Abstract
Adipocyte differentiation plays a pivotal role in maintaining the production of small-size adipocytes with insulin sensitivity, and impaired adipogenesis is implicated in insulin resistance. 4-Hydroxyderricin (4-HD), a phytochemical component of Angelica keiskei, possesses diverse biological properties such as anti-inflammatory, antidiabetic, and antitumor. In the present study, we investigated the effects of 4-HD on adipocyte differentiation. 4-HD promoted lipid accumulation in 3T3-L1 cells, upregulated both peroxisome proliferator-activated receptor (PPAR)-γ mRNA and protein expression, and acted as a ligand for PPARγ in the luciferase assay. Moreover, 4-HD increased the mRNA and protein expression levels of adiponectin. Additionally, it promoted insulin-dependent glucose uptake into 3T3-L1 adipocytes and increased Akt phosphorylation and glucose transporter (GLUT) 4 mRNA expression. In summary, these findings suggest that 4-HD, which promoted adipogenesis and insulin sensitivity in 3T3-L1 cells, might be a phytochemical with potent insulin-sensitizing effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes, a disorder involving chronic hyperglycemia, has become a global public health problem [1]. Type 2 diabetes begins with insulin resistance, a condition in which cells do not respond properly to insulin and therefore, fail to take up glucose from the bloodstream [2]. Adipose tissue, a highly insulin-responsive tissue, is crucial for whole body glucose homeostasis through its lipids storage and endocrine function [3, 4]. Alterations of adipose tissue, i.e. lipodystrophy (deficiency of fat) and obesity (excessive fat) are related with insulin resistance and hyperglycemia [5]. In the obese state, adipocytes become enlarged and less responsive to insulin [6]. Besides, enlarged adipocytes secret proinflammatory factors, such as tumor necrosis factors (TNF) α and monocyte chemoattractant protein (MCP)-1, which lead to insulin resistance and hyperglycemia [7]. Thus, maintenance of adipose function is critical in the regulation of glucose metabolism. Adipocyte differentiation or adipogenesis, a process during which preadipocytes become mature adipocytes, increases the number of insulin sensitive adipocytes. Impaired adipocyte differentiation, as indicated by decreased gene expression of adipogenesis markers and enlarged adipocyte size, is suggested to contribute to obesity associated insulin resistance [8, 9]. Therefore, the regulation of adipocyte differentiation is highly correlated with insulin resistance and metabolic syndromes.
Adipocyte differentiation is a complex process involving a series of transcription activators including Peroxisome proliferator-activated receptor (PPAR)γ and CCAAT/enhancer- binding proteins (C/EBP)α [10]. PPARγ, a member of the superfamily of nuclear receptors, is specifically expressed at high levels in adipose tissue, and plays a central role in adipocyte differentiation [11, 12]. PPARγ ligands such as the thiazolidinedione (TZD) class of drugs have been used clinically for the treatment insulin resistance [13]. TZD enhance insulin sensitivity through promoting adipocyte differentiation and elevating production of insulin-sensitizing adipocytokines such as adiponectin in adipocytes [14, 15]. TZD significantly elevate plasma adiponectin concentrations in insulin-resistant humans and rodents [14]. Adiponectin is predominately produced by adipocytes and directly sensitizes the body to insulin [16, 17]. Adiponectin levels are lower in animals and humans with insulin resistance and diabetes than they are in the healthy [18, 19]. Moreover, the treatment of ob/ob mice with adiponectin leads to decreased serum glucose [20]. Adiponectin is classified into three different full-length forms: low-molecular-weight (LMW, trimer), middle-molecular-weight (MMW), high-molecular-weight (HMW) forms, and all three circulate in the serum. Of the three forms, HMW adiponectin is better correlated with improvement in insulin sensitivity and low levels of HMW adiponectin is closely associated with type 2 diabetes compared to the other forms [21, 22].
Angelica keiskei, a Japanese herb, is consumed as a healthy vegetable and contains numerous phytochemicals including chalcone, flavanone, and coumarin [23]. Of all these bioactive substances, two chalcones named 4-hydroxyderricin (4-HD) and xanthoangelol (XA) occur in abundance and have been reported to exert several bioactivities including antitumor [24], antidiabetic [25, 26], and anti-inflammatory [27]. In this study, we investigated the effects of 4-HD and XA on adipocyte differentiation using 3T3-L1 cells, a well-established model of adipocyte differentiation. Our results showed that 4-HD but not XA promoted adipocyte triglyceride (TG) accumulation by activating PPARγ receptors and promoting PPARγ gene and protein expression levels. Moreover, treatment of 3T3-L1 cells with 4-HD enhanced not only total adiponectin secretion but also HMW adiponectin expression, which is better correlated with improvement of insulin sensitivity. Additionally, 4-HD increased insulin-dependent glucose uptake by adipocytes.
