Abstract
Dysregulation of adipokines is a hallmark of obesity. Polyunsaturated fatty acids in fish oil may exert anti-inflammatory effects on adipose tissue mitigating the dysregulation of adipokines thereby preventing obesity. This study investigated the effects of high-fat diets containing different amounts of n3 polyunsaturated fatty acids (PUFA) on adiposity and adipokine production in mice. Mice were fed a low-fat or a high-fat diet with 16 or 45 % of energy from corn oil (low n3 PUFA) in comparison with a high-fat diet containing soybean or high-oleic sunflower oil (adequate n3 PUFA) or flaxseed or fish oil (high n3 PUFA) for 11 weeks. High-fat diets, regardless of types of oils, significantly increased body fat mass and body weights compared to the low-fat diet. Adipose fatty acid composition and contents reflected dietary fatty acid profiles. The high-fat fish oil diet significantly increased adiponectin and reduced leptin concentrations in both plasma and adipose tissue; it did not elevate plasma insulin concentration compared to the high-fat corn oil diet. All high-fat diets elevated concentrations of plasminogen activator inhibitor-1 (PAI-1) and monocyte chemoattractant protein-1 (MCP-1) but lowered resistin concentrations in both plasma and adipose tissue. In conclusion, fish oil may be beneficial in improving insulin sensitivity by upregulation of adiponectin and downregulation of leptin production; n3 and n6 PUFA do not play a role at the dietary levels tested in reducing adiposity and production of pro-inflammatory cytokines (leptin, PAI-1, MCP-1 and resistin) and anti-inflammatory cytokine adiponectin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a growing health concern worldwide; it leads to an increased risk of chronic morbidities including type-2 diabetes, cardiovascular diseases and certain types of cancer [1]. Adipose tissue is considered an endocrine organ that produces adipokines (inflammatory cytokines) that participate in the regulation of food intake, adipose storage, adipogenesis and energy homeostasis. For example, the pro-inflammatory cytokine leptin regulates body weight through its effects on food intake and energy expenditure [2], circulating leptin concentrations are proportional to visceral adiposity in the body [3]. Adiponectin, an anti-inflammatory cytokine, modulates glucose regulation and fatty acid catabolism [4], plasma levels of adiponectin are reduced in obesity and elevated in those with normal body weights [5]. Furthermore, adipose tissue produced pro-inflammatory cytokines contribute to low-grade systemic inflammation and play important roles in the pathogenesis of obesity and obesity-related diseases. Overexpression of pro-inflammatory adipokines such as leptin [3], plasminogen activator inhibitor-1 (PAI-1) [6], monocyte chemoattractant protein-1 (MCP-1) [7], and resistin [8] are reported in obesity. Blood levels of these adipokines are elevated in obese humans [3, 6–8] and laboratory rodents fed a high-fat diet [9, 10]; weight loss reduces blood concentrations of these adipokines in humans [7, 11–13] and animals [14–16].
A growing body of evidence suggests that different types of fats in a diet may have differences in contributing to the development of obesity and regulating adipokine production. Diets high in saturated fat promote obesity [17, 18]. Diets high in α-linolenic acid (ALA; 18:3n3) or eicosapentaenoic acid (EPA; 20:5n3)/docosahexaenoic acid (DHA; 22:6n3) upregulate production of adiponectin [19–22] and downregulate production of pro-inflammatory cytokines such as PAI-1 and MCP-1 [23, 24]. In vitro studies showed that palmitic acid downregulates adiponectin production [25, 26] and that EPA increases adiponectin secretion in 3T3-L1 adipocytes [25]. The n3:n6 ratio is suggested to be important in disease development, for example, cardiovascular disease [27] and cancer [28, 29] in rodent models fed a high-fat diet.
Corn oil is a source of dietary fat commonly used in nutrition research, particularly in studies where a low quantity of n3 polyunsaturated fatty acids (PUFA) in a diet is preferred [30–32]. We reported that significant increases in plasma concentrations of pro-inflammatory adipokines (e.g., leptin, PAI-1, and MCP-1) and decreases in anti-inflammatory adipokines (adiponectin) are accompanied with metabolic disturbance in mice fed a corn oil based high-fat diet [9]. However, it remains to be determined whether a lack of ALA in corn oil contributes to the elevation in pro-inflammatory cytokine production in mice fed a high-fat corn oil diet. We hypothesized that differences in n3 PUFA in diet affect inflammatory cytokine production. To test this hypothesis, we compared high-fat diets containing different amounts of n3 PUFA on adiposity and adipokine production in mice.
