Abstract
The micellar properties of dodecyltrimethylammonium bromide (DTAB) in water and methanol water mixtures at different temperatures have been studied by conductivity and surface tension measurements. The critical micelle concentrations (CMC), degree of ionization (α), standard Gibbs free energy of micellization (\( \Delta G_{\text{m}}^{\text{o}} \)), standard enthalpy of micellization (\( \Delta H_{\text{m}}^{\text{o}} \)), standard entropy of micellization (\( \Delta S_{\text{m}}^{\text{o}} \)) and free energy of transfer (\( \Delta G_{\text{trans}}^{\text{o}} \)) were evaluated from conductivity data. The CMC, maximum excess surface concentration (\( \varGamma_{ \hbox{max} } \)), area occupied per surfactant molecule (\( A_{ \hbox{min} } \)), surface pressure at the CMC (\( {\pi}_{\text{cmc}} \)), packing parameter (P) and standard free energy interfacial adsorption \( (\Delta G_{\text{ads}}^{\text{o}} \)) were estimated from surface tension measurements. The CMC of DTAB was found to increase with increasing volume fraction of methanol and increasing temperature. Thermodynamic parameters and surface properties revealed that the addition of methanol changes the relevant physicochemical properties which affect the process of micellization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactant molecules are characterized by the presence of polar and non-polar parts. Under certain conditions they form aggregates called micelles in solution [1]. The concentration at which micelle are formed is called the critical micelle concentration (CMC). Micelle formation is an important characteristic property of surfactants which is caused by hydrophobic interaction of hydrocarbon tails with water balanced by electrostatic repulsions between the surfactant head groups [2]. Physicochemical properties such as CMC, degree of ionization and thermodynamics of micellization depend on the nature of the hydrophobic tail, the hydrophilic head group and the counter ion species [3].
Organic additives can significantly affect micelle formation. This has prompted investigations concerning the effect of organic additives on the micellization of individual surfactants [4]. The effect of alcohol on micellization has been extensively studied in the preparation of microemulsions [5]. The investigations show that alcohol interacts with the micelle in the surface region resulting in: (a) insertion of alcohol molecules between the ionic head groups of the micelle [6]; (b) a decrease in the dielectric constant at the micellar interface [7]; and (c) a change in the molecular order of the interfacial region of the micelle [5].
In this paper, we investigated the effect of methanol addition and temperature change on the micellization of dodecyltrimethylammonium bromide (DTAB) by conductivity and surface tension measurements.
Experimental Section
Materials
Dodecyltrimethylammonium bromide was purchased from Loba Chemie Private Limited (Mumbai, India). Methanol (E. Merck, India, 99 % pure) was distilled with phosphorous pentoxide and then redistilled over calcium hydride. The purified solvent had a density of 0.7772 g cm−3 and a co-efficient of viscosity of 0.4742 mPa s at 308.15 K which matches that in the literature [8].
DTAB was dried for 1 h and solutions were prepared using triply distilled water. The purity of DTAB was verified by CMC measurement using conductivity and surface tension at 298.15 K. The measured values are in close agreement with those in the literature [9]. Triply distilled water with a specific conductance of less than 10−6 S cm−1 at 308.15 K was used for the preparation of methanol–water mixed solvent media as well as the DTAB solutions.
Electrical Conductivity Measurements
The conductivity of freshly prepared DTAB solutions was measured using a digital conductivity meter (Systronics, India) with a dip type conductivity cell having a cell constant of 1.002 cm−1 and an uncertainty of 0.01 %. The cell was calibrated using aqueous potassium chloride solution [10].
Surface Tension
The surface tension of freshly prepared DTAB solutions was measured using a Borosil Mansingh Survismeter (calibration no. 06070582/1.01/c-0395, NPL, New Delhi) [11] by pendant drop number (PDN) as explained in the literature [12]. The temperature inside the Survismeter was controlled by a thermostat [13].