Materials and Methods
Materials
4-HD and XA were isolated from ethyl acetate extract of roots of A. keiskei using the method established by Baba et al. [28] and dissolved in dimethyl sulfoxide (DMSO). For all experimental groups, the final DMSO concentrations in the administered compounds were maintained at 0.1 %. Other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), Nacalai Tesque (Kyoto, Japan), or Wako Pure Chemicals (Osaka, Japan).
Cell Culture
The 3T3-L1 fibroblasts and monkey CV1 kidney cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and were cultured separately in Dulbecco’s modified Eagle’s medium (DMEM, high glucose) supplemented with 10 % fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin in a humidified 5 % CO2 atmosphere at 37 °C.
Two days after reaching confluence, 3T3-L1 were differentiated with basic medium plus 0.25 µM dexamethasone, 10 µg/mL insulin, 0.5 mM 1-methyl-3-isobutylxanthine for 48 h. Then the media was replaced with growth medium (basic medium containing 5 µg/mL insulin) every 2 days, as previously described [29–31]. XA or 4-HD was added throughout the experimental period at the indicated concentrations. DMSO (0.1 %) is used as the vehicle control. Adiponectin produced by the 3T3-L1 adipocytes in the medium was measured using the Mouse Adiponectin enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
Oil Red O Staining
Cells were fixed with 10 % formalin for 1 h and stained with 0.5 % Oil Red O solution for 60 min. After washing with PBS, the cells were photographed. Then Oil Red O retained in the cells was eluted with 100 % isopropanol and the absorbance was measured at 490 nm.
Nile Red staining
Cells were washed once by PBS, then added with lipophilic fluorescent dye Nile Red (5 µg/ml) and nuclear fluorescent dye Hechst 33342 (10 µM)/PBS. After incubation at 37 °C for 10 min, the fluorescence was visualized by fluorescence microscopy and photographed.
Glucose Uptake
The rate of cellular uptake of 2-deoxy-d-[3H] glucose (Amersham Biosciences, Piscataway, NJ, USA) was measured as described previously [29–31]. On day 3 after cell differentiation in the presence or absence of 4-HD, 3T3-L1 cells were washed twice and incubated with serum-free DMEM. After 18 h, cells were incubated with HEPES-Krebs–Ringer (HKR) buffer containing 0.1 % bovine serum albumin with or without 100 nM insulin for 20 min at 37 °C. Glucose uptake was initiated by the addition of 2-deoxy-d-[3H] glucose at a final concentration of 0.5 µCi/mL to each well. After 10 min, the supernatant was discarded, the cells were washed twice with cold PBS, and then solubilized in 0.1 N sodium hydroxide (NaOH). The radioactivity of cell lysate was then measured using a scintillation counter and normalized to the protein concentrations.
mRNA Extraction and Quantitation
Total RNA was isolated using a commercially available reagent (Sepasol-RNA I Super G, Nacalai Tesque) and reverse transcribed with the M-MLV Reverse Transcriptase (Promega) in accordance with the manufacturer’s instructions. The quantification of mRNA expression levels of target genes was performed using the LightCycler system (Roche Diagnostics, Mannheim, Germany) with SYBR Green fluorescence signals. The primers were as follows:
mC/EBPα (forward, 5ʹ-TGGACAAGAACAGCAACGAC-3ʹ and reverse, 5ʹ-TCACTGGTCA ACTCCAGCAC-3′), mPPARγ (forward, 5ʹ- GGAGATCTCCAGTGATATCGACCA-3′ and reverse, 5ʹ-ACGGCTTCTACGGATCGAAACT-3′), adipocyte fatty acid-binding protein 2 (maP2, forward, 5ʹ-AAGACAGCTCCTCCTCGAAGGTT-3ʹ and reverse, 5ʹ-TGACCAAA TCCCCATTTACGC-3′), adiponectin (forward, 5ʹ-TACAACCAACAGAATCATTATGACGG -3ʹ and reverse, 5ʹ-GAAAGCCAGTAAATAGAGTCGTTGA-3ʹ), glucose transporter 4 (mGlut4, forward, 5ʹ-TAGGAGCTGGTGTGGTCAATACG-3ʹ and reverse, 5ʹ-TAAAAGGGAAGG TGTCCGTCG-3ʹ), preadipocyte factor 1 (mPref1, forward, 5ʹ-GTGACCCCCAGTATGGAT TC-3ʹ and reverse, 5ʹ-AGGGAGAACCATTGATCACG-3ʹ), and m36B4 (forward, 5ʹ-TGTGTGTCTGCAGATCGGGTAC-3ʹ and reverse, 5ʹ-CTTTGGCGGGATTAGTCGAAG- 3ʹ).
