct interaction with proteins of the ETC, cardiolipin also serves a structural role in the IMM, including maintenance of membrane integrity. Perturbations that compromise IMM integrity would be anticipated to result in dissipation of the proton gradient that drives ADP phosphorylation. In keeping with this, BTHS mitochondria exhibit a decreased membrane potential, a decreased respiratory coupling index and increased proton leak [28, 29, 30]. Leakage of protons across the IMM can also lead to formation of free radicals and increased levels of reactive oxygen species which, in turn, exacerbate mitochondrial impairment [29, 30].
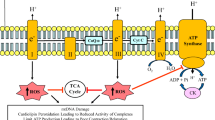
It is noteworthy that the IMM contains numerous folds and invaginations, termed cristae. Due to its distinctive cone-shaped structure, cardiolipin segregates to regions of negative membrane curvature [31, 32]. In BTHS, defective cardiolipin remodeling correlates with profound changes in cristae ultrastructure [11, 33, 34]. Furthermore, cardiolipin contributes to the stability of ETC super complexes, thereby promoting efficient electron transfer between individual complexes of the ETC [35, 36]. BTHS mitochondria have reduced levels of super complexes and display decreased stability of existing super complexes [30, 37]. In keeping with this, Huang et al. [38] reported that ETC super complexes are destabilized in cardiolipin-depleted mitochondria from taz knockdown mouse hearts, an effect that potentially has an adverse affect on metabolic channeling of reducing equivalents. Likewise, Kiebish et al. [39] reported that loss of tafazzin function in myocardium leads to changes in the mitochondrial lipidome, resulting in the dysregulated generation of oxidized derivatives of polyunsaturated fatty acids. A generalized model depicting the impact of cardiolipin impairment in BTHS on ETC activity is summarized in Fig. 2. The net effect of this impairment is a decreased ability to generate ATP through oxidation of the respiratory intermediates, NADH and FADH2.
Efficient ETC activity is required for sustained aerobic ATP production. Two key products of fuel metabolism in the matrix, NADH and FADH2, are oxidized to NAD+ and FAD+ by Complex I and II of the ETC, respectively. The flow of electrons to Complexes III and IV via coenzyme Q and cytochrome c, respectively, is coupled to proton movement to the inner membrane space (upper panel). The proton gradient thus formed drives oxidative phosphorylation via Complex V (F1 ATPase). When the content and composition of cardiolipin in the IMM is perturbed by TAZ mutations (lower panel) the orientation, stability and function of ETC complexes are affected in a manner that compromises electron flow (dashed lines). Likewise decreased membrane integrity can increase proton leakage (solid red line) (color figure online)
NADH/FADH2 Accumulation Inhibits TCA Cycle Enzymes
For each acetyl CoA oxidized via the TCA cycle, three NADH and one FADH2 are generated. As NADH and FADH2 are oxidized back to NAD+ and FAD+, respectively, electrons funnel into the ETC. Their passage from low to high standard reduction potential supports establishment of a proton gradient across the IMM that functions as the driving force for F1 ATPase-mediated phosphorylation of ADP. In a working muscle, this sequence of events is constantly repeated, providing muscle fibers with a steady supply of ATP. At rest, less ATP is required and, correspondingly, less fuel consumption occurs. Muscle has a limited capacity to store the energy of ATP (e.g., phosphocreatine) such that, when muscle activity increases, metabolic activity necessary to generate ATP also increases. In a normal mitochondrion, oxidation of NADH and FADH2 is highly efficient. High NADH ubiquinone oxidoreductase (Complex I) activity maintains NADH levels at very low levels [40]. Maintenance of this disproportionate ratio requires efficient movement of electrons from Complex I to Complex III (Coenzyme Q–cytochrome c reductase) and is essential for proper functioning of mitochondrial enzymes that employ NAD+ as substrate (e.g., isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, malate dehydrogenase and others). If NADH levels rise in the matrix, product inhibition of these enzymes will occur (Fig. 3) [41, 42]. In BTHS, TAZ mutation-induced alterations in cardiolipin impair ETC function, decreasing the efficiency of electron transfer reactions. When this occurs, oxidation of NADH does not keep pace with its production and the ratio of NAD+/NADH decreases [30]. As the concentration of NADH rises, product inhibition of TCA cycle enzymes that employ NAD+ as a substrate occurs.