Materials and Methods
Reagents and Assay Kits
Five different oils were used in the high fat diets. Corn oil was obtained from ACH Food Companies (Memphis, TN), fish oil was from MP Biomedicals, LLC (Solon, OH), flaxseed oil was from Dyets, Inc. (Bethlehem, PA), soybean oil was from ConAgra Foods, Inc. (Omaha, NE) and sunflower oil was from Natural Oils International, Inc. (Simi Valley, CA). All reagents for gas chromatography were obtained from Sigma Aldrich (St. Louis, MO) unless otherwise detailed. Ketamine and xylazine were obtained from Midwest Veterinary Supply (Lakeville, MN). Enzyme-linked immunosorbent assay (ELISA) kits for leptin, resistin, and MCP-1 were obtained from R&D Systems (Minneapolis, MN), that for PAI-1 was from Molecular Innovations, Inc. (Novi, MI) and those for adiponectin and insulin were from EMD Millipore Corp. (Billerica, MA).
Animals and Diets
Three-week-old male C57BL/6 mice were purchased from Harlan Laboratories (Madison, WI). Mice were housed, three per cage, in a pathogen-free room under a 12:12 h light–dark cycle and maintained at 22 ± 1 °C. Six diets were compared and gross energy of each diet (Table 1) was quantified using bomb calorimetry (Model 6200, oxygen bomb calorimeter, Parr Instrument, Moline, IL). The AIN93G diet [33] with 16 % of energy from corn oil was used as the low-fat control diet (0.001 mol ALA/kg, 0.13 mol LNA/kg). The high-fat diets, modified from the AIN93G diet, contained 45 % of energy from various oils to obtain different contents of ALA (18:3n3) or linoleic acid (LNA; 18:2n6). These diets were (1) corn oil diet: 45 % of energy from corn oil (0.005 mol ALA/kg, 0.5 mol LNA/kg); (2) fish oil diet: 35 % of energy from fish oil and 10 % of energy from corn oil (0.007 mol ALA/kg, 0.17 mol long-chain n3 fatty acids/kg, 0.11 mol LNA/kg); (3) flaxseed oil diet: 18 % of energy from flaxseed oil and 27 % of energy from corn oil (0.17 mol ALA/kg, 0.33 mol LNA/kg); (4) soybean oil diet: 45 % of energy from soybean oil (0.05 mol ALA/kg, 0.4 mol LNA/kg); (5) sunflower oil diet: 39 % of energy from high-oleic sunflower oil and 6 % of energy from flaxseed oil (0.057 mol ALA/kg, 0.103 mol LNA/kg). All diets were powder diets; stored at −20 °C before being provided to the mice. The amounts and composition of fatty acids of dietary oils and energy provided by fatty acids from each oil are presented in Tables 2 and 3, respectively.
Experimental Design
This study was approved by the Animal Care and Use Committee of the U.S. Department of Agriculture, Agricultural Research Service, Grand Forks Human Nutrition Research Center. The procedures followed the National Institutes of Health guidelines for the care and use of laboratory animals [34]. Following a one-week acclimation with the low-fat diet, mice were randomized into six groups of 12 each (3 per cage). Mice had free access to diets and deionized water throughout the experiment; they were weighed weekly. Four weeks after the initiation of experimental feeding, six mice from each group were individually housed and their food intake was recorded daily (5 days per week) for 4 consecutive weeks. Caloric intake was calculated on the basis of average daily food intake and diet caloric content (Table 1). The duration of the experiment was 11 weeks. At week 10, body composition analysis of fat and lean mass of conscious, immobilized mice was performed by using quantitative magnetic resonance imaging (Echo whole-body composition analyzer, Model 100, Echo Medical System, Houston, TX). At termination, mice were fasted for 8 h and euthanized with an intraperitoneal injection of a mixture of ketamine and xylazine. Blood was collected via cardiac puncture into a tube containing 0.05 mM EDTA; plasma was obtained by centrifuging the blood at 10,000g for 10 min. Plasma and epididymal adipose tissue collected were stored at −80 °C for quantification of adipokines and insulin.
Quantification of Fatty Acids in Dietary Oils and Adipose Tissue
Epididymal adipose tissue (50 mg) was homogenized with 1 mL of ice-cold 3:2 hexane:isopropanol solution containing 1 mM butylated hydroxytoluene (Sigma Aldrich, St. Louis, MO) and centrifuged to remove insoluble materials. A 200 µL portion of the supernatant was mixed with 100 µL of internal standard solution [nonadecanoic acid (2.94 mg/mL) and heneicosanoic acid (0.21 mg/mL) (Nu-Chek Prep Inc., Elysian, MN) in methanol], and the solvent was evaporated under a stream of nitrogen at 35 °C. Dried samples were refluxed at 80 °C for 2 h in 2 mL of anhydrous methanol containing 5 % acetyl chloride (v/v) [35]. After heating, samples were returned to room temperature, neutralized with saturated sodium bicarbonate solution (0.5 mL) and methyl esters were extracted in hexane (1 mL) for analysis. Dietary oils (Table 2) and adipose tissue (Table 5) were quantified for fatty acids by gas chromatography on a Thermo Trace-1310 equipped with a TriPlus RSH Autosampler (Thermo Fisher Scientific, Waltham, MA) and a Supelco SP-2560 capillary column (Supelco, Belfonte, PA). Instrument parameters were adapted from Masood et al. [36]. Samples were injected twice, first with a 100:1 split ratio to allow for accurate quantitation of the major fatty acid species with respect to the nonadecanoic acid internal standard, and a second time with a 20:1 split ratio to permit quantitation of the minority species with respect to the heneicosanoic acid internal standards.