All target genes were normalized to the housekeeping gene m36B4.
Western Blotting
Cells were lysed in lysis buffer containing 20 mM Tris-hydrochloride (HCl, pH 7.5), 150 mM sodium chloride (NaCl), 1 % Triton X-100, and a protease inhibitor cocktail (Nacalai Tesque), followed by centrifugation (15,000×g) at 4 °C for 10 min. The protein concentration of the supernatant was measured using the Bio-Rad DC protein assay. After denaturing in sodium dodecyl sulfate (SDS), equal amounts of protein were separated using 10 % SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidene difluoride transfer membranes (GE Healthcare, Buckinghamshire, UK). The membranes were incubated with primary antibodies (anti-PPARγ, or anti-β-actin antibody, or anti-Akt, Cell Signaling Technology, MA, USA; anti-Phospho-Akt (S473), R&D systems, Minneapolis, MN) at 4 °C overnight, blocked with 5 % skim milk in PBS, and then incubated with the secondary antibody (Santa Cruz Biotechnology, CA, USA) for 1 h. The secondary antibody staining was visualized using a chemiluminescent horseradish peroxidase (HRP) substrate (Millipore, MA, USA). The adiponectin multimerization in the supernatant of the 3T3-L1 cells was measured as described previously [29–31].
Transfection and Luciferase Assay
Monkey CV-1 cells were transfected with p4xUASg-tk-luc (a reporter plasmid), pM-hPPARγ (an expression plasmid for a chimera protein of the GAL4 DNA-binding domain and human PPARγ-ligand binding domain), and pRL-CMV (an internal control for normalizing transfection efficiency) using Lipofectamine (Invitrogen, CA, USA) for 6 h. The transfected CV-1 cells were incubated with 4-HD for 24 h and then subjected to luciferase assays using a Dual-luciferase reporter gene Assay system (Promega) according to the manufacturer′s protocol.
Statistical Analysis
All data are presented as means at standard error of the mean (SEM) and were analyzed using unpaired t-tests and one-way analysis of variance (ANOVA) followed by the Tukey–Kramer test when variances were heterogeneous. Differences were considered significant at P < 0.05.
Results
4-HD Stimulated Differentiation of 3T3-L1 Preadipocytes
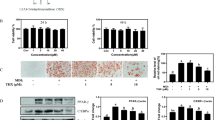
To evaluate the effects of 4-HD and XA on adipocyte differentiation, we first determined whether they promoted TG accumulation in 3T3-L1 preadipocytes (Fig. 1a). Differentiation of 3T3-L1 preadipocytes was induced in the presence of XA or 4-HD, and troglitazone (TRO) was used as a positive control. On day 10, we performed Oil-Red-O staining and Nile red staining, which demonstrated that 4-HD but not XA stimulated TG accumulation in 3T3-L1 cells, characterized by increased lipid droplets (Fig. 1b–d). Next, we measured adipogenic (aP2 and C/EBPα) and preadipocyte (Pref1) maker gene expression in 3T3-L1 cells treated with XA or 4-HD. Both C/EBPα and aP2 gene levels (Fig. 2a, b, respectively) were significantly elevated while Pref1 mRNA expression (Fig. 2c) declined in 4-HD-treated 3T3-L1 cells. The expression levels of all three genes remained unchanged in XA-treated 3T3-L1 cells compared with the control. These results (Fig. 2a–c) suggest that 4-HD treatment promoted differentiation of 3T3-L1 preadipocytes.