Effect of NADH/FADH2 accumulation on TCA cycle activity. When TAZ mutation-dependent alterations in cardiolipin content and composition interfere with ETC activity, NADH and FADH2 accumulate in the matrix space. Elevated levels of the reduced form of these coenzymes lead to product inhibition of TCA cycle enzymes, slowing/shutting down the cycle. Unable to enter the TCA cycle, acetyl CoA and propionyl CoA accumulate in the matrix space of skeletal muscle and cardiac tissue mitochondria
Inefficient Respiration Drives Anaerobic Glucose Metabolism/Lactic Acidosis
Demand for ATP by cardiac tissue is persistent. Even at rest, cardiac muscle contraction requires a continuous supply of ATP. Furthermore, any activity that accelerates the heart rate will increase demand for ATP. Normally, oxidative phosphorylation is the principal means by which ATP is generated and, as such, cardiac tissue contains abundant mitochondria that align adjacent to muscle fibers. As contractile activity increases, aerobic respiration and metabolic fuel consumption follow suit. In BTHS, however, a bottleneck occurs because alterations in cardiolipin content and composition that affect the IMM lead to ETC inefficiency, a decreased rate of NADH and FADH2 oxidation and subsequent inhibition of TCA cycle enzymes. The resulting slowdown in ATP production reaches a point wherein aerobic respiration fails to meet the energy demands of the tissue. Because survival depends on sustained cardiac muscle contraction, the tissue adapts by increasing anaerobic glycolysis and activating the Cori cycle (Fig. 4) [21]. Under normal circumstances, pyruvate dehydrogenase converts pyruvate to acetyl CoA that enters the TCA cycle and is metabolized to CO2. However, during anaerobic glycolysis, pyruvate is instead converted to lactic acid by lactate dehydrogenase. Lactic acid thus formed exits the cell and migrates to liver where it is converted to glucose via gluconeogenesis. Once formed, this glucose is secreted from hepatocytes and returns to muscle/heart where anaerobic glycolysis starts the cycle anew. Reliance on the Cori cycle to generate ATP for cardiac muscle contraction is problematic, however. As glucose utilization increases, lactic acid buildup leads to a decrease in blood pH (i.e. lactic acidosis). Furthermore, the entire process operates at an energy deficit since 6 ATP are required to generate glucose from lactic acid via gluconeogenesis in liver while anaerobic metabolism of glucose to lactic acid in muscle yields 2 ATP. Thus, although this process provides a stopgap means to generate ATP when oxidative metabolism is limiting, its meager ATP yield, buildup of lactic acid and increased demand for gluconeogenesis will lead to problems over time. Consistent with this, subjects with BTHS manifest lactic acidosis, skeletal muscle weakness, lethargy and cardiomyopathy [1, 43].
Inhibition of oxidative metabolism induces Cori cycle activity. As TCA cycle activity slows in cardiac and skeletal muscle tissue due to compromised ETC function and buildup of NADH and FADH2, a shift in metabolism occurs wherein anaerobic glycolysis increases and the pyruvate generated is converted to lactic acid. Lactic acid migrates to liver where gluconeogenesis converts lactate to glucose, at the expense of 6 ATP. Glucose generated in hepatocytes via this process transits back to muscle where anaerobic glycolysis yields 2 ATP for use in muscle contraction
TCA Cycle Inhibition Results in Metabolite Diversion into Organic Acids
As NADH and FADH2 levels rise in the matrix of skeletal muscle and cardiac tissue mitochondria, collateral metabolic repercussions occur. In these tissues, as TCA cycle activity slows, acetyl CoA and propionyl CoA accumulate. Above a threshold acetyl CoA concentration, the enzyme T2 thiolase reverses direction and catalyzes condensation of 2 acetyl CoA, forming acetoacetyl CoA plus CoASH. Acetoacetyl CoA subsequently reacts with another acetyl CoA to form 3-hydroxy 3-methylglutaryl CoA (HMG CoA) via HMG CoA synthase 2. Under the prevailing metabolic conditions in BTHS mitochondria, HMG CoA is diverted to 3-methylglutaconyl CoA (3MG CoA) via 3MG CoA hydratase, a leucine degradation pathway enzyme that normally functions in the reverse direction [44, 45]. As levels of 3MG CoA rise, this metabolite becomes a substrate for acyl CoA thioesterase-mediated hydrolysis, producing the dead end organic acid, 3-methylglutaconic acid (3MGA), that is abundant in urine of BTHS subjects but essentially absent in normal subjects (Fig. 5). In addition to 3MGA, a portion of the 3MG CoA pool is reduced to 3-methylglutaryl CoA followed by thioester hydrolysis to yield a second organic acid, 3-methylglutaric acid. At present the identity of the oxidoreductase responsible for conversion of 3MG CoA to 3-methylglutaryl CoA or the thioesterase that generates the product, 3-methylglutarate, are unknown. It is presumed, however, that 3MG CoA and 3-methylglutaryl CoA serve as alternate substrates for the enzyme(s) involved.