Quantification of Adipokines and Insulin
Enzyme-linked immunosorbent assays were used to quantify insulin in plasma and adipokines, leptin, adiponectin, PAI-1, MCP-1, and resistin, in both plasma and adipose tissue following manufacturers’ protocols. Ten samples were randomly chosen from each group for analysis, and samples were read within the linear range of the assay, and the accuracy of the analysis was confirmed by the controls provided in each kit.
Statistical Analyses
One-way analysis of variance (ANOVA) and Tukey’s contrasts were used to compare differences among the groups. All data are presented as means ± standard errors of the mean (SEM). Differences with a p value of 0.05 or less are considered significant. All statistical analyses were performed using SAS software (version 9.3, SAS Institute, Cary, NC).
Results
Body Weight, Body Composition and Caloric Intake
Feeding mice a high-fat diet, regardless of amounts of n3 PUFA, significantly increased body weight compared to mice fed the low-fat control diet (p < 0.05); the magnitude of increase was similar among all groups fed the high-fat diets (Fig. 1a–d).
Body weight changes in mice fed different high-fat diets. One-way ANOVA and Tukey’s contrasts were performed to test for differences among the groups. Values are means ± SEM (n = 12 per group). Mice fed the high-fat diets were heavier than those fed the low-fat control diet, and the difference was significant starting 5 weeks after the initiation of the experimental feeding (p ≤ 0.05). LF low-fat diet, HF high-fat diet
All high-fat diets significantly increased the percentage body fat mass (p < 0.05) and correspondingly reduced the percentage body lean mass (p < 0.05) compared to mice fed the low-fat diet; there were no significant differences in body fat mass among the high-fat diet-fed groups (Table 4). There were no significant differences in absolute lean mass weight among all dietary groups, except mice fed high-fat soybean oil diet which exhibited a small but significant increase compared to mice fed the low-fat diet (p < 0.05, Table 4). There were no significant differences in caloric intake among all groups regardless of diets (Table 4). Food intake of high-fat diet-fed groups were approximately 17–25 % lower than that of the low-fat control group (p < 0.05), there were no significant differences among groups fed the high-fat diets (Table 4).
Fatty Acid Concentrations of Epididymal Adipose Tissue
Fatty acid concentrations of epididymal adipose tissue (Table 5) reflected the fatty acid composition of the diets (Tables 2 and 3). Oleic acid contributed the highest concentrations of monounsaturated fatty acids (MUFA) to the adipose tissue. The highest concentrations of oleic acid were detected in mice fed the high-fat sunflower oil diet compared to all other groups (p < 0.05, Table 5). There were no differences in adipose oleic acid concentrations in any of the other dietary groups compared.
There were no significant differences in concentrations of saturated fatty acids (SFA) in adipose tissue between groups fed the low-fat and high-fat corn oil diets (Table 5). Palmitic acid contributed the highest concentration of SFA to adipose tissue, and the highest concentration of palmitic acid was detected in the high-fat fish oil group (Table 5). Compared to the high-fat fish oil diet, concentrations of palmitate were significantly lower in mice fed the corn oil and soybean oil diets by 37 and 49 % and flaxseed oil and sunflower oil diets each by 57 % (p < 0.05, Table 5). Similarly, stearic acid and lauric acid concentrations were significantly higher in mice fed the high-fat fish oil diet compared to all the other groups (Table 5).
Concentrations of n6 PUFA in adipose tissue were contributed largely by the high percentage of LNA in diets. Concentrations of LNA were significantly higher in mice fed the high-fat soybean oil diet compared to all other groups except the high-fat corn oil and flaxseed oil groups, and the lowest concentrations were observed in the sunflower oil group (p < 0.05, Table 5). Compared to the high-fat corn oil and soybean oil diets, LNA concentrations were 48 and 55 % lower respectively, in the fish oil group (p < 0.05) and 69 and 73 % lower respectively, in the sunflower oil group (p < 0.05, Table 5). Contributions of arachidonic acid and γ-linolenic acid to total n6 PUFA concentrations were small; however, mice fed the fish oil diet had the highest amounts of both compared to all other groups (p < 0.05, Table 5). Addition of corn oil to the fish oil and flaxseed oil diets contributed to concentrations of LNA observed in adipose tissue of mice fed those diets.