4-Hydroxyderricin (4-HD)-induced adipogenesis in 3T3-L1 cells. a Chemical structure of xanthoangelol (XA) and 4-HD. b Oil Red O staining of 3T3-L1 cells treated with XA, 4-HD (1 and 5 μM), or Tro (1 μM). c The cells were stained with Oil Red O and eluted with isopropyl alcohol, then qualified at 490 nm. Values are means ± SEM of 4–5 samples; *p < 0.05 and **p < 0.01 compared with control. d Nile Red staining of 3T3-L1 cells treated with 4-HD (1 and 5 μM), or Tro (1 μM)
4-Hydroxyderricin (4-HD) elevated the expression of adipogenesis marker genes, cytosine–cytosine–adenosine–adenosine–thymidine (CCAAT)/enhancer binding protein (C/EBPα) and adipocyte fatty acid binding-protein 2 (aP2) and downregulated that of preadipocyte marker gene, preadipocyte factor 1 (Pref1) in 3T3-L1 cells. a C/EBPα, b aP2, and c Pref1 gene expression levels in 3T3-L1 cells treated with xanthoangelol (XA) and 4-HD (1 and 5 μM) were normalized to 36B4 mRNA expression levels. Values are means ± SEM of 3–4 samples; *p < 0.05 and **p < 0.01 compared with control
4-HD Increased Expression Levels of PPARγ in 3T3-L1 Cells and Activate PPARγ Signaling
The presence and activation of PPARγ, a member of the nuclear hormone receptor superfamily, is required for adipogenesis both in vivo and in vitro [32]. To elucidate the effects of 4-HD on PPARγ, we first investigated whether 4-HD increased the expression levels of PPARγ mRNA and protein. Treatment with 4-HD greatly upregulated PPARγ mRNA (including PPARγ1 and 2), and PPARγ1 and PPARγ2 protein expression (Fig. 3a, b, respectively) in 3T3-L1 cells compared with control cells. Next, we examined whether 4-HD served as a ligand for PPARγ by performing a luciferase assay. As shown in Fig. 3c, 4-HD but not XA (data not shown) at concentrations of 1 and 5 µM significantly activated PPARγ by 2.5- and 6.5-fold higher, respectively, than no treatment did. The results indicate that 4-HD did not only promote PPARγ mRNA and protein expression but also served as a ligand of PPARγ.
4-Hydroxyderricin (4-HD) increased peroxisome proliferator-activated receptors (PPAR)-γ mRNA and protein expression levels and activated PPARγ. 3T3-L1 cells were induced to differentiate and then incubated in growth medium with or without 4-HD. mRNA expression of a PPARγ was measured on day 6, and b protein expression of PPARγ was determined on day 10. c Effect of 4-HD on PPARγ activity was measured using luciferase assay. Values are means ± SEM of 5 samples; *p < 0.05 and **p < 0.01 compared with control
4-HD Increased Adiponectin mRNA Expression and Secretion in 3T3-L1 Cells
The level of adiponectin, an insulin-sensitizing adipocytokine derived from adipocytes, increases during adipogenesis. Therefore, next, we determined the effects of 4-HD on adiponectin expression in 3T3-L1 cells. Treatment of 3T3-L1 cells with 5 μM 4-HD significantly enhanced adiponectin gene expression during differentiation with a 3-fold increase on day 6 compared to untreated cells (Fig. 4a). Consistent with the mRNA expression, the secretion of adiponectin in the medium of cells exposed to 4-HD was significantly upregulated on day 10 (Fig. 4b). Interestingly, 4-HD effectively enhanced the secretion of HMW adiponectin, which is better correlated with improvement of insulin sensitivity compared with the other forms of adiponectin (Fig. 4c). These data suggest that 4-HD treatment during adipocyte differentiation promoted adiponectin mRNA expression and protein secretion in adipocytes.
4-Hydroxyderricin (4-HD) promoted adiponectin mRNA expression and secretion. 3T3-L1 cells were induced to differentiate and then incubated in growth medium with or without 4-HD. a mRNA expression of adiponectin was measured on day 6. b Total and c high-molecular-weight (HMW) adiponectin in 3T3-L1 cell supernatants were measured on day 10. Values are means ± SEM of 5–6 samples; *p < 0.05 and **p < 0.01 compared with control
4-HD Promoted Insulin-Stimulated Glucose Uptake and GLUT4 mRNA Expression in 3T3-L1 Cells
To elucidate the effects of 4-HD on glucose utilization in 3T3-L1 adipocytes, we examined insulin-independent- and -dependent glucose uptake in 4-HD-treated adipocytes. Following treatment with 4-HD, insulin-dependent glucose uptake was significantly upregulated while insulin-independent glucose uptake remained unchanged (Fig. 5a). 4-HD treatment also increased the phosphorylation of Akt, a major protein kinase involved in insulin-stimulated glucose transport, in adipocytes. Besides, the mRNA expression of GLUT4, a key regulator of insulin-stimulated glucose uptake and whole-body glucose homeostasis was markedly elevated in 3T3-L1 cells treated with 4-HD (Fig. 5b). The results demonstrate that 4-HD increased insulin-stimulated glucose uptake accompanied by the upregulation of mRNA level of GLUT4 and Akt phosphorylation in adipocytes.