Solution densities required for the calculation of surface tension were determined using a thermostated 25 cm3 Sprengel-Ostwald pycnometer. The temperature control had an accuracy of ±0.1 °C and the reproducibility was ±5 × 10−5 g/cm3 [14].
Results and Discussion
Critical Micelle Concentration (CMC) and Degree of Ionization (α)
Cationic surfactants are generally tertiary ammonium salts of long-chain paraffins which act as strong electrolytes in dilute solution [1]. The critical micelle concentration (CMC) of DTAB was determined by conductometric and tensiometric methods. In the conductometric method, the CMC was obtained from the intersection of the two straight lines of the conductivity-concentration plots [13]. The ratio of the slopes of the linear fragments above and below the break gives an estimate of the degree of micelle ionization, α.
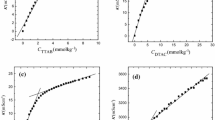
The dependence of specific conductivity and surface tension with DTAB concentration in pure water and in different volume fractions (0.1, 0.2, 0.3 and 0.4) of methanol–water mixtures at 298.15 K are shown in Figs. 1 and 2 respectively. Figure 1 shows that the conductivity of DTAB solutions decreases both in the pre- and post-micellar regions with an increasing volume fraction of methanol. This behavior is caused by two effects of the solvent-medium properties. Alcohol is known to have structure breaking effects on water and the increase in viscosity of the medium with increasing the methanol content [15, 16].
The data from conductivity and surface tension measurements are summarized in Table 1. The CMC as well as α of DTAB in water are in close resemblance to literature data at 298.15 K [3, 9]. Both the CMC and α increase with increasing volume fraction of methanol at all temperatures.
Increase in α with increasing volume fraction of methanol can be explained by two effects. Intercalation of methanol molecules between the DTAB ions in the micelle causes the average distance between ionic head groups to increase and the micellar surface charge density and ionization decreases [17]. The second effect concerns the dielectric constant of the palisade layer. Methanol probably replaces the water molecules from the palisade layer of the micelle which would decrease the dielectric constant [18]. The measurements done by Zana et al. [19], have shown that addition of alcohol to micellar solutions of tetradecyltrimethylammonium bromide (TTAB) brings about a decrease in polarity which increases the repulsion between ionic head groups and decreases micelle stability. Hence the corresponding charge density decreases and results in an increase of degree of ionization (α).
Increasing temperature may increase the steric volume of the N-atom-head in the micelle and hence degree of ionization (α) is affected [20]. Similar types of variations are found in this investigation in all volume fractions of methanol–water. According to Kabir-Ud-Din et al. [21], two forces, coulombic and thermal, are responsible for the increase in α. The former force is attributed to the attraction of the counter ions to the polar head and the second force is dependent on the temperature. When temperature is increased, the thermal force predominates over the coulombic force and the value of α increases.
Alcohols have lower dielectric constants than pure water and the dielectric constant of the medium decreases when alcohol is added to water. Lower dielectric constant decreases the hydrophobic interaction and increases the CMC [22]. This effect is seen with other hydrogen bonded organic solvents in aqueous medium. The presence of glycols in aqueous medium reveals water structure breaking properties which decreases the relative permittivity of the medium and stabilizes surfactant monomers [23]. In addition, hydrogen bonded organic solvents like alkoxyethanols in aqueous medium makes the medium less hydrophilic and increases surfactant monomer solubility [24]. Increasing temperature disrupts the water structured around the surfactant molecules and increases the CMC.
