Abstract
A novel anionic gemini surfactant containing an ester bond in the spacer group was synthesized using cardanol as the raw material and characterized by IR, 1H NMR and 13C NMR. The surface properties of the gemini surfactant were investigated and compared with its corresponding single chain surfactant counterpart. It was found that this novel gemini surfactant exhibited a low critical micelle concentration value (1.9 mM) and good efficiency in reducing surface tension of water (33.6 mN/m). The gemini surfactant was found to have antimicrobial activity against Gram-negative (Escherichia coli and Pseudomonas aeruginosa), Gram-positive (Bacillus subtilis and Staphylococcus aureus) bacteria and fungi (Aspergillus niger, Aspergillus flavus, Candida albicans and Rhizopus stolonifer). The gemini as well as the corresponding single chain surfactant showed good antimicrobial activity against all pathogenic microorganisms studied and can be employed as an antimicrobial agent. The synthesized novel anionic gemini surfactant possesses an excellent wettability and low foamability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gemini surfactants (dimer) are a new class of surfactants composed of two monomeric surfactant molecules chemically bonded together by a spacer at or near their head groups. The spacer can be hydrophobic or hydrophilic, flexible (alkyl or ether group) or rigid (aromatic moieties) in nature [1, 2]. Compared with conventional surfactants, gemini surfactants have much lower critical micelle concentrations (CMC), greater efficiency in decreasing surface tension and excellent wetting properties [3, 4]. These surfactants often possess better foaming, aggregation and rheological properties at low concentration. Geminis are known to have numerous applications such as solubilizing agents, antifoaming agents, emulsifiers, bactericidal agents, dispersants, detergents, etc. [5, 6]. Gemini surfactants invite attention for cosmetics and toiletries especially in shampoo and personal care products, because of their mildness, soft feeling and low skin irritation [7]. Gemini Surfactants are markedly more surface-active than their comparable conventional surfactants. Their outstanding physico-chemical properties and widespread applications have attracted chemists in search of new surfactants.
Gemini surfactants with enhanced wetting properties play an important role in personal care products, textiles, oil industry, paper industry, pesticide or herbicide formulations, paints and coatings. Anionic surfactants are often used as wetting agents in commercial applications [8, 9]. Amphiphilic compounds having dimeric moieties are well-known and effective antimicrobial agents [4].
Owing to environmental concern, it is essential to design biocompatible surfactants containing biodegradable functionalities such as ester and amide groups that can be easily degraded after use. Several investigations have recommended that surfactants containing ester bonds show good biodegradability as the polar bond contributes to the high water solubility. Therefore, readily cleavable gemini surfactants are more suitable for use compared to conventional surfactants. Although many chemists have reported studies of gemini or cleavable surfactants, only limited information is available regarding cleavable gemini surfactants [10,11,12,13,14].
Cashew nut shell liquid (CNSL) is a reddish brown colored, viscous, poisonous liquid obtained as a by-product from the extraction of cashew nut shells. Cardanol and anacardic acid are the major phenolic constituents present in natural CNSL (Anacardium occidentale species). Cardanol is a low cost, eco-friendly and biodegradable renewable biomass resource. It is a mixture of four different meta-alkyl phenols differing in the degree of unsaturation in the side chain: 5% saturated [3-(pentadecyl)-phenol], 49% monoene [3-(8Z-pentadecenyl)-phenol], 17% diene [3-(8Z,11Z-pentadecadienyl)-phenol] and 29% triene [3-(8Z,11Z,14-pentadecatrienyl)-phenol]. It has both the characteristics of phenolic compounds and flexibility of aliphatic compounds and is widely used as raw material in polymer, adhesives, coatings and composite manufacturing industries [15,16,17]. Recently, many surfactants with high surface activity have been synthesized using cardanol. There are numerous reports published on monomeric surfactants prepared from cardanol, however, there are few reports on cardanol-based gemini surfactants [18]. This is the first report on an ester-linked anionic gemini surfactant with cardanol as starting material.