Concentrations of n3 PUFA in adipose tissue were largely contributed by ALA in all diets except the fish oil diet in which contributing fatty acids were EPA, docosapentaenoic acids (DPA; 22:5n3) and DHA (Table 5). There were no significant differences in adipose ALA concentrations among groups fed the low-fat corn oil, high-fat corn oil and high-fat fish oil diets (Table 5). Concentrations of ALA in adipose tissue were highest in mice fed the high-fat flaxseed oil diet, followed by mice fed the high-fat soybean oil or high-fat sunflower oil diet (Table 5). The significant elevation of ALA in mice fed the sunflower oil diet was due to the addition of flaxseed oil to the diet. Mice fed the fish oil diet had the highest concentrations of DHA, DPA and EPA in adipose tissue compared to all other groups (p < 0.05, Table 5). There were no significant differences in concentrations of DHA, DPA and EPA among all other dietary groups (Table 5).
Insulin and Adipokine Concentrations in Plasma and Adipose Tissue
Adipose-produced inflammatory cytokines participate in energy metabolism and contribute to obesity [3, 5] and obesity-related diseases [9, 30, 31]. To understand roles of high-fat diets containing different amounts of n3 PUFA in adipokine production, we quantified leptin, adiponectin, PAI-1, MCP-1 and resistin in plasma and adipose tissue in mice fed different diets.
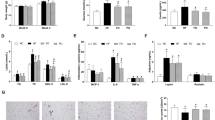
Compared to the low-fat corn oil diet, the high-fat corn oil diet increased concentrations of leptin in plasma by 2.1-fold (p < 0.05, Fig. 2a) and in adipose tissue by 2.5-fold (p < 0.05, Fig. 2b). The high-fat fish oil diet, in comparison to the high-fat corn oil diet, significantly reduced concentrations of leptin by 27 % in plasma (p < 0.05, Fig. 2a) but not in adipose tissue (Fig. 2b). The high-fat soybean oil diet reduced leptin in plasma by 23 % (p < 0.05, Fig. 3a) but not in adipose tissue (Fig. 2b).
Plasma and adipose concentrations of leptin (a, b) and adiponectin (c, d) of mice fed different high-fat diets. One-way ANOVA and Tukey’s contrasts were performed to test for differences among the groups. Values (mean ± SEM) with different letters are significantly different at p ≤ 0.05 (n = 10 per group).  ctl: low-fat corn oil control,
ctl: low-fat corn oil control,  corn: corn oil,
corn: corn oil,  fish: fish oil,
fish: fish oil,  flax: flaxseed oil,
flax: flaxseed oil,  soy: soybean oil,
soy: soybean oil,  sun: sunflower oil
sun: sunflower oil
Plasma and adipose concentrations of PAI-1 (a, b), MCP-1 (c, d) and resistin (e, f) of mice fed different high-fats diets. One-way ANOVA and Tukey’s contrasts were performed to test for differences among the groups. Values (mean ± SEM) with different letters are significantly different at p ≤ 0.05 (n = 10 per group).  ctl: low-fat corn oil control,
ctl: low-fat corn oil control,  corn: corn oil,
corn: corn oil,  fish: fish oil,
fish: fish oil,  flax: flaxseed oil,
flax: flaxseed oil,  soy: soybean oil,
soy: soybean oil,  sun: sunflower oil
sun: sunflower oil
The high-fat corn oil diet, in comparison to the low-fat corn oil diet, significantly reduced concentrations of adiponectin in plasma by 17 % (p < 0.05, Fig. 2c) but not in adipose tissue (Fig. 2d). Compared to the high-fat corn oil diet, the high-fat fish oil diet significantly increased concentrations of adiponectin in plasma by 43 % (p < 0.05, Fig. 2c) and in adipose tissue by two-fold (p < 0.05, Fig. 2d); the high-fat flaxseed oil diet significantly increased adiponectin concentrations in adipose tissue by 63 % (p < 0.05, Fig. 2d) but not in plasma (Fig. 2c).
All high-fat diets significantly increased concentrations of PAI-1 in plasma compared to the low-fat diet (Fig. 3a). In adipose tissue, there was no significant difference in PAI-1 between groups fed the low-fat and high-fat corn oil diets (Fig. 3b); the high-fat flaxseed oil diet increased PAI-1 concentration by 2.3-folds compared to the high-fat corn oil diet (p < 0.05, Fig. 3b).
Both the high-fat corn oil and fish oil diets elevated plasma MCP-1 concentrations by twofold compared to the low-fat corn oil diet (p < 0.05, Fig. 3c). There was no significant difference in adipose MCP-1 concentration between groups fed the low-fat and high-fat corn oil diets; the high-fat fish oil diet significantly elevated MCP-1 concentration by 85 % compared to the high-fat corn oil diet (p < 0.05, Fig. 3d).
The high-fat diets, regardless of types of oils, lowered plasma concentrations of resistin compared to the low-fat diet; the reduction by the high-fat corn oil diet (28 %) and flaxseed oil diet (34 %) were statistically significant (p < 0.05, Fig. 3e). In adipose tissue, all high-fat diets significantly reduced resistin concentrations compared to the low-fat diet (Fig. 3f).
