Abstract
The synthesis of a homologous series of alanine-based surfactants, namely sodium salts of n-alkanesulfonamido-2-propanoic acids in which n-alkane is n-dodecane, n-tetradecane, n–hexadecane, and n-octadecane having the formula RSO2NHCH (CH3)COO−Na+, is described. The starting materials used were a mixture of secondary positional isomers of n-alkanesulfonyl chlorides obtained by photosulfochlorination reaction using sulfuryl chloride and a catalyst. Surface properties of the aqueous solutions of the synthesized surfactants, including the critical micelle concentration and minimal surface tension δmin, were determined using surface tension measurements at 25 °C. The surface excess Γ and minimum area per molecule (A min) where calculated using the Gibbs equation. The foaming power was also determined by the Bartsh method, and the R 5 parameter was calculated to estimate the stability of the foam formed. The results obtained were compared to those of a commercial surfactant, sodium dodecylsulfate, and a series of synthesized glycine-based surfactants. The results obtained clearly show that the alanine-based surfactants possess good surface properties. The investigations highlight the influence on the surface properties of the addition of a methyl group in the hydrophilic part.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amino acid-based surfactants are of a great interest in the field of novel surfactants research because of their environmentally friendly character. They are characterized by low toxicity and quick biodegradation [1]. They can be produced either by biotechnological or chemical methods using renewable raw materials such as amino acids and vegetable oils [2–5]. They are defined as surfactants having an amino acid or its residue as hydrophilic head [1]. These surfactants with a carboxylate group are recommended as detergents, emulsifiers, and wetting and antistatic agents. They are also used as lubricants and finishing agents for glass and rock fibers [6, 7]. Another important property of these surfactants is their ability to cause solubilization [8, 9]. These surfactants are also found to be active against various diseases [10–12]. They are somewhat stronger than soaps and have good surfactant and detergent properties [6, 7]. The most frequent amino acids used in the synthesis of amino acid-based surfactants are alanine, serine, and leucine owing to the properties of the condensates with various fatty acids [6, 7]. As a result of the various applications of sulfonamide surfactants as detergent agents, different methods for their synthesis have been reported. The sulfonylation of amines in the presence of a base is the most typical method for preparing sulfonamides. Different reactions of synthesized alkanesulfonamides using ammonia as starting materiel were described [13]. As an example, the reaction of methyl or benzylsulfonyl chloride dissolved in toluene or benzene and gaseous ammonia was described, but this method is limited by the use of a gas and by a drop in the yield caused by crystallization in toluene [13]. Another method consists of the direct oxidative conversion of thiols into sulfonamides with H2O2/SOCl2 and amines [14]. A modification of the reaction of sulfur dioxide with various organometallic reagents to give sulfinic acid salts has been reported. It is proposed to treat organometallic reagents directly with sulfuryl chloride and amine to furnish sulfonamides in good yields. But in this reaction, a bench-stable colorless solid charge transfer complex is needed. It is obtained from the combination of DABCO (1,4-diazabicyclo[2.2.2]octane) and sulfur dioxide, and so can replace gaseous sulfur dioxide in organic synthesis [14]. Most of these reported syntheses need various products and steps. In previous works, amino acid-based surfactants and n-alkanesulfonamides with chain length C12–C18 were prepared using glycine and N,N-diethylamine, respectively [15–20]. In the current work, a simple method was used. It consists in the conversion of n-alkanesulfonyl chlorides synthesized into sodium salts of n-alkanesulfonamido-2-propanoic acids in only one step. The compounds obtained are new amino acid (alanine)-based surfactants. They contain in their hydrophilic head an additional methyl group when compared to glycine-based surfactants. The alanine-based surfactants are obtained by the reaction of n-alkanesulfonyl chlorides RSO2Cl (R = C12–C18), a mixture of secondary positional isomers for each length chain, with alanine to give a series of anionic surfactants of general formula RSO2NHCH(CH3)COO−Na+. The surface properties of their aqueous solutions at different concentrations were studied at 25 °C using surface tension measurements. The critical micelle concentration (CMC), minimum area per molecule (A min) at the air/water interface, and superficial concentrations (Γ) were evaluated. The foaming power was also determined by the Bartsch method. The results obtained were compared to those of a commercial surfactant, sodium dodecylsulfate (SDS), and those of glycine-based surfactants prepared in previous works [15]. The influence of the structure of the hydrophilic part on the surfactant properties was also examined.