In the present study, a cardanol-based novel anionic gemini surfactant containing an ester linkage in the spacer group has been synthesized (Fig. 2) and its structure confirmed by TLC, IR, 1H NMR and 13C NMR. The surface properties such as critical micelle concentration (CMC), surface tension, foam power and wettability were investigated. Also, the antimicrobial activity of newly synthesized anionic gemini surfactant against pathogenic bacteria and fungi are reported. The values obtained were compared with its corresponding monomeric surfactant.
Experimental Section
Materials and Methods
Cardanol (3-pentadecyl phenol) was obtained from the South Asia Cashew Corporation, Goa, India and vacuum distilled (at 225 ± 4 °C, 0.005 mm Hg) before use. Adipic acid, chlorosulfuric acid, Sudan IV (M. S) dye, sulfuric acid, sodium hydroxide and sodium carbonate were of analytical reagent grade, purchased from S. D. Fine Chemicals Limited, India. All the solvents were of analytical reagent grade and used without further purification. Distilled water (conductance = 0.005 mS/cm) was employed in all experiments. Deuterated solvents such as CDCl3, D2O and DMSO-d6 were purchased from Sigma Aldrich, India. A UV–Visible spectrophotometer (Agilent technologies Model-8453, India) was used for measurement of dye absorption spectra.
The structure of synthesized cardanol sulfonate monomer and novel ester-linked gemini surfactant were confirmed by using IR spectrometry (Bruker FTIR spectrometer), 1H NMR and 13C NMR (Agilent Technologies, 500 MHz). The two amphiphiles synthesized were designated as CSMS and CSGS.
Synthesis of Cardanol Sulfonate Monomeric Surfactant (CSMS)
The schematic route for synthesis of amphiphile CSMS is shown in Fig. 1. Cardanol (12.4 mmol in 25 ml dichloromethane) was charged to a three-necked round-bottom reaction kettle equipped with a water condenser and the solution was cooled in an ice bath to 0–5 °C. To this solution, chlorosulfuric acid (42.9 mmol) was added dropwise over a period of 1 h. The reaction mass was stirred for 3 h at room temperature [19]. The dichloromethane was separated from the reaction mass and the product was neutralized with ice cold 20% NaOH to pH = 8. The product obtained was washed with excess hexane and extracted with methanol, dried over anhydrous sodium sulfate and the solvent was evaporated using a rotary evaporator. The yield of the isolated product was 87%.
Synthesis of Novel Ester-linked Cardanol Sulfonate Gemini Surfactant (CSGS)
The synthesis of the cardanol sulfonate gemini surfactant containing ester linkage in spacer group was carried out using a two-step procedure and the reaction steps used are summarized in Fig. 2.
In a typical experiment, a three-necked round-bottom reaction kettle fitted with a mechanical stirrer, Dean-Stark trap with water condenser and dropping funnel was charged with 20 mmol adipic acid and 40 mmol of concentrated sulfuric acid in toluene. The reaction mixture was refluxed for 10 min at 150–160 °C until the complete dissolution of the adipic acid. The reaction mass was cooled to room temperature before addition of cardanol (40 mmol), dissolved in 25 ml toluene. The reaction mass was refluxed at 110–115 °C for 6 h till the complete removal of water. The product was washed with excess hexane to remove unreacted cardanol and extracted with ethyl acetate, dried over anhydrous sodium sulfate and the solvent was evaporated using a rotary evaporator. The isolated product was further purified with a silica gel column and elution with hexane/ethyl acetate. The yield of the isolated product was 78%.