Feeding mice a high-fat diet significantly increased plasma concentrations of insulin compared to the low-fat diet, except the high-fat fish oil diet maintained concentrations of insulin unchanged compared to the low-fat diet (Fig. 4).
Plasma concentrations of insulin from mice fed different high-fat diets. One-way ANOVA and Tukey’s contrasts were performed to test for differences among the groups. Values (mean ± SEM) with different letters are significantly different at p ≤ 0.05 (n = 10 per group).  ctl: low-fat corn oil control,
ctl: low-fat corn oil control,  corn: corn oil,
corn: corn oil,  fish: fish oil,
fish: fish oil,  flax: flaxseed oil,
flax: flaxseed oil,  soy: soybean oil,
soy: soybean oil,  sun: sunflower oil
sun: sunflower oil
Discussion
The present study investigated the effects of high-fat diets containing different amounts of n3 PUFA on adiposity and production of inflammatory cytokines in mice. Consumption of a high-fat diet, regardless of the type of oil, increased body fat mass, which was responsible for increases in body weights in mice. These results indicate that different types and amounts of n3 or n6 PUFA at dietary levels tested do not affect adipogenesis and that high-fat diet-induced adiposity is independent from dietary n3 and n6 PUFA.
It is well documented that leptin regulates satiety and energy intake [2] and that concentrations of leptin in blood increase in obesity and decline in weight loss [2]. On the other hand, adiponectin regulates lipid and glucose metabolism, increases insulin sensitivity and protects against chronic inflammation [37, 38]. We found that the high-fat fish oil diet significantly reduced leptin and increased adiponectin concentrations in both plasma and adipose tissue; furthermore it did not elevate insulin concentration in plasma. These results indicate that fish oil or fish oil components attenuate high-fat diet-induced metabolic disturbance and improve insulin sensitivity. Previous studies showed that replacing corn oil with fish oil elevated plasma adiponectin concentrations in sucrose-fed, insulin resistant rats [39]. Consumption of a high-fat diet enriched with EPA/DHA increased plasma adiponectin concentrations in C57BL/6 mice [20] and increased adiponectin mRNA expression and improved insulin tolerance in ob/ob mice [21]. Dietary supplementation with EPA/DHA negatively correlated to plasma leptin concentrations in men, and intake of a diet containing long-chain n3 PUFA reduced leptin concentrations in plasma and leptin mRNA expression in adipose tissue in rats [17]. In vitro studies showed that EPA and DHA inhibited insulin–stimulated leptin secretion in isolated white adipocytes [40] and leptin mRNA expression in trophoblast cells [17]. Our results agree with these reports that fish oil improves insulin sensitivity by positively regulating adipokine profiles and support that n3 PUFA may be responsible for such beneficial effects. Our results of significant increases in adipose concentrations of adiponectin in the flaxseed oil diet-fed mice further support this concept. However, we do not agree with the reports suggesting that such improvements by fish oil were associated with its reduction in adipose mass [41–43], since we did not observe reductions in body fat mass in the fish oil diet-fed mice in the present study.
Elevation of PAI-1 and MCP-1 are observed in obese humans [7, 44] and laboratory rodents fed a high-fat diet [9, 45]. In the present study, feeding mice high-fat diets containing different amounts of n3 PUFA did not result in reduction in either PAI-1 or MCP-1. Changes in fatty acid composition and contents in adipose tissue corresponded to dietary fatty acid profiles. These changes indicate that n3 PUFA at levels tested in the present study do not downregulate pro-inflammatory cytokines PAI-1 and MCP-1 or they are insufficient at the levels tested for such a downregulation. Furthermore, the lack of reductions in plasma and adipose PAI-1 and MCP-1 concentrations in mice fed the fish oil diet indicates that mechanisms regulating PAI-1 and MCP-1 may be different from those regulating adiponectin and leptin in these mice. It is important to note that the production of inflammatory cytokines, e.g. PAI-1 and MCP-1, can be up-regulated by inflammation. For example, concentrations of PAI-1 in mouse liver are relatively low [46]; however, its gene expression in liver is up-regulated by inflammatory mediators, e.g. endotoxin [46, 47]. Adipose produced PAI-1 and MCP-1 contributed to their concentrations in plasma. However, systemic inflammation related to increases in body fat mass in high-fat diet-fed groups may up-regulate production of PAI-1 and MCP-1 in other organs, e.g. liver, which may further increase plasma concentrations of these inflammatory cytokines.
Resistin, a pro-inflammatory adipokine, is associated with insulin resistance and inflammation in obesity [48–50]. Serum concentrations of resistin are elevated in human obese subjects [8] and in laboratory rodents of diet-induced and genetic forms of obesity [10]. The findings that concentrations of resistin were lower in both plasma and adipose tissue of high-fat diet-fed groups than in low-fat diet-fed controls do not support those observations. However, our results are in agreement with the reports that obesity reduces resistin levels. Feeding mice a high-fat fish oil diet reduced serum concentrations of resistin and leptin and improved the obesity phenotype [51]. Resistin expression in adipose tissue is severely depressed in genetic obese models including ob/ob, db/db and tub/tub mice [50]. While reasons for the different results from available studies on obesity and resistin remain to be elucidated, our results indicate that the mode of action for reductions in resistin across all groups fed high-fat diets may be independent from n3 and n6 PUFA at the dietary levels tested.