4-Hydroxyderricin (4-HD) stimulated insulin-dependent glucose uptake and upregulated mRNA expression of glucose transporter 4 (GLUT4) and protein levels of AKT phosphorylation. 3T3-L1 cells were induced to differentiate, and then incubated in growth medium with or without 4-HD. a 2-Deoxyglucose uptake into 4-HD treated cells was determined on day 3. b The level of AKT phosphorylation and the expression of total protein were measured by immunoblotting. On day 3 after differentiation, the cells were washed with serum free medium for three times and incubated in serum free medium overnight, then stimulated with 3.4 nM insulin and extracted for immunoblot assay. c mRNA expression of GLUT4 was measured on day 6. Values are means ± SEM of 5–6 samples; *p < 0.05 and **p < 0.01 compared with control
Discussion
Insulin-responsive adipose tissue plays a key role in regulating insulin sensitivity and the risk for diabetes by its fats storage and endocrine functions [3, 33]. Adipocyte differentiation contributes to increasing insulin sensitive adipocytes, which are thought to be more insulin sensitive in comparison with large adipocytes. Failure of adipocyte differentiation might contribute to insulin resistance and type 2 diabetes [8]. In the present study, we examined the effects of 4-HD on adipogenesis in 3T3-L1 cells. 4-HD significantly enhanced adipogenesis as evidenced by the elevated TG accumulation of the 3T3-L1 cells. The mRNA expression of adipogenic markers such as C/EBPα and aP2 were significantly upregulated while the gene levels of the preadipocyte marker Pref1 significantly decreased in adipocytes treated with 4-HD. Based on these results, we proposed that 4-HD exhibited adipogenic effects in 3T3-L1 cells.
PPARγ, the major regulator involved in adipocyte differentiation, has been reported to be required for the differentiation of adipose tissue in vivo and in vitro [32]. In the present study, 4-HD elevated both mRNA and protein expression levels of PPARγ in 3T3-L1 cells during differentiation. Additionally, 4-HD upregulated PPARγ activity in the luciferase assay and the results suggest that the effect of 4-HD on adipogenesis might be mediated by the increase in ligand activation of PPARγ.
In addition, some interesting observations needed further discussion in the study. One is that, XA, with the same main structure as 4-HD, exhibited weaker adipogenic activity, although similar effects of XA and 4-HD at the same concentration on promoting glucose uptake in L6 cell and anti-inflammation in LPS-stimulated RAW cells were observed before [26, 27]. The diverse effects of XA and 4-HD on adipocyte differentiation might contribute to the difference of structure on the side hydrocarbon chain, which leads to distinct PPARγ ligand activity.
Another observation is that our results are contrary to the conclusion of Zhang et al. [34] who reported that 4-HD suppressed adipocyte differentiation of 3T3-L1 cells at a concentration of 5 µM. This discrepancy might be due to differences in exposure time (2 vs 3 days) and the differentiation inducer cocktail. In our study, we used the most common agents for differentiation, including dexamethasone, insulin, and 1-methyl-3-isobutylxanthine [35, 36]. Besides of these common agent, ascorbic acid was also listed in Zhang′s study. Although ascorbic acid has been reported to promote adipocyte differentiation in previous study [37], some study has shown that ascorbic acid suppress adipocyte differentiation [38]. Therefore, ascorbic acid might effect adipogenic activity of 4-HD.
PPARγ agonists such as TZD are used in the therapy of insulin resistance and type 2 diabetes [12]. Treatment of patients with diabetes using TZD is accompanied with elevated plasma adiponectin, which contributes to enhancing insulin sensitivity [14, 15]. Adiponectin is predominantly produced in white adipose tissue and increases during adipocyte differentiation. In the present study, treatment of 3T3-L1 cells with 4-HD also upregulated both mRNA and protein expression levels of adiponectin. Moreover, we firstly shown that 4-HD increased not only total adiponectin protein production but also HMW adiponectin secretion, which is the active form. The HMW adiponectin proportion rather than total adiponectin is a more accurate reflection of the association of adiponectin with insulin resistance [20]. Collectively, these results suggest that 4-HD might increase adiponectin production in adipose tissue via activation of PPARγ signaling.