Diversion of acetyl CoA to organic acid waste products. The inability to oxidize acetyl CoA via the TCA cycle leads to transient accumulation of this metabolite. Above a threshold concentration the enzyme T2 thiolase reverses direction and condenses 2 acetyl CoA into acetoacetyl CoA plus CoASH. Further condensation of acetoacetyl CoA and acetyl CoA generates HMG CoA that is converted to 3-methylglutaconyl CoA (3MG CoA) by 3MG CoA hydratase. As the concentration of 3MG CoA increases it becomes a substrate for thioesterase-mediated hydrolysis (forming 3-methylglutaconic acid; 3MGA) or oxidoreductase-mediated conversion to 3-methylglutaryl CoA. This latter metabolite is subsequently hydrolyzed to 3-methylglutarate and excreted in urine along with 3MGA
Whereas 3MGA and 3-methylglutarate are derived from acetyl CoA, elevated levels of propionyl CoA are largely responsible for formation of a third, less abundant organic acid. Despite some progress, the identity of this organic acid is not yet clear. In 1991, Kelley et al. [43] reported the presence of 2-ethylhydracrylic acid (2-EHA) in urine of BTHS subjects. A relatively rare organic acid, 2-EHA is proposed to arise from an obscure alternate pathway of isoleucine metabolism [46, 47]. Insofar as no isoleucine metabolism pathway defects have been reported in BTHS, the molecular basis for production of 2-EHA is not readily apparent. Of interest, however, is the fact that T2 thiolase not only functions as the terminal enzyme in β-oxidation, it also catalyzes the final step of isoleucine degradation, cleaving 2-methylacetoacetyl CoA into propionyl CoA plus acetyl CoA. As propionyl CoA levels rise in the presence of acetyl CoA, T2 thiolase reverses direction and catalyzes a more complex set of reactions [48]. Condensation of acetyl CoA and propionyl CoA can generate the isoleucine degradation pathway intermediate, 2-methylacetoacetyl CoA and buildup of this metabolite (or 3-hydroxy, 2-methylbutyryl CoA) could interfere with isoleucine-derived metabolite flux. When this occurs, proximal intermediates in the isoleucine degradation pathway can undergo enol–keto tautomerization, converting (S) enantiomers to the corresponding (R) intermediates that, subsequently, are metabolized by three valine metabolic pathway enzymes as alternate substrates, leading to formation of 2-EHA [49]. By the same token, if propionyl CoA loads onto T2 thiolase before acetyl CoA, the expected condensation product is n-3-ketovaleryl CoA. Under the prevailing metabolic conditions in BTHS, NADH dependent dehydrogenation will generate n-3-hydroxyvaleryl CoA and subsequent thioester hydrolysis will generate n-3-hydroxyvaleric acid, an organic acid previously observed in propionyl CoA carboxylase deficiency and methylmalonyl CoA mutase deficiency [50, 51, 52]. Insofar as the molecular mass of 2-EHA and n-3-hydroxyvalerate are identical (118 Da), careful organic acid analysis will be necessary to distinguish their relative contributions to the profile observed in urine of BTHS subjects.