The cardanol gemini ester (4.2 mmol in 15 ml dichloromethane) prepared in the first step was charged into a three-necked round-bottom reaction kettle and stirred at 0–5 °C for 10 min. To this solution, chlorosulfuric acid (25.2 mmol) was added dropwise over 30 min. The reaction mass was stirred for 3 h at room temperature. The dichloromethane containing an excess of unreacted sulfuric acid was separated from the reaction mass. The product obtained was neutralized with ice cold 10% Na2CO3 to pH = 8. The neutralization reaction was carried out for an hour at room temperature. The product obtained was washed with excess hexane and extracted with methanol. The organic layer was concentrated using a rotary evaporator. The yield of the isolated product was 80%.
Chemical Analysis
Cardanol—pale lemon color, liquid, FTIR—wavelength (cm−1) = 3341.9 (O–H, phenolic compound), 3006.29 (=C–H, aromatic ring), 2922.9 and 2852.88 (–C–H, aliphatic chain), 1588.69 (C=C, long aliphatic chain), 1455.32 (C=C, aromatic), 1263.1−1072.82 (C–O, phenol). GC–MS (m/z, relative intensity %) = 302.28 (M, 18), 206.18 (4), 161.11 (4), 133.09 (12), 120 (44), 108 (100), 91 (20), 77 (46), 55 (54). 1H NMR (500 MHz, CDCl3, TMS) δ ppm = 6.65 (2H, d, Ar-Hc,g), 6.76 (1H, Ar–He), 7.13(1H, t, Ar–Hf), 5.28 (1H, s, Ar-OHa), 4.89–5.42 (4H, m, side chain HC=CH), 0.90–2.79 (27H, alkyl side chain proton). 13C NMR (500 MHz, CDCl3, TMS) δ ppm = 155.4 (ArC-OH), 144.9 (ArC-alkyl chain), 112.45–130.22 (C=C, Ar and aliphatic chain), 13.80–35.8 (aliphatic chain).
CSMS—yellow color, solid, hygroscopic, FTIR- wavelength (cm−1) = 3594.52, 3531.95 (O–H, phenolic compound), 3091.47 (=C–H, aromatic ring), 2927.92 and 2859.94 (–C–H, aliphatic chain), 1679.72−1584.56 (C=C), 1425 and 1182.37 (S=O, sulfonate), 1049.39 (C–O, phenol), 693.79 (C–S stretching). 1H NMR (500 MHz, DMSO-d6, TMS) δ ppm = 8.10 (1H, s, Ar-OHa), 7.94 (1H, s, Ar–Hf), 7.38–7.47 (2H, d, Ar-Hc,e), 6.46–6.58 (2H, d, side chain), 0.74–1.92 (27H, aliphatic chain).
Cardanol gemini ester—brown-black color, liquid, FTIR- wavelength (cm−1) = 2925.43 and 2854.34 (–C–H, aliphatic chain), 1614.27 − 1573.43 (C=C), 1734.44 (C=O, ester spacer group), 1015.23–1239.08 (C–O, ester). 1H NMR (500 MHz, DMSO-d6, TMS) δ ppm = 7.02–7.92 (8H, Ar-Hc,e,f,g), 6.53 (4H, aliphatic chain), 0.82–3.4 (62H). 13C NMR (500 MHz, DMSO-d6, TMS) δ ppm = 172.78 (2C, C=O, ester), 150.76–153.48 (2C, ArC–O), 145.99–146.21 (2C, ArC–alkyl chain) 115.62–138.92 (C=C, Ar and aliphatic chain), 14.22–29.55 (aliphatic chain)
CSGS—brown color, solid, FTIR—wavelength (cm−1) = 2924.39 and 2856.88 (–C–H, aliphatic chain), 1637.34−1588.35 (C=C), 1764.98 (C=O, ester spacer group) 1435.39 and 1140.47 (S=O, sulfonate), 1049.39 (C–O, phenol), 619.30 (C–S stretching). 1H NMR (500 MHz, D2O, TMS) δ ppm = 8.28–8.29 (2H, Ar–Hf), 7.88 (4H, Ar-Hc,e), 6.40 (4H, aliphatic chain), 0.71–3.58 (62H) [20, 21].