Experimental Procedures
Synthesis
Synthesis of n-Alkanesulfonyl Chlorides
The n-alkanesulfonyl chlorides (RSO2Cl) which are considered as raw material, were obtained by photosulfochlorination. Thus, 0.172 mol of sulfuryl chloride (Fluka, 97 % pure), freshly distilled under a stream of nitrogen until colorless, was added drop by drop to 0.345 mol of n-alkane (n-dodecane, n-tetradecane, n-hexadecane, or n-octadecane) (Fluka, 99 % pure) in the presence of pyridine (Fluka, 99.8 % pure) as catalyst (10−2 M) under visible light irradiation (150 W). The reaction temperature was maintained between 25 and 35 °C. The reaction mixture was bubbled with nitrogen before the addition of SO2Cl2 and at the end of the reaction. The obtained n-alkanesulfonyl chlorides, a mixture of primary and secondary isomers, were separated from the reaction mixture by solvent extraction using acetonitrile (Fluka, 97 % pure), which was then evaporated leading to a yellow liquid. They were then purified on a silica gel column. The resulting n-alkanesulfonyl chlorides (n-dodecane, n-tetradecane, n-hexadecane, and n-octadecane) were analyzed by FTIR and GC/MS/IE after their derivatization into the more thermally stable N,N-diethyl n-alkanesulfonamides.
Synthesis of N,N-Diethyl n-Alkanesulfonamides
The n-alkanesulfonyl chlorides produced were then derivatized into the corresponding N,N-diethyl n-alkanesulfonamides by addition of diethylamine (Aldrich, 99.5 % pure) using Berthold’s method [16]. The primary isomers were identified in GC analysis by cross injection with pure samples obtained by Grignard reaction using commercial 1-chloroalkanes (Fluka, >99 % pure).The secondary sulfonamide derivatives were identify by GC/MS/EI.
Synthesis of Sodium Salts of n-Alkanesulfonamido-2-Propanoic Acids
Alanine (4.6 mmol; Merck, 99 % pure) was treated with 15 mL of 10 % NaOH. Then, a solution of 1.86 mmol of n-alkanesulfonyl chlorides (C12–C18) in 40 mL of dichloromethane (Merck, 99 % pure) was added drop by drop under magnetic agitation at 0 °C. At the end of the addition, the mixture was heated to reflux for 90 min. The mixture was cooled, and a white solid was collected by filtration. The products were then purified by recrystallization in petroleum ether and the obtained yields were about 83, 78, 85, and 64 % for the following mixture of positional isomers of alanine-based surfactants respectively:
-
C12-Ala: sodium salts of n-dodecanesulfonamido-2-propanoic acids
-
C14-Ala: sodium salt of n-tetradecanesulfonamido-2-propanoic acids
-
C16-Ala: sodium salt of n-hexadecanesulfonamido-2-propanoic acids
-
C18-Ala: sodium salt of n-octadecanesulfonamido-2-propanoic acids
These compounds were characterized by FTIR and LC-HRMS.
Instrumentation
Fourier Transform Infrared Spectroscopy (FTIR)
The presence of the different functions in the synthesized products was confirmed by FTIR using a Perkin Elmer Paragon 500 spectrophotometer using KBr.