Thermodynamics of Micellization
On the basis of a pseudo-phase separation model [25, 26] the standard Gibbs free energy of micellization, \( \Delta G_{\text{m}}^{\text{o}} \), is calculated from Eq. 1:
where \( X_{\text{cmc}} \) is the mole fraction of surfactant at the CMC, R is the universal gas constant and T is the temperature. Standard enthalpies of micelle formation, \( \Delta H_{\text{m}}^{\text{o}} \), can be calculated from Gibbs–Helmholtz equation [25, 26]
The term \( [ {{\raise0.7ex\hbox{${\partial \ln X_{\text{cmc}} }$} \!\mathord{\left/ {\vphantom {{\partial \ln X_{\text{cmc}} } {\partial T}}}\right.\kern-0pt} \!\lower0.7ex\hbox{${\partial T}$}}} ]_{\text P} \) is calculated by fitting the plot of \( \ln X_{\text{cmc}} \) versus temperature and taking the corresponding temperature derivative. From the values of \( \Delta G_{\text{m}}^{\text{o}} \) and \( \Delta H_{\text{m}}^{\text{o}} \), the standard entropy of micellization, \( \Delta S_{\text{m}}^{\text{o}} \) can be calculated using Eq. 3.
In addition, the effect of additives on the micellization process can be studied by means of free energy of surfactant tail transfer, \( \Delta G_{\text{trans}}^{\text{o}} \), which is defined by [26]
Thermodynamic properties of micellization such as standard free energy of micellization \( (\Delta G_{\text{m}}^{\text{o}} ) \), standard enthalpy of micellization \( (\Delta H_{\text{m}}^{\text{o}} ) \), the standard entropy of micellization \( (\Delta S_{\text{m}}^{\text{o}} ) \) and standard free energy of transfer \( \Delta G_{\text{trans}}^{\text{o}} \) are calculated from Eqs. 1 to 4 respectively and the values are displayed in Table 1 in pure water, 0.1, 0.2, 0.3 and 0.4 volume fractions of methanol at 298.15, 308.15, 318.15 and 323.15 K. The free energy of micellization signifies the spontaneity of the micellization process. The more negative the standard free energy change the greater the spontaneity of micellization. It is seen from the data of Table 1 that the standard free energy of micellization is negative in water as well as in methanol–water mixed solvent media at all investigated temperatures. Moreover, the \( \Delta G_{\text{m}}^{\text{o}} \) values become less negative with increasing volume fraction of methanol–water at constant temperature indicating that addition of methanol makes the micellization less favorable. It can also be observed that with the increase in temperature \( \Delta G_{\text{m}}^{\text{o}} \) values become less negative indicating less spontaneity of micellization at higher temperature. This phenomenon can be attributed to agitation of the micelle due to thermal forces at higher temperature [27].
According to the theory of surfactant self-assembly [28], the major contribution to the standard free energy of micellization is associated with transfer of the surfactant tail from solvent into the micelle \( \Delta G_{\text{trans}}^{\text{o}} \). The \( \Delta G_{\text{trans}}^{\text{o}} \) values in Table 1 are all positive and increase with the increasing volume fraction of methanol in water indicating the transfer of the surfactant tail from the bulk into the micelle is less favorable. It is also seen that \( \Delta G_{\text{trans}}^{\text{o}} \) values decrease with increasing temperature. The organic solvent–water mixed solvent media is a better solvent for the surfactant molecules [29]. This makes the hydrophobic tail transfer from the bulk phase into the micelle less favorable. It can also be understood on the basis of a reduction in the solvophobic interactions which leads to an increase in the solubility of hydrocarbon tails in the presence of methanol, and consequently to an increase in the CMC.
The values of \( \Delta H_{\text{m}}^{\text{o}} \) of DTAB in aqueous as well as in methanol–water are also negative. The values become more negative with increasing temperature, suggesting that the micellization of the surfactant is exothermic. The observed decrease of \( \Delta H_{\text{m}}^{\text{o}} \) with increasing temperature is probably due to destruction of the ordered aqueous region diminishing hydrogen bonding between water molecules surrounding the hydrocarbon chain of the surfactant [30]. The negative \( \Delta H_{\text{m}}^{\text{o}} \) values can be taken as evidence that London-Dispersion interactions play a more predominant role as the temperature increases [27].