Results of adipose fatty acid analyses are consistent with the existing knowledge that fatty acids in adipose tissue correlate with types of fatty acids consumed from a diet [52, 53]. Adipose MUFA, SFA and n6 PUFA were contributed mainly by dietary oleate, palmitate, and linoleate, respectively. Similarly, adipose n3 PUFA were contributed mainly by dietary ALA and by EPA, DHA and DPA in the fish oil diet-fed mice. The amounts of LNA in adipose tissue of mice fed the flaxseed oil and fish oil diets were largely due to the addition of corn oil to the diets to adjust the amounts of n6 PUFA. Similarly, concentrations of ALA in adipose tissue of mice fed the sunflower oil diet were due to the addition of flaxseed oil to the diet to adjust the amounts of n3 PUFA.
In summary, consumption of high-fat diets significantly increased body fat mass and body weights. Adipose fatty acid composition and contents reflected dietary fatty acid profiles. Fish oil had favorable effects on downregulation of leptin and upregulation of adiponectin, which may be responsible for its improvement in insulin sensitivity compared to other types of oils. The lack of reductions in pro-inflammatory cytokines, PAI-1 and MCP-1, in groups fed the high-fat diets containing different amounts of n3 and n6 PUFA, in comparison to mice fed the high-fat corn oil diet containing lower amounts of n3 PUFA, suggests that n3 or n6 PUFA do not play a role in downregulation of PAI-1 and MCP-1 or they are insufficient at the dietary levels tested for such a downregulation. Together with the finding of lower concentrations of resistin in all groups fed the high-fat diets, we conclude that the actions of the high-fat diets on adiposity and regulation of inflammatory cytokine production are independent from dietary contents of n3 PUFA and n6 PUFA.
Abbreviations
- ALA :
-
α-Linolenic acid 18:3n3
- DHA :
-
Docosahexaenoic acid 22:6n3
- DPA :
-
Docosapentaenoic acid 22:5n3
- ELISA:
-
Enzyme-linked immunosorbent assay
- EPA :
-
Eicosapentaenoic acid 20:5n3
- LNA :
-
Linoleic acid 18:2n6
- MCP-1:
-
Monocyte chemoattractant protein-1
- MUFA:
-
Monounsaturated fatty acids
- PAI-1:
-
Plasminogen activator inhibitor-1
- PUFA:
-
Polyunsaturated fatty acids
- SFA:
-
Saturated fatty acids
References
FastStats (2014) Obesity and Overweights. CDC; http://www.cdc.gov/nchs/fastats/obesity-overweight.htm
Williams KW, Scott MM, Elmquist JK (2009) From observation to experimentation: leptin action in the mediobasal hypothalamus. Am J Clin Nutr 89:985S–990S
Staiger H, Tschritter O, Machann J, Thamer C, Fritsche A, Maerker E, Schick F, Haring HU, Stumvoll M (2003) Relationship of serum adiponectin and leptin concentrations with body fat distribution in humans. Obes Res 11:368–372
Diez JJ, Iglesias P (2003) The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol 148:293–300
Tajtakova M, Petrasova D, Petrovicova J, Pytliak M, Semanova Z (2006) Adiponectin as a biomarker of clinical manifestation of metabolic syndrome. Endocr Regul 40:15–19
Eriksson P, Reynisdottir S, Lonnqvist F, Stemme V, Hamsten A, Arner P (1998) Adipose tissue secretion of plasminogen activator inhibitor-1 in non-obese and obese individuals. Diabetologia 41:65–71
Christiansen T, Richelsen B, Bruun JM (2005) Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes 29:146–150
Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, Zhu Q, Considine RV (2003) Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab 88:5452–5455
Yan L, Graef GL, Claycombe KJ, Johnson LK (2013) Effects of voluntary running and soy supplementation on diet-induced metabolic disturbances and inflammation in mice. J Agric Food Chem 61:9373–9379
Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA (2001) The hormone resistin links obesity to diabetes. Nature 409:307–312
Monzillo LU, Hamdy O, Horton ES, Ledbury S, Mullooly C, Jarema C, Porter S, Ovalle K, Moussa A, Mantzoros CS (2003) Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res 11:1048–1054
Lopez HL, Ziegenfuss TN, Hofheins JE, Habowski SM, Arent SM, Weir JP, Ferrando AA (2013) Eight weeks of supplementation with a multi-ingredient weight loss product enhances body composition, reduces hip and waist girth, and increases energy levels in overweight men and women. J Int Soc Sports Nutr 10:22