Gas Chromatography (GC)
GC separations were performed with a Perkin Elmer Clarus 500 gas chromatograph. An ULTRA 2, a poly (5 % phenyl/95 % methylsiloxane) capillary column 25 m × 0.20 mm I.D., 0.33 µm film thickness (Hewlett-Packard) was used with N2 carrier gas (0.6 mL/s). The analysis of the reaction mixture was carried out with the following temperature program: initial temperature 180 °C and then increased at a rate of 20 °C/min to 270 °C. Injector and flame ionization detector (FID) temperatures were 300 °C.
GC/MS/EI
A gas chromatograph, Hewlett-Packard model 6890, was coupled to an MSD 5973 mass spectrometer. An HP-5ms capillary column 30 m × 0.25 mm I.D. was used with N2 carrier gas (0.6 mL/s). The products were detected by EI (70 eV).
LC/HRMS
A liquid chromatograph coupled to a high resolution mass spectrometer was used (LC/HRMS). The LC method was realized on an ACQUITY UPLC WATERS-SN 475 M, and the separation was performed using an ACQUITY UPLC BEH C18: 1.7 μm 2.1 × 50 mm column. Then, 5-μL aliquots of each sample which were filtered using regenerated glucose (0.2 μm), diluted 100 times, and stored at room temperature were then injected using mobile phase which consists of HCOONH4/10 mM, pH 8/CH3CN, delivered at a flow of 0.45 mL/min. The mass spectrometric detection was performed with a Xevo Q-Tof WATERS-SN: YAA122 and fitted with an electrospray interface (ESI).
Surface Tension Measurements
The measurements were performed at 25 °C for freshly prepared solutions of alanine-based surfactants in the concentration ranges of 10−1–10−4 mol/L using a tensiometer (Prolabo Tensimat-Densimat TD 2000) equipped with a platinum Wilhelmy plate. The pH value of the aqueous surfactant solution is 8. All measurements were repeated three times. In most cases, the accuracy of surface tension measurements was ±0.1 mN/m.
Foaming Power
The study of foam power and foam stability of aqueous solutions of the alanine-based surfactants for different concentrations (from 10−2 M until the disappearance of the foam) reported here has been carried at 25 °C using the Bartsh method [15, 21]. Thus 10 mL aliquots of aqueous surfactant solutions were prepared and introduced into a 100-mL graduated cylinder. The cylinder containing the solution was turned upside down a total of ten times at a rate of one turn every 2 s [15]. The foaming stability is determined by calculating the R 5 parameter. It represents the quotient of the foam height after 5 min to the initial foam [15, 21, 22]. So the initial foam height, H 5, after 5 min was measured for all surfactants. The residual foam ratio R 5 % was calculated as follows in Eq. 1:
where H 0 is the initial foam height and H 5 the foam height measured after 5 min.
Results and Discussion
Synthesis and Characterizations of n-Alkanesulfonyl Chlorides and N,N-Diethyl n-Alkanesulfonamides
The starting materials, i.e., n-alkanesulfonyl chlorides (RSO2Cl where R = C12–C18) obtained by photosulfochlorination of n-alkanes using sulfuryl chloride, were first analyzed by FTIR. The results show the characteristic absorption bands of the SO2 stretching vibrations between 1368 and 1160 cm−1, which is in good agreement with literature data [18]. As long-chain n-alkanesulfonyl chlorides can hardly be analyzed directly by GC because of their instability and their low volatility, they were derivatized into N,N-diethylsulfonamides for their analysis by GC and GC–MS in electron impact (EI) mode. The isomeric compositions were well determined by GC analysis; the ratios of primary/secondary isomers are 20:80, 23:77, 22:78, and 18:82 corresponding respectively to the compounds C12H25SO2N(C2H5)2, C14H29SO2N(C2H5)2, C16H33SO2N(C2H5)2, and C18H37SO2N(C2H5)2. The results showed the presence of the positional isomers for each length chain. Indeed, it was possible to separate the six isomers for n-C12H25SO2N(C2H5)2. We were also able to separate six of the seven isomers for C14H29SO2N(C2H5)2, seven of the eight isomers for C16H33SO2N(C2H5)2, and eight of the nine isomers for C18H37SO2N(C2H5)2 as a result of the co-elution of the internal position isomers. The GC/MS/EI analysis with EI mode was useful for analyzing the position isomers of these N,N-diethylsulfonamide derivatives. The most important peaks of mass spectra in EI mode characterizing the presence of N,N-diethylsulfonamide group in the molecules for all isomers of each alkyl chain length were m/z = 73 for [HN(CH2CH3)2] +, m/z = 58 for [CH3CH2HN = CH2]+, m/z = 107 for [HN (CH2)(C2H5)]+, m/z = 122 for [HSO2NH(CH2)(C2H5)]+, and m/z = 137 for [HSO2N(CH2CH3)2] +. The peak m/z 73 was present with a relative intensity of 100 % for all isomers.