Insufficient ATP Production Initiates Cellular/Tissue Damage Leading to Dilated Cardiomyopathy
In addition to its occurrence in BTHS, decreased cardiolipin content, altered acyl chain composition and lipid peroxidation are associated with mitochondrial dysfunction in chronic conditions ranging from ischemia, hypothyroidism, heart failure and aging [53]. In particular, age-related heart failure is associated with decreased levels of tetralinoleoylcardiolipin and myocardial dysfunction [18, 54]. The aged heart exhibits impaired metabolic efficiency, decreased capacity to oxidize fatty acids and enhanced dependence on glucose metabolism. Furthermore, aging impairs mitochondrial oxidative phosphorylation due to a decrease in the activity of Complexes III and IV [55]. Thus, although BTHS is a rare disorder, it shares biochemical features with major chronic diseases and aging related processes. Moreover, since BTHS is caused by a single gene defect, studies of this disorder may provide unique mechanistic insight about how cardiolipin profile defects lead to cardiac dysfunction.
When cardiac muscle is unable to contract efficiently, over time, tissue damage occurs that is manifest as cardiomyopathy. Characteristic cardiac abnormalities associated with BTHS normally present in the first year of life, usually as dilated cardiomyopathy or left ventricular non-compaction (LVNC) [21]. LVNC is a distinct cardiomyopathy caused by disruption of the endocardium and myocardium during embryogenesis. Characteristic features of LVNC include trabeculations and intertrabecular recesses in the ventricular myocardium. The fact that these anatomical defects as well as compromised mitochondrial energy metabolism both lead to LVNC suggests this condition can arise from different origins [56]. Hemodynamic analysis of BTHS subjects normally reveals a reduced ejection fraction and increased left ventricular internal dimension in diastole [26, 57]. The link between mitochondrial cardiolipin defects and these morphologic/functional myocardial features are poorly understood. Notwithstanding the possible contribution of cardiomyocyte apoptosis, it is likely that an inability to sustain muscle contraction, owing to a limiting supply of ATP, underlies the phenotype [58]. Moreover, the observation that mutations in TAZ can lead to LVNC is in keeping with various metabolic effects described above and points to a direct connection between compromised energy metabolism and cardiomyopathy.
A contracted muscle is up to one-third shorter than its extended length due to a decrease in the length of sarcomeres. The basic unit of striated muscle, sarcomeres are comprised of long fibrous proteins organized as filaments that slide past each other during contraction and relaxation. The mechanics of the process, which involves interaction between myosin and actin, is driven by ATP hydrolysis. In the absence of ATP, myosin does not bind actin and fails to undergo the conformational change that pulls against actin (i.e. the power stroke). When sufficient quantities of ATP are not available, sarcomeres cannot contract uniformly. When this occurs the tissue weakens and ejection volumes decrease. Left ventricle filling, followed by a partial or incomplete contractile event, increases chamber volume, stretches the tissue and promotes thinning of the ventricle wall (i.e. dilation). Support for this general concept is seen by distinctive mutations in myosin. Interestingly, mutations that increase the myosin power stroke above normal lead to hypertrophic cardiomyopathy while others that decrease the power stroke result in dilated cardiomyopathy. For example, Phe764Leu or Ser532Pro mutations in myosin correlate with diminished power production and are associated with dilated cardiomyopathy [59]. Analogous to these myosin mutations that directly affect contractility, mutations in TAZ elicit a similar, albeit indirect, effect by compromising the ability of mitochondria to generate ATP. Whereas the left ventricle is the first tissue site to exhibit pathology, as damage spreads to the right ventricle and atria, the disease worsens. As the heart chambers dilate, contractility declines further and blood circulation decreases, exacerbating the physiological impairment. In the absence of intervention, the heart will continue to weaken and, ultimately, fail.
In normal subjects, cardiomyocytes respond to an increase in heart rate by increasing production of ATP via aerobic respiration. Whereas BTHS cardiac tissue may be able to satisfy tissue needs for ATP under resting conditions, it is unable to adequately ramp up production to meet an increase in demand [21]. An interesting question relates to why cardiac crises in BTHS subjects often present at key life stage junctions including the first few months of life, 2–4 years of age and puberty. One possibility is that these crises are precipitated by rapid growth that is characteristic of these developmental stages [60]. Insofar as the heart normally has one of the highest metabolic rates of any organ [61], the increased energy requirements of tissue growth and expansion may place extra strain on cardiac tissue such that damage/failure is more likely to occur. An inability to increase aerobic respiration in response to physiological need is also directly responsible for two hallmark phenotypic features of BTHS, lactic acidosis and organic aciduria. Thus, their presence is a telltale sign that the tissue is laboring to meet the physiological demand for ATP and, unless this demand is reduced, tissue damage (i.e. cardiomyopathy) will result.