Critical Micelle Concentration Determination
The critical micelle concentration (CMC) was estimated by tensiometry, conductometry and dye solubilization method. The surface tension experiments were performed by a Wilhelmy plate method using a Krüss K11 tensiometer at room temperature (303 ± 1 K). The accuracy of the measurement was within ±0.2 mN/m. The platinum plate used for the measurement was cleaned with acetone, washed thoroughly with distilled water and then heated using a piezo gas burner before use. The instrument was calibrated using distilled water having a surface tension value of 71 ± 1.0 mN/m. Each surface tension value was a mean average of three readings at an interval of 30 s. All the experiments were run in triplicate with an accuracy of ±0.3 mN/m.
The conductivity of aqueous solutions of surfactants was measured using a Labtronics digital conductivity meter (Model LT 23, India). The instrument was standardized using 0.01 M potassium chloride having conductance 1.4 mS/cm.
The dye solubilization was studied using water insoluble Sudan IV dye. The ultraviolet, visible absorption spectra were recorded on a single beam UV–Visible spectrophotometer. The Sudan IV dye (λ max = 514 nm) shows a shift in the maximum wavelength (λ max = 531 nm) due to the presence of micelles. The typical procedure is to add an excess of powdered dye to aqueous surfactant solutions, stir the suspension until equilibrium, separate the non-solubilized dye by filtration or centrifugation and record the absorbance. Plot of surfactant concentration versus the absorbance at the maximum wavelength (Fig. 4) was used to estimate the CMC values of synthesized CSMS and CSGS.
Wettability
The contact angle measurements were carried out by a static sessile drop method using a Krüss G-10 goniometer with an accuracy of ±2°. The instrument consists of a horizontal stage to mount a solid or liquid sample, a micrometer pipette to form a liquid drop, an illumination source and a telescope equipped with a protractor eyepiece. The contact angle measurements were performed by simply aligning a tangent of the sessile drop with the solid surface at the contact point and the reading was taken with a protractor eyepiece. Wettability of the aqueous CSGS and CSMS solutions (2 mM) on hydrophilic/hydrophobic surfaces such as glass, Teflon and stainless steel was studied.
Evaluation of Antimicrobial Activity
The antimicrobial efficacy of newly synthesized ester-linked gemini surfactant and its corresponding monomeric surfactant was determined by using the Kirby-Bauer disc-diffusion method as per NCCLS guidelines (National Committee for Clinical Laboratory Standards) [22]. The CSMS, CSGS and CTAB were tested against bacterial strains such as Staphylococcus aureus (S. aureus, ATCC 6538), Escherichia coli (E. coli, ATCC 10536), Pseudomonas aeruginosa (P. aeruginosa, ATCC 9027) and Bacillus subtilis (B. subtilis, ATCC 6633). The fungal strains used were Aspergillus niger (A. niger, ATCC 6275), Candida albicans (C. albicans, ATCC 14037), Aspergillus flavus (A. flavus, ATCC 9643) and Rhizopus stolonifer (ATCC 10231). CTAB was used as a conventional reference antibacterial agent. Amphotericin B discs (AP 20 mcg/disc) were used for antimicrobial susceptibility testing of fungal culture.
The CSMS, CSGS and CTAB (test samples) were in distilled water (100 mg in 500 μL) and thoroughly homogenized in Vortex mixer. The test microbes of approximately 107–108 CFU/ml were individually spread over the face of Trypticase soy agar (TSA) by a sterile cotton swab. Then 30 μL test samples of above concentration were smeared on the sterile disc. The disc containing test preparation was placed on seeded plates. The plates were then incubated at 37 °C for 24 h. The antimicrobial activity was evaluated by measuring the diameter of the growth inhibition zone formed around each disc. After the incubation period, the zones of inhibition (mm) were measured by a calibrated ruler.