Synthesis and Characterization of Alanine-Based Surfactants
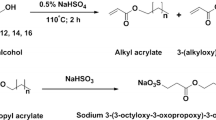
Scheme 1 presents the synthesis of sodium salts of n-alkanesulfonamido-2-propanoic acids. This homologous series of surfactants corresponding to a mixture of primary and secondary isomers for each length chain was obtained in a one-step procedure (Scheme 1).
The alanine-based surfactants were obtained from a convenient reaction of sodium hydroxide and alanine with a yield of ca. 90 % and allowed to react with a mixture of secondary alkanesulfonyl chlorides to give the final sodium n-alkanesulfonamido-2-propanoate surfactants (C12-Ala, C14-Ala, C16-Ala, and C18-Ala). All the synthesized compounds were purified by crystallization in petroleum ether, leading to a crystalline solid form with yields from 64 to 85 %. Their purity was confirmed by the absence of minima near the CMC in the surface tension plots. The chemical structure of these compounds was checked by FTIR and LC/HRMS. For the four synthesized surfactants, the FTIR spectra showed the expected absorption bands of the N–H stretching vibrations at 3453, 3436, 3453, and 3495 cm−1. The O=C–O asymmetric stretch appeared at 1590–1630 cm−1, the O=C–O symmetric stretch at 1450–1460 cm−1, and the stretching of the sulfonate group at 1175–1180 cm−1. The band at 2925–2850 cm−1 is assigned to symmetric and asymmetric stretches of CH2 of linear hydrocarbon chain. These results are in agreement with the data given in the literature [15].
The liquid chromatograms of LC/HRMS presented in Fig. 1 show many well-separated and resolved peaks related to different positional isomers for each chain length (Fig. 1a–d). The main observation, for all the chromatograms, is that one isomer is not well separated for each chain length. This behavior has already been observed in the case of GC and GC/MS analysis of N,N-diethyl n-alkanesulfonamides where the obtained results led to the conclusion that the internal positional isomers are often co-eluted [18–20]. As seen in Fig. 1a, it was possible to separate five of six isomers for C12-Ala as a result of the co-elution of isomers 5 and 6. For the other samples, it was possible to separate six of the seven isomers for C14-Ala, seven of the eight isomers for C16-Ala, and eight of the nine isomers for C18-Ala (Fig. 1a–d). In the last case, the separation was not as good as for the other surfactants in spite of changing the concentration and the injected volume. This could be due to the low solubility of C18-Ala in the used mobile phase.
It is clear from the mass spectrometry analyses of LC/HRMS that all the mass spectra exhibited the peak corresponding to the molecular ion [M]− with the general formula RSO2NHCH(CH3)COO−Na+ of each sample, at m/z = 320, 348, 376, and 404 corresponding to C12-Ala, C14-Ala, C16-Ala, and C18-Ala, respectively (Fig. 2). These peaks with relatively high intensity allow a confirmation of the molecular weight of the synthesized surfactants. The elemental composition report of the LC/HRMS also confirmed that the proposed formula corresponds to that of these alanine-based surfactants. The presence of the sulfonamide function belonging to the alanine moiety in all the isomer structures was also confirmed by the presence of the characteristic fragments at m/z 249, 221, and 277 corresponding to [C7H14SO2NHCH(CH3)COO]−, [C5H10SO2NHCH(CH3)COO]−, and [C9H17SO2NC(HCH3COO)]−, respectively.