Siklova-Vitkova M, Klimcakova E, Polak J, Kovacova Z, Tencerova M, Rossmeislova L, Bajzova M, Langin D, Stich V (2012) Adipose tissue secretion and expression of adipocyte-produced and stromavascular fraction-produced adipokines vary during multiple phases of weight-reducing dietary intervention in obese women. J Clin Endocrinol Metab 97:E1176–E1181
Yan L, DeMars LC, Johnson LK (2012) Long-term voluntary running improves diet-induced adiposity in young adult mice. Nutr Res 32:458–465
Wang HT, Liu CF, Tsai TH, Chen YL, Chang HW, Tsai CY, Leu S, Zhen YY, Chai HT, Chung SY, Chua S, Yen CH, Yip HK (2012) Effect of obesity reduction on preservation of heart function and attenuation of left ventricular remodeling, oxidative stress and inflammation in obese mice. J Transl Med 10:145
Soares FL, de Oliveira Matoso R, Teixeira LG, Menezes Z, Pereira SS, Alves AC, Batista NV, de Faria AM, Cara DC, Ferreira AV, Alvarez-Leite JI (2013) Gluten-free diet reduces adiposity, inflammation and insulin resistance associated with the induction of PPAR-alpha and PPAR-gamma expression. J Nutr Biochem 24:1105–1111
Reseland JE, Haugen F, Hollung K, Solvoll K, Halvorsen B, Brude IR, Nenseter MS, Christiansen EN, Drevon CA (2001) Reduction of leptin gene expression by dietary polyunsaturated fatty acids. J Lipid Res 42:743–750
Kalupahana NS, Voy BH, Saxton AM, Moustaid-Moussa N (2011) Energy-restricted high-fat diets only partially improve markers of systemic and adipose tissue inflammation. Obesity 19:245–254
Sekine S, Sasanuki S, Murano Y, Aoyama T, Takeuchi H (2008) Alpha-linolenic acid-rich flaxseed oil ingestion increases plasma adiponectin level in rats. Int J Vitam Nutr Res 78:223–229
Flachs P, Mohamed-Ali V, Horakova O, Rossmeisl M, Hosseinzadeh-Attar MJ, Hensler M, Ruzickova J, Kopecky J (2006) Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia 49:394–397
Gonzalez-Periz A, Horrillo R, Ferre N, Gronert K, Dong B, Moran-Salvador E, Titos E, Martinez-Clemente M, Lopez-Parra M, Arroyo V, Claria J (2009) Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J 23:1946–1957
Lozano A, Perez-Martinez P, Marin C, Tinahones FJ, Delgado-Lista J, Cruz-Teno C, Gomez-Luna P, Rodriguez-Cantalejo F, Perez-Jimenez F, Lopez-Miranda J (2013) An acute intake of a walnut-enriched meal improves postprandial adiponectin response in healthy young adults. Nutr Res 33:1012–1018
Kalupahana NS, Claycombe K, Newman SJ, Stewart T, Siriwardhana N, Matthan N, Lichtenstein AH, Moustaid-Moussa N (2010) Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr 140:1915–1922
Baranowski M, Enns J, Blewett H, Yakandawala U, Zahradka P, Taylor CG (2012) Dietary flaxseed oil reduces adipocyte size, adipose monocyte chemoattractant protein-1 levels and T-cell infiltration in obese, insulin-resistant rats. Cytokine 59:382–391
Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, Yuan X, Tanaka M, Kawano H, Yano T, Aoe S, Takeya M, Shimatsu A, Kuzuya H, Kamei Y, Ogawa Y (2007) Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol 27:1918–1925
Bradley RL, Fisher FF, Maratos-Flier E (2008) Dietary fatty acids differentially regulate production of TNF-alpha and IL-10 by murine 3T3-L1 adipocytes. Obesity 16:938–944
Poudyal H, Panchal SK, Waanders J, Ward L, Brown L (2012) Lipid redistribution by alpha-linolenic acid-rich chia seed inhibits stearoyl-CoA desaturase-1 and induces cardiac and hepatic protection in diet-induced obese rats. J Nutr Biochem 23:153–162
Fay MP, Freedman LS, Clifford CK, Midthune DN (1997) Effect of different types and amounts of fat on the development of mammary tumors in rodents: a review. Cancer Res 57:3979–3988
Rose DP (1997) Dietary fatty acids and cancer. Am J Clin Nutr 66:998S–1003S
Yan L, Combs GFJ (2014) Consumption of a high-fat diet abrogates inhibitory effects of methylseleninic acid on spontaneous metastasis of Lewis lung carcinoma in mice. Carcinogenesis 35:2308–2313
Yan L, DeMars LC (2014) Effects of a high-fat diet on spontaneous metastasis of Lewis lung carcinoma in plasminogen activator inhibitor-1 deficient and wild-type mice. PLoS ONE 9:e110869
Mason JK, Chen J, Thompson LU (2010) Flaxseed oil-trastuzumab interaction in breast cancer. Food Chem Toxicol 48:2223–2226
Reeves PG, Nielsen FH, Fahey GCJ (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Institute for Laboratory Animal Research (2011) Guide for the care and use of laboratory animals. National Academies Press, Washington, DC