Foamability Study
The foaming properties of synthesized CSMS and CSGS surfactants (2 mM) were determined by the standard Ross-Miles pour foam test method (ASTM designation D-1173-53). In this method, 50 ml surfactant solution was taken in the foam receiver provided with a water jacket and the solution height was adjusted with a stopcock. The foam pipette was filled with 200 ml of same surfactant solution. The pipette solution was allowed to fall into the foam receiver from a height of 90 cm. The foam height in the foam receiver was measured immediately after the last drop of the solution fell from the pipette. The temperature (303 ± 1 K) was maintained with the help of a water jacket. The foamability was measured in terms of the height (mm) of the foam produced initially and the foam stability was measured in terms of the height (mm) after 5 min [16].
Foamability was also tested using a horizontal impinging jet apparatus [23]. In this method, surfactant solution with a constant flow rate is made to impinge horizontally on a specially designed flat surface covered with a thin layer of the same solution, which generates polydispersed foam. The fine bubbles get carried towards the bottom of the column, whereas larger ones drift to the top and intermediates accumulate in between. The fine foam is collected in the collector arm where the volume is noted. The rate of fine foam generation is taken as a measure of foamability. All the experiments were triplicated.
Results and Discussion
Surface Tension and CMC Measurements
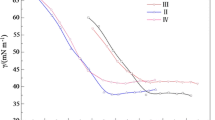
The CMC value was estimated by fitting the surface tension curve at a premicellar concentration with the Szyszkowski Eq. (1) and at postmicellar concentration with linear graph. Figure 3 shows the graph of surface tension against concentration.
where \(\gamma_{0}\) is the surface tension of the pure water and π is the surface pressure, \(\varGamma_{\hbox{max} }\) is the maximum surface excess concentration of surfactant, c is the concentration of the surfactant solution, R is the universal gas constant, T is the absolute temperature, K is the adsorption constant and n is the number of dissociating ionic species whose concentration at the interface varies with the change in the surfactant concentration in the solution [24]. In case of gemini surfactant having a divalent surfactant ion and two univalent counter ions, the value of n employed is 3.
The efficiency (pC20) of the synthesized anionic gemini surfactant was also estimated. The pC20 value is the negative logarithm of surfactant concentration (C20), required to reduce the surface tension by 20 mN/m. \(\frac{\text{CMC}}{{C_{20} }}\) compares micellization and adsorption phenomena [4]. The minimum surface area per molecule (A min) for synthesized surfactants was calculated using Eq. (2).
where \(N_{A}\) is Avogadro’s number. CMC values, surface tension at CMC (γCMC), C 20, \(\varGamma_{ \hbox{max} }\) and A min are summarized in Table 1. It is expected that the gemini surfactants have a lower CMC than their corresponding monomeric surfactants. The CMC of the pure CSMS and CSGS from surface tension plot was obtained as 8.1 and 1.9 mM respectively. The lower C 20 value for CSMS and CSGS indicated that cardanol based surfactants are efficient in reducing surface tension. Higher \(\frac{\text{CMC}}{{C_{20} }}\) ratio obtained for CSMS (28.9) and CSGS (43.2) indicates that adsorption is facilitated more than micellization. It also indicates the presence of a larger hydrophilic head group area [4].
Conductivity measurements were carried out for CSMS and CSGS to calculate the CMC and the plot is shown in Fig. 3. The CMC value was obtained from the intersection point of two straight lines. These values are in good agreement with the CMC values obtained from the surface tension method. The slope in the premicellar region (s 1 ) is greater than that in the postmicellar region (s 2 ) and the ratio \(\frac{{s_{2} }}{{s_{1} }}\) gives the counterion dissociation constant, \(\alpha_{\text{cb}}\) and counterion binding coefficient, \(\beta_{\text{cb}}\) (\(1 - \alpha_{\text{cb}}\)) [25]. The degree of binding (\(\beta_{\text{cb}}\)) is related to the surface area per head group in the ionic micelle. The lower the value of \(\beta_{\text{cb}}\), higher would be the surface head group area [4]. CSMS and CSGS have low \(\beta_{\text{cb}}\) which indicates a larger surface head group area as mentioned in Table 1.