To estimate the performance of these novel alanine-based surfactants, synthetic glycine-based surfactants and sodium dodecylsulfate were used as reference surfactants.
Surface Properties
CMC and Related Parameters
The curve of the variation of surface tension values versus log concentration values for different aqueous solutions of alanine-based surfactants show sharp breaks (Fig. 3), which were used to determine CMC values of these surfactants (Table 1). The surface tension of the surfactant solutions decreases as their concentrations increase as shown in Fig. 3. Above the CMC, the surface tension of surfactant solutions reaches a constant minimum value, which indicates that the interface is saturated with the surfactants. The saturation adsorption values Γ max (Gibbs surface excess) at the air/water interface can be obtained by using the Gibbs adsorption equation. On the basis of δ-log (concentration) plots, Γ max (mol m−2) can be obtained from the slope of the isotherms using the Gibbs adsorption isotherm [23–25] as shown in Eqs. 2 and 3:
N A is Avogadro’s number, R is the gas constant (8.31 J mol−1 K−1), T is the absolute temperature in kelvin, and C is the surfactant concentration.
The value of n in the Gibbs equation is the number of ionic species whose concentrations varied with the surfactant concentrations. In this study, n = 2. Γ max, A min, and δ min values are given in Table 1.
It appears from Table 1 that the surface tension increases when the chain length of the hydrophobic part of these synthesized surfactants increases, which is in good agreement with literature data [25–27]. Table 1 shows also that CMC values decrease with increasing tail length. It shows also that increasing the chain length leads to an increase of A min, and consequently, the Γ max values decrease. The same tendency has been observed for glycine-based surfactants as reported in Table 1. Concerning the influence of the addition of a methyl group in the hydrophilic part, the measured surface tensions are compared well to those of glycine-based surfactants, but the CMC values are more important. This can be explained by the probable steric effect of the hydrophilic part which results in the delay of the CMC. As expected, A min values of alanine-based surfactants increased approximately twofold, in comparison with those of glycine-based surfactants, which explains the rapid saturation of the surface.
As expected, Fig. 4 shows that the CMC values decrease linearly with the increase of the number of carbon atoms for the two series of amino acid-based surfactants. As is known, the CMC indeed depends on the hydrophobic part.
Foaming Power and Stability
Different methods are generally used to determine foam power and stability. Forster used sparge tube technique or gas flow [28], Koczó et al. foam beating and gas flow [29], and Patel et al. a “whipping” method [30]. The most widely used test for foaminess adopted by the ASTM as a standard method is the Ross–Miles method [30, 31] and Bartsh method [15–17]. The results collected for the foam ability of the synthesized surfactants are presented in Fig. 5. As can be seen, the foam height decreases as the time increase, and the foams become stable. The initial volume and stability of foam of aqueous solutions depend on the variation of surface tension: the lower surface tension is, the greatest the foam volume is, which is in good agreement with literature data [32].