Concluding Remarks
In this review, we have navigated the circuitous path from mutations in TAZ, a gene encoding a phospholipid transacylase, to manifestation of cardiomyopathy. The presence of key phenotypic features, including alterations in the content and composition of cardiolipin, lactic acidosis, organic aciduria and muscle fatigue, fit into a model of defective mitochondrial ATP production that leads to an energy deficit. In turn, lack of sufficient ATP limits muscle contractility and underlies cellular and tissue damage that manifests as LVNC or dilated cardiomyopathy. The remarkable finding that mutations in a seemingly innocuous phospholipid transacylase have such a profound impact on cardiac/skeletal muscle metabolism and contractile function reveals a set of tightly interconnected processes that underpin efficient muscle physiology and metabolism. Moreover, although BTHS subjects can function “normally” at rest, when stressed (e.g. sustained increase in heart rate) disease is manifest by failure to generate sufficient quantities of ATP. When this occurs, compensatory metabolic and cell biological changes occur that lead to tissue damage. To assess whether fitness training can improve outcomes, or be curative, for BTHS, Cade et al. [62] investigated the effect of endurance exercise training on exercise tolerance by measuring skeletal muscle oxygen extraction, cardiac function, and quality of life in young adults with BTHS. Whereas twelve weeks of endurance exercise training was found to be safe, only minor improvements in peak exercise tolerance were recorded. Moreover, endurance training did not appear to improve skeletal muscle oxygen extraction or cardiac function to a significant extent. While exercise training may not be the best means of combating BTHS-related symptoms, interventions aimed at improving mitochondrial function and energy balance are likely to be beneficial in management of this disease.
Abbreviations
- TAZ :
-
The tafazzin gene
- BTHS:
-
Barth syndrome
- IMM:
-
Inner mitochondrial membrane
- ETC:
-
Electron transport chain
- ER:
-
Endoplasmic reticulum
- ALCAT1:
-
Acyl CoA lysocardiolipin acyltransferase
- MLCAT:
-
Monolysocardiolipin acyltransferase
- HMG CoA:
-
3-Hydroxy 3-methylglutaryl CoA
- 3MG CoA:
-
3-Methylglutaconyl CoA
- 3MGA:
-
3-Methylglutaconic acid
- 2-EHA:
-
2-Ethylhydracrylic acid
- LVNC:
-
Left ventricular non-compaction
References
Clarke SL, Bowron A, Gonzalez IL, Groves SJ, Newbury-Ecob R, Clayton N, Martin RP, Tsai-Goodman B, Garratt V, Ashworth M, Bowen VM, McCurdy KR, Damin MK, Spencer CT, Toth MJ, Kelley RI, Steward CG (2013) Barth syndrome. Orphanet J Rare Dis 8:23
Bione S, D’Adamo P, Maestrini E, Gedeon AK, Bolhuis PA, Toniolo D (1996) A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nat Genet 12:385–389
Whited K, Baile MG, Currier P, Claypool SM (2013) Seven functional classes of Barth syndrome mutation. Hum Mol Genet 22:483–492
Xu Y, Kelley RI, Blanck TJ, Schlame M (2003) Remodeling of cardiolipin by phospholipid transacylation. J Biol Chem 278:51380–51385
Xu Y, Malhotra A, Ren M, Schlame M (2006) The enzymatic function of tafazzin. J Biol Chem 281:39217–39224
Valianpour F, Mitsakos V, Schlemmer D, Towbin JA, Taylor JM, Ekert PG, Thorburn DR, Munnich A, Wanders RJ, Barth PG, Vaz FM (2005) Monolysocardiolipins accumulate in Barth syndrome but do not lead to enhanced apoptosis. J Lipid Res 46:1182–1195
Gu Z, Valianpour F, Chen S, Vaz FM, Hakkaart GA, Wanders RJ, Greenberg ML (2004) Aberrant cardiolipin metabolism in the yeast taz1 mutant: a model for Barth syndrome. Mol Microbiol 51:149–158