The dye solubilization is one of the methods used for determination of the CMC. Plot of absorbance of the solubilized dye (λ max = 531 nm) as a function of surfactant concentration is shown in Fig. 4. The inflection point in λ max is taken as the CMC [26]. The dye solubilization is related to the structure of both surfactant and dyes. The CMC values obtained from dye micellization are lower than the CMC values obtained from tensiometry and conductometry (Table 1). It was observed that CSMS and CSGS have higher dye solubilization power. The reason for the good performance of these surfactants is that they allow dyes to be assimilated not only in the inner hydrocarbon part of the micelle but also in the head group shell which resulted in a lower CMC value [27].
Wetting Power
Contact angle measurements are commonly used to evaluate the degree of spreading/wettability of a liquid on a surface [28]. Wetting agents that lower the surface tension of the liquid, allow easier spreading or penetration into the solid if it is porous [29]. Figure 5 shows that a small contact angle (θ) is noticed when the liquid spreads on the surface, while a large contact angle is noticed when the liquid beads on the surface. When the contact angle of the pure water is smaller than 90°, the solid surface is considered to be hydrophilic; when the contact angle of the pure water is greater than 90°, the solid surface is hydrophobic [30]. It was observed that the CSGS has a very low contact angle 20°, 52° and 30° on glass, Teflon and stainless steel respectively. The contact angle of CSMS on glass, Teflon and stainless steel was found to be 25°, 76° and 41° respectively. The contact angle results were taken as an average of five measurements and are listed in Table 2. The synthesized ester-linked gemini surfactant (CSGS) as well as its corresponding monomeric surfactant (CSMS) derived from cardanol possess excellent wetting properties and can be used as wetting agents.
Antimicrobial Activity
A zone of inhibition signifies the presence of antimicrobial activity. The larger the zone of inhibition, the greater the diffusible antimicrobial activity. The antibacterial and antifungal activity of the CSGS and CSMS was assessed. Results of inhibition zone values are presented in Table 3. CSGS showed favorable antifungal activity in disc diffusion method, whereas absence of growth inhibition zone was observed in case of CSMS. An equivalent antifungal activity was observed with CSGS against C. albicans, A. niger, A. flavus and Rhizopus stolonifer in comparison with Amphotericin B. Both CSGS and CSMS were exhibited antibacterial activity comparable to CTAB. It was found that CSGS showed good antibacterial activity against both Gram-positive and Gram-negative bacteria as well as antifungal activity against all fungi studied. Hence, it can be employed as antimicrobial agents against plant, animal and human microbial pathogens.
Foamability
The foaming efficiency of the CSMS and CSGS was evaluated by the well-known Ross-Miles method as well as the horizontal impinging jet method. Foamability of the surfactant solution is a very important property both in the consumer and non-consumer goods industries. Generally anionic surfactants are known to be highly foaming surfactants [31]. The foam capacity and foam stability of the CSGS, CSMS and SDBS are mentioned in Table 4. The foam produced with CSMS is as stable as that observed with SDBS. It can be seen from Fig. 6 that CSMS and SDBS show similar foaming properties suggesting CSMS can be used as an alternative to SDBS. However, the foamability studies revealed that the synthesized ester-linked gemini surfactant falls in the class of low foam producing surfactant and thus may be used in a variety of applications such as washing machine laundry, spray cleaners or additives in oilfield applications.