The residual foam height ratio R 5, calculated using Eq. 1, as a function of concentration is given in Table 2. The reported values show that the foam of synthesized surfactants is stable as R 5 values are greater than 50 %; R 5 = 50 % indicates a metastable foam, whereas lower values of R 5 indicate foams of low stability [23]. Table 3 shows the foam volume of the alanine- and glycine-based surfactants at the same concentrations and foam conditions. As shown, the volume of foam also seems to depend on the nature of the hydrophilic group. Indeed, the addition of a methyl group increases the initial foam volume, which is in agreement with the low surface tension values obtained, but decreases the foam stability after 30 min in comparison with glycine-based surfactants. The high initial volume foam value of C14-Ala could be explained by the interchain cohesion. This latter imparts elastic and mechanical strength to liquid lamellar enclosing the gas in the foam while it increases with the increase of the length of the hydrophobic part [28, 33]. The surfactants with longer length chain C16-Ala and C18-Ala have low water solubility at 25 °C which may probably explain the reason for the low height and stability of their foam. Finally and as shown in Table 3, the addition of one methyl group in the hydrophilic part of these surfactants has an important influence on their foam properties except for C12-Ala which possesses better foam properties than the corresponding C12-Gly.
Conclusion
In this work a series of alanine-based surfactants, namely sodium salts of n-alkanesulfonamido-2-propanoic acids, was synthesized with good yields by a simple method using synthesized secondary n-alkanesulfonyl chlorides and alanine as starting materials. The structures of these surfactants were determined using FTIR, GC/MS/EI, and LC/HRMS methods. The study of their physicochemical properties shows that the surface tension values decrease with the increase of surfactant hydrophobic lengths and with the concentration of surfactants. The addition of one methyl group to the hydrophilic part therefore has an important influence on the surface parameters as the CMC, Γ max, and A min. From the foam study, it appears that the sodium salts of n-alkanesulfonamido-2-propanoic acids possess good foam properties, but that the addition of a methyl group influences the foam stability over time.
References
Infante MR, Pérez L, Pinazo A, Clapés P, Morán MC, Angelet M, García MT, Vinardell MP (2004) Amino acid-based surfactants. CR Chimie 7:583–592
Furutani T, Ooshima H, Kato J (1997) Preparation of N-O-diacyl ethanolamine from N-acyl ethanolamine using lipase preparations. Enzyme Microbiol Technol 20:214–220
Gallot B, Hassan HH (1989) Lyotropic lipo-amino-acids: synthesis and structural study. Mol Cryst Liq Cryst 170:195–214
Foley P, Kermanshahi-pour A, Beach SE, Zimmerman JB (2012) Derivation and synthesis of renewable surfactants. Chem Soc Rev 41:1499–1518
Walia NK, Cameotra SS (2015) Lipopeptides: biosynthesis and applications. J Microb Biochem Technol 7:103–107
Mohamed AS, Mohamed MZ, Ismail DA (2004) Alanine based surfactants: synthesis and some surface properties. J Surfact Deterg 7(4):415–419
Al-Sabagh AM, Nasser NM, El-Azabawy OE, El-Tabey A (2016) Corrosion inhibition behavior of new synthesized nonionic surfactants based on amino acid on carbon steel in acid media. J Mol Liq 219:1078–1088
Baier G, Baki A, Tomcin S, Mailänder V (2014) Stabilization of nanoparticules synthesized by miniemulsion polymerization using “green” amino-acid based surfactant. Macromol Symp 337:9–17
Katz JS, Tan Y, Kuppannan K, Song Y, Brennan DJ, Young T, Yao L, Jordan S (2016) Amino-acid-incorporating nonionic surfactants for stabilization of protein pharmaceuticals. ACS Biomater Sci Eng 2(7):1093–1096. doi:10.1021/acsbiomaterials.6b00245