Xu Y, Condell M, Plesken H, Edelman-Novemsky I, Ma J, Ren M, Schlame M (2006) A drosophila model of Barth syndrome. Proc Natl Acad Sci 103:11584–11588
Khuchua Z, Yue Z, Batts L, Strauss AW (2006) A zebrafish model of human Barth syndrome reveals the essential role of tafazzin in cardiac development and function. Circ Res 99:201–208
Acehan D, Vaz F, Houtkooper RH, James J, Moore V, Tokunaga C, Kulik W, Wansapura J, Toth MJ, Strauss A, Khuchua Z (2011) Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem 286:899–908
Acehan D, Khuchua Z, Houtkooper RH, Malhotra A, Kaufman J, Vaz FM, Ren M, Rockman HA, Stokes DL, Schlame M (2009) Distinct effects of tafazzin deletion in differentiated and undifferentiated mitochondria. Mitochondrion 9:86–95
Houtkooper RH, Vaz FM (2008) Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci 65:2493–2506
Schlame M (2009) Formation of molecular species of mitochondrial cardiolipin 2. A mathematical model of pattern formation by phospholipid transacylation. Biochim Biophys Acta 1791:321–325
Malhotra A, Xu Y, Ren M, Schlame M (2009) Formation of molecular species of mitochondrial cardiolipin. 1. A novel transacylation mechanism to shuttle fatty acids between sn-1 and sn-2 positions of multiple phospholipid species. Biochim Biophys Acta 1791:314–320
Gaspard GJ, McMaster CR (2015) Cardiolipin metabolism and its causal role in the etiology of the inherited cardiomyopathy Barth syndrome. Chem Phys Lipids 193:1–10
Cao J, Liu Y, Lockwood J, Burn P, Shi Y (2004) A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. J Biol Chem 279:31727–31734
Taylor WA, Hatch GM (2009) Identification of the human mitochondrial linoleoyl-coenzyme A monolysocardiolipin acyltransferase (MLCL AT-1). J Biol Chem 284:30360–30371
Mejia EM, Cole LK, Hatch GM (2014) Cardiolipin metabolism and the role it plays in heart failure and mitochondrial supercomplex formation. Cardiovasc Hematol Disord 14:98–106
Ye C, Shen Z, Greenberg ML (2016) Cardiolipin remodeling: a regulatory hub for modulating cardiolipin metabolism and function. J Bioenerg Biomembr 48:113–123
Ronvelia D, Greenwood J, Platt J, Hakim S, Zaragoza MV (2012) Intrafamilial variability for novel TAZ gene mutation: Barth syndrome with dilated cardiomyopathy and heart failure in an infant and left ventricular noncompaction in his great-uncle. Mol Genet Metab 107:428–432
Spencer CT, Byrne BJ, Bryant RM, Margossian R, Maisenbacher M, Breitenger P, Benni PB, Redfearn S, Marcus E, Cade WT (2011) Impaired cardiac reserve and severely diminished skeletal muscle O(2) utilization mediate exercise intolerance in Barth syndrome. Am J Physiol Heart Circ Physiol 301:H2122–H2129
Fry M, Green DE (1981) Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem 256:1874–1880
Eble KS, Coleman WB, Hantgan RR, Cunningham CC (1990) Tightly associated cardiolipin in the bovine heart mitochondrial ATP synthase as analyzed by 31P nuclear magnetic resonance spectroscopy. J Biol Chem 265:19434–19440
Robinson NC, Zborowski J, Talbert LH (1990) Cardiolipin-depleted bovine heart cytochrome c oxidase: binding stoichiometry and affinity for cardiolipin derivatives. Biochemistry 29:8962–8969
Hoffmann B, Stöckl A, Schlame M, Beyer K, Klingenberg M (1994) The reconstituted ADP/ATP carrier activity has an absolute requirement for cardiolipin as shown in cysteine mutants. J Biol Chem 269:1940–1944
Christodoulou J, McInnes RR, Jay V, Wilson G, Becker LE, Lehotay DC, Platt BA, Bridge PJ, Robinson BH, Clarke JT (1994) Barth syndrome–clinical observations and genetic-linkage studies. Amer J Med Gen 50:255–264