Pioglitazone belongs to the TZD class of drugs and potently promotes glucose utilization in adipose tissue by increasing the uptake and metabolism of glucose via activation of regulatory genes involved in glucose transport (GLUT4) and metabolism of lipids (fatty acid synthase, FAS) and phosphoenolpyruvate carboxykinase (PEPCK) [39]. GLUT4 plays a pivotal role in insulin-dependent glucose uptake and is impaired during insulin resistance [40]. Insulin promotes glucose transport in adipocytes mainly by enhancing the translocation of GLUT4 from intracellular sites to the membrane [41]. Akt is a major effector of the insulin response and capable of stimulating the translocation of GLUT4 to membrane [42]. In our study, 4-HD significantly increased insulin-dependent but not insulin-independent glucose uptake in 3T3-L1 cells during differentiation at a concentration of 5 µM. 4-HD treatment also elevated GLUT4 gene expression and Akt phosphorylation in 3T3-L1 adipocytes. Our results suggest that 4-HD increased insulin-dependent glucose transport by elevating GLUT4 expression and Akt phosphorylation. Two hours treatment of 3T3-L1 adipocytes with 20 µM 4-HD was reported to promote GLUT4 translocation to the plasma membrane [43]. These results suggest that 4-HD promotes insulin-stimulated glucose uptake though Akt/GLUT4 signaling pathway. 4-HD was reported to elevate glucose uptake in L6 myotubes by stimulating GLUT4 translocation with Akt-independent manner [26]. It suggests that 4-HD might promote glucose uptake via different mechanisms in different cell types.
Previous studies have demonstrated that 4-HD was rapidly absorbed in mice and distributed preferentially to adipose tissue [44], and decreased blood glucose in KK-Ay mice [25]. They suggest that 4-HD might promote glucose uptake and increase insulin sensitivity in adipose tissue of mice. In addition, TZD promote adipocyte differentiation by generating small insulin sensitive adipocytes [45]. Moreover, adipose tissue distribution influences metabolic disorder, the ratio of visceral adipose tissue and subcutaneous adipose tissue is positively associated with insulin resistance [46]. PPARγ agonist rosiglitazone was reported to elevate glucose uptake and intracellular metabolism in subcutaneous fat through redistribution of triacylglycerol from visceral adipose tissue to subcutaneous adipose tissue in a PPARγ-dependent way [47]. Nevertheless, the effects of 4-HD on glucose metabolism, generation of small adipocytes within adipose tissue and redistribution of fat between different adipose depots in vivo remain to be clarified in future.
Taken together, we demonstrated that 4-HD promoted adipocyte differentiation by activating PPARγ signaling. Additionally, it stimulated adiponectin production in adipocytes and enhanced insulin-dependent glucose uptake into adipocytes. These results collectively suggest that 4-HD might be a potent phytochemical with the potential to regulate adipocyte function.
Abbreviations
- 4-HD:
-
4-Hydroxyderricin
- aP2:
-
Adipocyte fatty acid-binding protein 2
- CCAAT:
-
Cytosine–cytosine–adenosine–adenosine–thymidine
- C/EBP:
-
CCAAT/enhancer binding protein
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
Fetal bovine serum
- HKR:
-
HEPES-Krebs–Ringer
- HMW:
-
High molecular weight
- IBMX:
-
1-Methyl-3-isobutylxanthine
- LMW:
-
Low molecular weight
- MCP-1:
-
Monocyte chemoattractant protein-1
- PBS:
-
Phosphate-buffered saline
- PPAR:
-
Peroxisome proliferator-activated receptors
- Pref1:
-
Preadipocyte factor-1
- SDS–PAGE:
-
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- TG:
-
Triglyceride
- TRO:
-
Troglitazone
- TNFα:
-
Tumor necrosis factors α
- TZD:
-
Thiazolidinedione
- XA:
-
Xanthoangelol
References
Hu FB (2011) Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 34:1249–1257
Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L (2014) The many faces of diabetes: a disease with increasing heterogeneity. Lancet 383:1084–1094
Scherer PE (2006) Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 55:1537–1545
Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89:2548–2556
Rosen ED, Spiegelman BM (2006) Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444:847–853