Yokota H, Sagawa K, Eguchi C, Takehara M, Ogino K, Shibayama T (1985) New amphoteric surfactants derived from lysine. I. Preparation and properties of Nε-acyllysine derivatives-acyllysine derivatives. J Am Oil Chem Soc 62:1716–1719
Braun DB (1989) Developments with lipoamino acids and their salts. Cosmet Toilet 104:87–96
Pinazo A, Manresa MA, Marques AM, Bustelo M, Espuny MJ, Perez L (2015) Amino acid–based surfactants: new antimicrobial agents. Adv Colloid Interface Sci 228:17–39. doi:10.1016/j.cis.2015.11.007
Sandler SR, Schon SG, Gardner DM (1991) Process for the preparation of alkane and arenesulfonamides. US Patent 5068427
Kołaczek A, Fusiarz I, Ławecka J, Branowska D (2014) Biological activity and synthesis of sulfonamide derivatives: a brief review. Chemik 68(7):620–628
Mousli R, Tazerouti A (2011) Synthesis and some surface properties of glycine-based surfactants. J Surfact Deterg 14:65–72
Tazerouti A, Rahal S, Soumillion JPh (1992) Simultaneous gas chromatographic analysis of heptyl chloride-heptanesulfonyl chloride isomeric mixtures. J Chromatogr A 596:132–137
Tazerouti A, Rahal S, Soumillion JPh (1994) The photochemical chlorosulfonation of heptane by sulfuryl chloride: the role of solvent and catalyst-a reinvestigation. J Chem Res 1:1101–1119
Azira H, Assassi N, Tazerouti A (2003) Synthesis of long-chain alkanesulfonates by photosulfochlorination using sulfuryl chloride. J Surfact Deterg 6:55–59
Assassi N, Tazerouti A, Soumillion JP (2005) Analysis of chlorinated, sulfochlorinated and sulfonamide derivatives of n-tetradecane by gas chromatography/mass spectrometry. J Chromatogr A 1071:71–80
Fekarcha L, Tazerouti A (2012) Surface activities, foam properties, HLB, and Krafft points of some n-alkanesulfonates (C14-C18) with different isomeric distributions. J Surfact Deterg 15:419–431
Piispanen PS, Persson M, Claesson M, Norin T (2004) Surface properties of surfactants derived from natural products. Part 2: structure/property relationships—foaming, dispersion, and wetting. J Surfact Deterg 7(2):161–167
Lunkenheimer K, Malysa K (2003) Simple and generally applicable method of determining and evaluation of foam properties. J Surfact Deterg 6:69–74
Tamura T, Kaneko Y, Ohyama M (1995) Dynamic surface tension and foaming properties of aqueous polyoxyethylene n-dodecyl ether solutions. J Colloid Interf Sci 173:493–499
Myers D (2006) Surfactant science and technology, 3rd edn. Wiley, New Jersey
Rosen MJ, Kunjappu JT (2012) Adsorption of surface-active agents at interfaces the electrical double layer. In: Surfactants and interfacial phenomena, 4th edn, Wiley, New Jersey
Holmberg K (2002) Handbook of colloid and surface chemistry, Part 1. Wiley, London
Dahanayake M, Cohen AW, Rosen MJ (1986) Relationship of structure to properties of surfactants. 13. Surface and thermodynamic properties of some oxyethylenated sulfates and sulfonates. J Phys Chem 90:2413–2418
Forster CF (1989) Foam and the activated sludge process. In: Wilson AJ (ed) Foams: physics, chemistry and structure. Springer, London, p 167
Koczó K, Rácz G (1991) Foaming properties of surfactant solutions. Colloid Surf 56:59–82
Patel PD, Stripp AM, Fry JC (1988) Whipping test for the determination of foaming capacity of protein: a collaborative study. Int J Food Sci Tech 23:57–63
Ross J, Miles G (1941) An apparatus for comparison of foaming properties of soaps and detergents. Oil Soap 18:99–102
Rosen M, Solash J (1969) Factors affecting initial foam height in the Ross-Miles foam test. J Am Oil Chem Soc 46:399–402
Rosen M (2004) Foaming and antifoaming by aqueous solution of surfactants. In: Surfactants and interfacial phenomena, 3rd edn. Wiley, New York
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bougueroua, M., Mousli, R. & Tazerouti, A. Synthesis and Physicochemical Properties of Alanine-Based Surfactants. J Surfact Deterg 19, 1121–1131 (2016). https://doi.org/10.1007/s11743-016-1878-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1878-8