Barth PG, Van Den Bogert C, Bolhuis PA, Scholte HR, van Gennip AH, Schutgens RB, Ketel AG (1996) X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): respiratory-chain abnormalities in cultured fibroblasts. J Inherit Metabol Dis 19:157–160
Xu Y, Sutachan JJ, Plesken H, Kelley RI, Schlame M (2005) Characterization of lymphoblast mitochondria from patients with Barth syndrome. Lab Invest 85:823–830
Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang DZ, Li K, Wang J, Wanders RJ, Kulik W, Vaz FM, Laflamme MA, Murry CE, Chien KR, Kelley RI, Church GM, Parker KK, Pu WT (2014) Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med 20:616–623
Gonzalvez F, D’Aurelio M, Boutant M, Moustapha A, Puech JP, Landes T, Arnauné-Pelloquin L, Vial G, Taleux N, Slomianny C, Wanders RJ, Houtkooper RH, Bellenguer P, Møller IM, Gottlieb E, Vaz FM, Manfredi G, Petit PX (2013) Barth syndrome: cellular compensation of mitochondrial dysfunction and apoptosis inhibition due to changes in cardiolipin remodeling linked to tafazzin (TAZ) gene mutation. Biochim Biophys Acta 1832:1194–1206
Schlame M, Acehan D, Berno B, Xu Y, Valvo S, Ren M, Stokes DL, Epand RM (2012) The physical state of lipid substrates provides transacylation specificity for tafazzin. Nat Chem Biol 8:862–869
Renner LD, Weibel DB (2011) Cardiolipin microdomains localize to negatively curved regions of Escherichia coli membranes. Proc Natl Acad Sci 108:6264–6269
Bissler JJ, Tsorads M, Goring HH, Hug P, Chuck G, Tombragel E, McGraw C, Schlotman J, Ralston MA, Hug G (2002) Infantile dilated X-linked cardiomyopathy, G4. 5 mutations, altered lipids, and ultrastructural malformations of mitochondria in heart, liver, and skeletal muscle. Lab Invest 82:335–344
Acehan D, Xu Y, Stokes DL, Schlame M (2007) Comparison of lymphoblast mitochondria from normal subjects and patients with Barth syndrome using electron microscopic tomography. Lab Invest 87:40–48
Zhang M, Mileykovskaya E, Dowhan W (2005) Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J Biol Chem 280:29403–29408
Bazan S, Mileykovskaya E, Mallampalli VK, Heacock P, Sparagna GC, Dowhan W (2013) Cardiolipin-dependent reconstitution of respiratory supercomplexes from purified Saccharomyces cerevisiae complexes III and IV. J Biol Chem 288:401–411
McKenzie M, Lazarou M, Thorburn DR, Ryan MT (2006) Mitochondrial respiratory chain supercomplexes are destabilized in Barth syndrome patients. J Mol Biol 361:462–469
Huang Y, Powers C, Madala SK, Greis KD, Haffey WD, Towbin JA, Purevjav E, Javadov S, Strauss AW, Khuchua Z (2015) Cardiac metabolic pathways affected in the mouse model of Barth syndrome. PLoS One 10:e0128561
Kiebish MA, Yang K, Liu X, Mancuso DJ, Guan S, Zhao Z, Sims HF, Cerqua R, Cade WT, Han X, Gross RW (2013) Dysfunctional cardiac mitochondrial bioenergetic, lipidomic, and signaling in a murine model of Barth syndrome. J Lipid Res 54:1312–1325
Williamson DH, Lund P, Krebs HA (1967) The redox state of free nicotinamide–adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J 103:514–527
Gabriel JL, Plaut GW (1984) Inhibition of bovine heart NAD-specific isocitrate dehydrogenase by reduced pyridine nucleotides: modulation of inhibition by ADP, NAD+, calcium (2+), citrate, and isocitrate. Biochemistry 23:2773–2778
Smith CM, Bryla J, Williamson JR (1974) Regulation of mitochondrial alpha-ketoglutarate metabolism by product inhibition at alpha-ketoglutarate dehydrogenase. J Biol Chem 249:1497–1505
Kelley RI, Cheatham JP, Clark BJ, Nigro MA, Powell BR, Sherwood GW, Sladky JT, Swisher WP (1991) X-linked dilated cardiomyopathy with neutropenia, growth retardation, and 3-methylglutaconic aciduria. J Pediatr 119:738–747