The \( \Delta S_{\text{m}}^{\text{o}} \) values are positive and decrease with an increasing volume fraction of methanol. The positive entropy change indicates that the micellization process is favored by entropy gain, associated with the destruction of the iceberg structure around the hydrophobic alkyl chain, a pre-requisite condition for micelle formation [31]. The \( \Delta S_{\text{m}}^{\text{o}} \) values shows a decreasing trend with increasing temperature. This implies that disordering of water molecules becomes less pronounced due to the destruction of the iceberg water structure around the alkyl group with increasing temperature [25, 26].
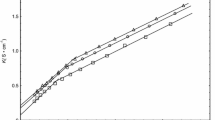
It is well known that there is enthalpy-entropy compensation for micellization of surfactants [26]. The enthalpy-entropy compensation plot is displayed in Fig. 3. It shows a linear co-relation between enthalpy and entropy over all the volume fractions studied. It is seen that as the volume fraction of methanol increases the enthalpic contribution becomes lower indicating a less favorable condition for micellization process.
Plot of variation of \( {\text{T}}\Delta S_{\text{m}}^{\text{o}} \) with \( \Delta H_{\text{m}}^{\text{o}} \) for DTAB in pure water (open circles) different volume fractions of methanol–water mixtures (open squares, 0.10 methanol; open inverted triangles, 0.20 methanol; closed circles, 0.3 methanol; closed squares, 0.40 methanol)
Surface Properties
The maximum surface excess concentration at the air/methanol–water interface \( (\varGamma_{\hbox{max} } ) \), has been calculated by applying the Gibbs adsorption isotherm [32]:
where γ denotes the surface tension, R is the gas constant (8.314 J mol−1 K−1), T is the absolute temperature, C is the surfactant concentration, (\( \frac{{\text{d} \gamma }}{{\text{d} \log C}} \)) is the slope of the γ versus log C plot taken at the CMC. For conventional univalent ionic surfactants the constant n takes a value of 2. The area occupied per surfactant molecule \( (A_{\hbox{min} } ) \) at the air/methanol–water interface [33] has been obtained by,
where N is Avogadro’s number. Low values of \( A_{\hbox{min} } \) suggest that the orientation of the surfactant molecule at the interface is almost perpendicular to the interface [34]. The value of the surface pressure at the CMC \( (\pi_{\text{cmc}} ) \) is obtained as:
where \( \gamma_{\text{o} } \) and \( \gamma_{\text{cmc}} \) are the values of surface tension of water and the surfactant solution at the CMC respectively. The surface excess concentration \( (\varGamma_{\hbox{max} } ) \) is an effective measure of adsorption at air/solution interface. It measures how much the air/solution interface has been changed by surfactant adsorption and depends on the molecular structures of surfactants. The standard free energy interfacial adsorption at the air/saturated monolayer interface can be evaluated from the relation [34].
Israelachvili et al. [34] proposed that the micellar shape is mainly governed by the geometry of the surfactant and its packing. The surface area of amphiphiles in mixed micelles and micellar growth (spherical–nonspherical) can be used to calculate the packing parameters (P):
where \( V_{\text{o} } \) is the volume of exclusion per monomer in the micelle, given by Tanford’s formula [2]. \( V_{\text{o} } = [27.4 + 26.9(n_{\text{c} } - 1)]2{\AA}^{3} \), \( l_{\text{c} } = [1.54 + 1.26\left( {n_{\text{c} } - 1} \right)]{\AA} \), is the maximum chain length and n c is the number of carbon atoms in the hydrocarbon chain. The packing parameter (P) gives information about the geometry of micelles and indicates minimum size of aggregates in solution, due to which the Gibbs free energy of micellization (\( \Delta G_{\text{m}}^{\text{o}} \)) is minimized.