Conclusions
A novel anionic gemini surfactant derived from cardanol containing ester-linkage in spacer group was synthesized. The surface activity of the gemini surfactant is higher than its corresponding single chain surfactant. The synthesized gemini surfactant shows excellent wettability and antimicrobial potency and has low foamability. It can be used as an antimicrobial agent for treatment against infectious organisms. This novel ester-linked gemini surfactant is suggested as having an extensive potential for industrial applications.
Abbreviations
- CMC:
-
Critical micelle concentration (mM)
- CSMS:
-
Cardanol sulfonate monomeric surfactant
- CSGS:
-
Cardanol sulfonate gemini surfactant
- SDBS:
-
Sodium dodecyl benzene sulphonic acid
- CTAB:
-
Cetyl trimethyl ammonium bromide
- TLC:
-
Thin layer chromatography
- 13C NMR:
-
Carbon nuclear magnetic resonance
- 1H NMR:
-
Proton nuclear magnetic resonance
- D2O:
-
Deuterated water
- DMSO-d6 :
-
Hexadeuterated dimethyl sulfoxide
- CDCl3 :
-
Deuterated chloroform
- IR:
-
Infrared spectrometry
- GC–MS:
-
Gas chromatography mass spectroscopy
- γ:
-
Surface tension (mN/m)
- γCMC:
-
Surface tension at CMC (mN/m)
- Γ max :
-
Maximum surface excess concentration (mol/cm2)
- A min :
-
Minimum surface area per molecule (nm2)
- C 20 :
-
Concentration of surfactant required to reduce surface tension by 20 mN/m
- λmax :
-
Wavelength maximum (nm)
- β cb :
-
Counterion binding coefficient
- α cb :
-
Counterion dissociation coefficient
- θ :
-
Contact angle (°)
- R :
-
Universal gas constant
- T :
-
Absolute temperature
- N A :
-
Avogadro’s number (6.022 × 1023 mol−1)
References
Menger FM, Littau CA. Gemini-surfactants: synthesis and properties. J Am Chem Soc. 1991;113:1451–2.
Holmberg K, Tehrani-Bagha AR. Cationic ester-containing gemini surfactants: physical-chemical properties. Langmuir. 2010;26:9276–82.
Hait SK, Moulik SP. Gemini surfactants: a distinct class of self-assembling molecules. Curr Sci. 2002;82:1101–11.
Rosen MJ. Surfactants and interfacial phenomena. 3rd ed. New York: Wiley; 2004.
Sikiric M, Primozic I, Talmon Y, Filipovic-Vincekovic N. Effect of the spacer length on the association and adsorption behavior of dissymmetric gemini surfactants. J Colloid Interface Sci. 2005;281:473–81.
Zana R. Gemini (dimeric) surfactants. Curr Opin Colloid Interface Sci. 1996;1:566–71.
Villa C, Baldassari S, Martino DF, Spinella A, Caponetti E. Green synthesis, molecular characterization, and associative behavior of some gemini surfactants without a spacer group. Materials. 2013;6:1506–19.
Falbe J: Surfactants in consumer products: theory, technology and application. SSBM; 2012.
Schramm LL, Stasiuk EN, Marangoni DG. Surfactants and their applications. Annu Rep Sect C Phys Chem. 2003;99:3–48.
Gao Z, Tai S, Zhang Q, Zhao Y, Lü B, Ge Y, Huang L, Tang X. Synthesis and surface activity of biquaternary ammonium salt gemini surfactants with ester bond. Wuhan Univ J Nat Sci. 2008;13:227–31.
Kuo CJ, Lin L, Dong M, Chang W, Chen K. Preparation and properties of new ester-linked cleavable gemini surfactants. J Surfactants Deterg. 2011;14:195–201.
Bhadani A, Tani M, Endo T, Sakai K, Abe M, Sakai H. New ester based gemini surfactants: the effect of different cationic headgroups on micellization properties and viscosity of aqueous micellar solution. Phys Chem Chem Phys. 2015;17:19474–83.