Yang J, Eliasson B, Smith U, Cushman SW, Sherman AS (2012) The size of large adipose cells is a predictor of insulin resistance in first-degree relatives of type 2 diabetic patients. Obesity 20:932–938
Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Investig 116:1793–1801
McLaughlin T, Sherman A, Tsao P, Gonzalez O, Yee G, Lamendola C, Reaven GM, Cushman SW (2007) Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia 50:1707–1715
Yang X, Jansson PA, Nagaev I, Jack MM, Carvalho E, Sunnerhagen KS, Cam MC, Cushman SW, Smith U (2004) Evidence of impaired adipogenesis in insulin resistance. Biochem Biophys Res Commun 317:1045–1051
Gregoire FM, Smas CM, Sul HS (1998) Understanding adipocyte differentiation. Physiol Rev 78:783–809
Lowell BB (1999) PPARgamma: an essential regulator of adipogenesis and modulator of fat cell function. Cell 99:239–242
Schoonjans K, Staels B, Auwerx J (1996) The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta 1302:93–109
Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA (1995) An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 270:12953–12956
Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM (2002) The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes 51:2968–2974
Hallakou S, Doare L, Foufelle F, Kergoat M, Guerre-Millo M, Berthault MF, Dugail I, Morin J, Auwerx J, Ferre P (1997) Pioglitazone induces in vivo adipocyte differentiation in the obese Zucker fa/fa rat. Diabetes 46:1393–1399
Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270:26746–26749
Berg AH, Combs TP, Scherer PE (2002) ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab TEM 13:84–89
Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, Matsuzawa Y (2001) Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 50:1126–1133
Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, Knowler WC, Krakoff J (2002) Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 360:57–58
Berg AH, Combs TP, Du X, Brownlee M, Scherer PE (2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7:947–953
Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE (2004) Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279:12152–12162
Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE (2003) Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem 278:9073–9085
Akihisa T, Tokuda H, Ukiya M, Iizuka M, Schneider S, Ogasawara K, Mukainaka T, Iwatsuki K, Suzuki T, Nishino H (2003) Chalcones, coumarins, and flavanones from the exudate of Angelica keiskei and their chemopreventive effects. Cancer Lett 201:133–137
Kimura Y, Taniguchi M, Baba K (2004) Antitumor and antimetastatic activities of 4-hydroxyderricin isolated from Angelica keiskei roots. Planta Med 70:211–219
Enoki T, Ohnogi H, Nagamine K, Kudo Y, Sugiyama K, Tanabe M, Kobayashi E, Sagawa H, Kato I (2007) Antidiabetic activities of chalcones isolated from a Japanese herb, Angelica keiskei. J Agric Food Chem 55:6013–6017
Kawabata K, Sawada K, Ikeda K, Fukuda I, Kawasaki K, Yamamoto N, Ashida H (2011) Prenylated chalcones 4-hydroxyderricin and xanthoangelol stimulate glucose uptake in skeletal muscle cells by inducing GLUT4 translocation. Mol Nutr Food Res 55:467–475
Yasuda M, Kawabata K, Miyashita M, Okumura M, Yamamoto N, Takahashi M, Ashida H, Ohigashi H (2014) Inhibitory effects of 4-hydroxyderricin and xanthoangelol on lipopolysaccharide-induced inflammatory responses in RAW264 macrophages. J Agric Food Chem 62:462–467
Baba K, Nakata K, Taniguchi M, Kido T, Kozawa M (1990) Chemical-components of Angelica Keiskei. 7. Chalcones from Angelica Keiskei. Phytochemistry 29:3907–3910
Goto T, Kim YI, Furuzono T, Takahashi N, Yamakuni K, Yang HE, Li Y, Ohue R, Nomura W, Sugawara T, Yu R, Kitamura N, Park SB, Kishino S, Ogawa J, Kawada T (2015) 10-oxo-12(Z)-octadecenoic acid, a linoleic acid metabolite produced by gut lactic acid bacteria, potently activates PPARgamma and stimulates adipogenesis. Biochem Biophys Res Commun 459:597–603
Takahashi H, Hara H, Goto T, Kamakari K, Wataru N, Mohri S, Takahashi N, Suzuki H, Shibata D, Kawada T (2015) 13-Oxo-9(Z),11(E),15(Z)-octadecatrienoic acid activates peroxisome proliferator-activated receptor gamma in adipocytes. Lipids 50:3–12