Su B, Ryan RO (2014) Metabolic biology of 3-methylglutaconic acid-uria: a new perspective. J Inherit Metab Dis 37:359–368
Ikon N, Ryan RO (2016) On the origin of 3-methylglutaconic acid in disorders of mitochondrial energy metabolism. J Inherit Metab Dis 39:749–756
Mamer OA, Tjoa SS, Scriver CR, Klasen GA (1976) Demonstration of a new mammalian isoleucine catabolic pathway yielding an R series of metabolites. Biochem J 160:417–426
Korman SH, Andresen BS, Zeharia A, Gutman A, Boneh A, Pitt JJ (2005) 2-ethylhydracrylic aciduria in short/branched-chain acyl-CoA dehydrogenase deficiency: application to diagnosis and implications for the R-pathway of isoleucine oxidation. Clin Chem 51:610–617
Haapalainen AM, Meriläinen G, Wierenga RK (2006) The thiolase superfamily: condensing enzymes with diverse reaction specificities. Trends Biochem Sci 31:64–71
Ryan RO (2015) Metabolic annotation of 2-ethylhydracrylic acid. Clin Chim Acta 448:91–97
Sweetman L, Weyler W, Nyhan WL, de Céspedes C, Loria AR, Estrada Y (1978) Abnormal metabolites of isoleucine in a patient with propionyl-CoA carboxylase deficiency. Biomed Mass Spectrom 5:198–207
Kuhara T, Matsumoto I (1980) Studies on the urinary acidic metabolites from three patients with methylmalonic aciduria. Biomed Mass Spectrom 7:424–428
Goodman SI, McCabe ER, Fennessey PV, Miles BS, Mace JW, Jellum E (1978) Methylmalonic/beta-hydroxy-n-valeric aciduria due to methylmalonyl-CoA mutase deficiency. Clin Chim Acta 87:441–449
Chicco AJ, Sparagna GC (2007) Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol 292:C33–C44
Shen Z, Ye C, McCain K, Greenberg ML (2015) The role of cardiolipin in cardiovascular health. Biomed Res Int. 891707
Lesnefsky EJ, Chen Q, Hoppel CL (2016) Mitochondrial Metabolism in Aging Heart. Circ Res 118:1593–1611
Towbin JA, Lorts A, Jefferies JL (2015) Left ventricular non-compaction cardiomyopathy. Lancet 386:813–825
Spencer CT, Bryant RM, Day J, Gonzalez IL, Colan SD, Thompson WR, Berthy J, Redfearn SP, Byrne BJ (2006) Cardiac and clinical phenotype in Barth syndrome. Pediatrics 118:e337–e346
Guertl B, Noehammer C, Hoefler G (2000) Metabolic cardiomyopathies. Int J Exp Pathol 81:349–372
Debold EP, Schmitt JP, Patlak JB, Beck SE, Moore JR, Seidman JG, Seidman C, Warshaw DM (2007) Hypertrophic and dilated cardiomyopathy mutations differentially affect the molecular force generation of mouse alpha-cardiac myosin in the laser trap assay. Am J Physiol Heart Circ Physiol 293:H284–H291
Janz KF, Dawson JD, Mahoney LT (2000) Predicting heart growth during puberty: the muscatine study. Pediatrics 105:e63
Wang Z, Ying Z, Bosy-Westphal A, Zhang J, Schautz B, Later W, Heymsfield SB, Müller MJ (2010) Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr 92:1369–1377
Cade WT, Reeds DN, Peterson LR, Bohnert KL, Tinius RA, Benni PB, Byrne BJ, Taylor CL (2016) Endurance exercise training in young adults with Barth syndrome: a pilot study JIMD Rep Jun 11 [Epub ahead of print]
Acknowledgements
This work was supported by a grant from the US National Institutes of Health (R37 HL64159). NI was supported by NIH training Grant T32 DK061918.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nikita Ikon and Robert O. Ryan declare that they have no conflict of interest.
About this article
Cite this article
Ikon, N., Ryan, R.O. Barth Syndrome: Connecting Cardiolipin to Cardiomyopathy. Lipids 52, 99–108 (2017). https://doi.org/10.1007/s11745-016-4229-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-016-4229-7