Infante MR, Perez L, Pinazo A, Clapes P, Moran MC, Angelet M, Gracia MT, Vinardell MP. Amino acid-based surfactants. C R Chim. 2004;7:583–92.
Castro M, Kovensky J, Cirelli AF. New family of nonionic gemini surfactants. Determination and analysis of interfacial properties. Langmuir. 2002;18:2477–82.
Yang XH, Wang ZM, Jing F, Hu LH, Zhou YH. A brief review of cardanol-based surfactants. AMM. 2014;483:83–7.
Li H, Wang J, Liu C, Han J, Li C, Ning M. Synthesis and surface activity of cashew-based anion-nonionic surfactants. OJAppS. 2012;2:93.
Balachandran VS, Jadhav SR, Vemula PK, John G. Recent advances in cardanol chemistry in a nutshell: from a nut to nanomaterials. Chem Soc Rev. 2013;42:427.
Shi W, Wang P, Li C, Li J, Li H, Zhang Z, Wu S, Wang J. Synthesis of cardanol sulfonate gemini surfactant and enthalpy-entropy compensation of micellization in aqueous solutions. OJAppS. 2014;4:360.
Wang J, Wang YW, Li CQ, Li J. Synthesis and surface activity of biomass cardanol sulfonate surfactant. AMR. 2011;183:1534–8.
Badertscher M, Buhlmann P, Pretsch E. Structure determination of organic compounds. 4th ed. Berlin Heidelberg: Springer; 2008.
Silverstein RM, Webster FX, Kiemle DJ, Bryce DL. Spectrometric identification of organic compounds. 7th ed. New York: Wiley; 2014.
Lalitha MK. Manual on antimicrobial susceptibility testing. Perform Stand Antimicrob Test Twelfth Inf Suppl. 2004;56238:454–6.
Powale RS, Bhagwat SS. Influence of electrolytes on the foaming of sodium lauryl sulphate. J Disper Sci Technol. 2006;27:1181–6.
Clint JH. Surfactant aggregation. Berlin: Springer; 2012.
Bakshi MS. Cationic mixed micelles in the presence of β-cyclodextrin: a host–guest study. J Colloid Interface Sci. 2000;227:78–83.
Patist A, Bhagwat SS, Penfield KW, Aikens P, Shah DO. On the measurement of critical micelle concentrations of pure and technical-grade nonionic surfactants. J Surfactants Deterg. 2000;3:53–8.
Tehrani-Bagha AR, Holmberg K. Solubilization of hydrophobic dyes in surfactant solutions. Materials. 2013;6:580–608.
Khan MI, Nasef MM. Spreading behaviour of silicone oil and glycerol drops on coated papers. Leonardo J Sci. 2009;14:18–30.
Hill RM. Superspreading. Curr Opin Colloid Interface Sci. 1998;3:247–54.
Zhang X, Shi F, Niu J, Jiang Y, Wang Z. Superhydrophobic surfaces: from structural control to functional application. J Mater Chem. 2008;18:621–33.
Saiyad AH, Bhat SGT, Rakshit AK. Physicochemical properties of mixed surfactant systems: sodium dodecyl benzene sulfonate with triton X 100. Colloid Polym Sci. 1998;276:913–9.
Acknowledgements
The authors would like to gratefully acknowledge the Department of Science and Technology (DST), Government of India for the financial support under the DST-FIST program (SR/FST/ETII 007-2007). One of the authors (M. B. Ahire), would like to acknowledge the University Grants Commission (UGC), Government of India for providing a fellowship (F.4-1/2006 (BSR)/8-10/2007/BSR).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ahire, M.B., Bhagwat, S.S. Novel Ester-linked Anionic Gemini Surfactant: Synthesis, Surface-Active Properties and Antimicrobial Study. J Surfact Deterg 20, 789–797 (2017). https://doi.org/10.1007/s11743-017-1977-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-017-1977-1