Takahashi N, Yao R, Kang MS, Senda M, Ando C, Nishimura K, Goto T, Hirai S, Ezaki Y, Kawada T (2011) Dehydroabietic acid activates peroxisome proliferator-activated receptor-gamma and stimulates insulin-dependent glucose uptake into 3T3-L1 adipocytes. BioFactors 37:309–314
Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM (1999) PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4:611–617
Kahn BB, Flier JS (2000) Obesity and insulin resistance. J Clin Investig 106:473–481
Zhang T, Sawada K, Yamamoto N, Ashida H (2013) 4-Hydroxyderricin and xanthoangelol from Ashitaba (Angelica keiskei) suppress differentiation of preadipocytes to adipocytes via AMPK and MAPK pathways. Mol Nutr Food Res 57:1729–1740
Kim AY, Park YJ, Pan X, Shin KC, Kwak SH, Bassas AF, Sallam RM, Park KS, Alfadda AA, Xu A, Kim JB (2015) Obesity-induced DNA hypermethylation of the adiponectin gene mediates insulin resistance. Nat Commun 6:7585
Takagi M, Uno H, Nishi R, Sugimoto M, Hasegawa S, Piao J, Ihara N, Kanai S, Kakei S, Tamura Y, Suganami T, Kamei Y, Shimizu T, Yasuda A, Ogawa Y, and Mizutani S (2015) ATM regulates adipocyte differentiation and contributes to glucose homeostasis. Cell Rep
Kim B, Choi KM, Yim HS, Lee MG (2013) Ascorbic acid enhances adipogenesis of 3T3-L1 murine preadipocyte through differential expression of collagens. Lipids Health Dis 12:182
Rahman F, Al Frouh F, Bordignon B, Fraterno M, Landrier JF, Peiretti F, Fontes M (2014) Ascorbic acid is a dose-dependent inhibitor of adipocyte differentiation, probably by reducing cAMP pool. Front Cell Dev Biol 2:29
Kubota N, Terauchi Y, Kubota T, Kumagai H, Itoh S, Satoh H, Yano W, Ogata H, Tokuyama K, Takamoto I, Mineyama T, Ishikawa M, Moroi M, Sugi K, Yamauchi T, Ueki K, Tobe K, Noda T, Nagai R, Kadowaki T (2006) Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. J Biol Chem 281:8748–8755
Leguisamo NM, Lehnen AM, Machado UF, Okamoto MM, Markoski MM, Pinto GH, Schaan BD (2012) GLUT4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovasc Diabetol 11:100
Leto D, Saltiel AR (2012) Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol 13:383–396
Mackenzie RW, Elliott BT (2014) Akt/PKB activation and insulin signaling: a novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab Syndr Obes Targets Ther 7:55–64
Ohta M, Fujinami A, Kobayashi N, Amano A, Ishigami A, Tokuda H, Suzuki N, Ito F, Mori T, Sawada M, Iwasa K, Kitawaki J, Ohnishi K, Tsujikawa M, Obayashi H (2015) Two chalcones, 4-hydroxyderricin and xanthoangelol, stimulate GLUT4-dependent glucose uptake through the LKB1/AMP-activated protein kinase signaling pathway in 3T3-L1 adipocytes. Nutr Res 35:618–625
Nakamura T, Tokushima T, Kawabata K, Yamamoto N, Miyamoto M, Ashida H (2012) Absorption and metabolism of 4-hydroxyderricin and xanthoangelol after oral administration of Angelica keiskei (Ashitaba) extract in mice. Arch Biochem Biophys 521:71–76
Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H, Yazaki Y, Kadowaki T (1998) Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Investig 101:1354–1361
Wajchenberg BL (2000) Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21:697–738
Festuccia WT, Blanchard PG, Turcotte V, Laplante M, Sariahmetoglu M, Brindley DN, Deshaies Y (2009) Depot-specific effects of the PPARgamma agonist rosiglitazone on adipose tissue glucose uptake and metabolism. J Lipid Res 50:1185–1194
Acknowledgments
The authors thank S. Shinotoh and M. Sakai for their secretarial and technical support, respectively. This study was supported by Grants-in-Aid for Scientific Research Grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (22228001 and 24688015). This study was also supported by a Grant from the Taisho Pharmaceutical Co. Ltd, Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing financial interests.
About this article
Cite this article
Li, Y., Goto, T., Yamakuni, K. et al. 4-Hydroxyderricin, as a PPARγ Agonist, Promotes Adipogenesis, Adiponectin Secretion, and Glucose Uptake in 3T3-L1 Cells. Lipids 51, 787–795 (2016). https://doi.org/10.1007/s11745-016-4154-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-016-4154-9