Lepage G, Roy CC (1986) Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27:114–120
Masood A, Stark KD, Salem NJ (2005) A simplified and efficient method for the analysis of fatty acid methyl esters suitable for large clinical studies. J Lipid Res 46:2299–2305
Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE (2004) Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279:12152–12162
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941–946
Rossi AS, Lombardo YB, Lacorte JM, Chicco AG, Rouault C, Slama G, Rizkalla SW (2005) Dietary fish oil positively regulates plasma leptin and adiponectin levels in sucrose-fed, insulin-resistant rats. Am J Physiol Regul Integr Comp Physiol 289:R486–R494
Cammisotto PG, Gelinas Y, Deshaies Y, Bukowiecki LJ (2003) Regulation of leptin secretion from white adipocytes by free fatty acids. Am J Physiol Endocrinol Metab 285:E521–E526
Couet C, Delarue J, Ritz P, Antoine JM, Lamisse F (1997) Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int J Obes Relat Metab Disord 21:637–643
Raclot T, Groscolas R, Langin D, Ferre P (1997) Site-specific regulation of gene expression by n-3 polyunsaturated fatty acids in rat white adipose tissues. J Lipid Res 38:1963–1972
Belzung F, Raclot T, Groscolas R (1993) Fish oil n-3 fatty acids selectively limit the hypertrophy of abdominal fat depots in growing rats fed high-fat diets. Am J Physiol 264:R1111–R1118
Alessi MC, Bastelica D, Morange P, Berthet B, Leduc I, Verdier M, Geel O, Juhan-Vague I (2000) Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes 49:1374–1380
Sartipy P, Loskutoff DJ (2003) Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA 100:7265–7270
Sawdey MS, Loskutoff DJ (1991) Regulation of murine type 1 plasminogen activator inhibitor gene expression in vivo. Tissue specificity and induction by lipopolysaccharide, tumor necrosis factor-alpha, and transforming growth factor-beta. J Clin Invest 88:1346–1353
Quax PH, van den Hoogen CM, Verheijen JH, Padro T, Zeheb R, Gelehrter TD, van Berkel TJ, Kuiper J, Emeis JJ (1990) Endotoxin induction of plasminogen activator and plasminogen activator inhibitor type 1 mRNA in rat tissues in vivo. J Biol Chem 265:15560–15563
Catta-Preta M, Martins MA, Cunha Brunini TM, Mendes-Ribeiro AC, Mandarim-de-Lacerda CA, Aguila MB (2012) Modulation of cytokines, resistin, and distribution of adipose tissue in C57BL/6 mice by different high-fat diets. Nutrition 28:212–219
Ribot J, Rodriguez AM, Rodriguez E, Palou A (2008) Adiponectin and resistin response in the onset of obesity in male and female rats. Obesity 16:723–730
Way JM, Gorgun CZ, Tong Q, Uysal KT, Brown KK, Harrington WW, Oliver WR Jr, Willson TM, Kliewer SA, Hotamisligil GS (2001) Adipose tissue resistin expression is severely suppressed in obesity and stimulated by peroxisome proliferator-activated receptor gamma agonists. J Biol Chem 276:25651–25653
Monk JM, Hou TY, Turk HF, Weeks B, Wu C, McMurray DN, Chapkin RS (2012) Dietary n-3 polyunsaturated fatty acids (PUFA) decrease obesity-associated Th17 cell-mediated inflammation during colitis. PLoS ONE 7:e49739
Hodson L, Skeaff CM, Fielding BA (2008) Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 47:348–380
Abbott SK, Else PL, Atkins TA, Hulbert AJ (2012) Fatty acid composition of membrane bilayers: importance of diet polyunsaturated fat balance. Biochim Biophys Acta 1818:1309–1317
Acknowledgments
The authors gratefully acknowledge the assistance of the following staff of the Grand Forks Human Nutrition Research Center: Lana DeMars and Kay Keehr for technical support, James Lindlauf for preparing experimental diets and vivarium staff for providing high quality animal care. This work was supported by the U.S. Department of Agriculture, ARS, research project 5450-51000-050-00D.
The U.S. Department of Agriculture, ARS, Plains Area, is an equal opportunity/affirmative action employer and all agency services are available without discrimination. Mention of trade names or commercial products in this article is solely for providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
About this article
Cite this article
Sundaram, S., Bukowski, M.R., Lie, WR. et al. High-Fat Diets Containing Different Amounts of n3 and n6 Polyunsaturated Fatty Acids Modulate Inflammatory Cytokine Production in Mice. Lipids 51, 571–582 (2016). https://doi.org/10.1007/s11745-015-4093-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-015-4093-x