The maximum surface excess concentration at the air/methanol–water interface \( (\varGamma_{\hbox{max} } \)), area occupied per surfactant molecule (\( A_{\hbox{min} } \), surface pressure at the CMC (\( \pi_{\text{cmc}} \)), standard free energy interfacial adsorption (\( \Delta G_{\text{ads}}^{\text{o}} \)) and the packing parameters (P) calculated by Eqs. 5 to 9 are displayed in Table 2. The data shows that \( \varGamma_{\hbox{max} } \) as well as \( \pi_{\text{cmc}} \) values decrease with increase in volume fraction of methanol at a constant temperature indicating a reduced population of surfactant molecules at the interface as the volume fraction of methanol is increased. However, \( A_{\hbox{min} } \) values increase with increasing volume fraction of methanol which indicates that the surfactant molecule occupies more area as the methanol content is increased. Negative values of \( \Delta G_{\text{ads}}^{\text{o}} \) indicate that the adsorption of surfactant molecules on the surface is spontaneous and is more spontaneous that micellization. The \( \Delta G_{\text{ads}}^{\text{o}} \) values become less negative with increasing volume fraction of methanol at constant temperature which indicates less spontaneity of adsorption of surfactant molecules on the surface. Similar types of investigations are found in the literature [9, 16].
Data from Table 2 suggests that the surface properties of DTAB in water and in the presence of methanol are highly dependent on temperature. As a general rule, when the temperature of a system increases, thermal expansion changes several properties of the system. In our case, there is a decrease in \( \varGamma_{\hbox{max} } \), an increase in \( A_{ \hbox{min} } \), a decrease in \( \pi_{\text{cmc}} \) and decrease in P with increasing temperature. These variations can be understood on the basis of thermal expansion of the solution with increase in temperature. Similar variations are observed by others in the literature [9].
Israelachvili et al. [34] have proposed that depending on the value of the packing parameter (P), surfactant aggregates acquire different shapes. They showed that, in general, micelles are spherical for P < 1/3. In our investigation, P is less than 1/3 in all the cases suggesting the presence of spherical micelles. The P values decrease with an increasing volume fraction of methanol at constant temperature. Previous investigations have shown that the micelle aggregation number (N agg) increases with increasing volume fraction of methanol [35]. The decrease in N agg and P values suggest that micellar aggregates get smaller when methanol is added. Pan et al. [35] observed that there is no evidence of micelle formation when the volume fraction exceeds 50 %.
Conclusion
Thermodynamic and surface properties of DTAB in water and methanol–water mixtures at different temperatures were determined using conductometry and tensiometry. Methanol is miscible in water in all proportions and breaks down the three-dimensional H-bonded water structure and alters the micellization of DTAB. The CMC and degree of ionization (α) of DTAB increases with increasing volume fraction of methanol. Methanol decreases the cohesiveness of water making the medium more acceptable to the alkyl chain of DTAB making self-assembly more difficult. Thermodynamic parameters suggest that the driving force for the hydrophobic effect, which is required for micelle formation is due to the cohesive force of the solvent. The change in cohesive force due to methanol addition slows down the aggregation of DTAB. Surface properties suggest that methanol acts as a surface active agent and alters the surface properties by competing with the surfactant molecules for interfacial adsorption. More importantly, the size of the micelle decreases with an increasing volume fraction of methanol.
References
Flockhart BD (1957) The critical micelle concentration of sodiumdodecyl sulfate in ethanol-water mixtures. J Coll Sci 12:557–565
Tanford C (1980) The hydrophobic effect-formation of micelles and Biological membranes, 2nd edn. Wiley, New York
Mata J, Varade D, Bahadur P (2005) Aggregation behavior of quaternary salt based cationic surfactants. Thermochemica Acta 428:147–155
Rafati AA, Maleki H (2007) Mixed micellization of tetradecyltrimethylammonium bromide and triton X-100 in water-ethanol mixtures, using potentiometric and surface tension techniques. J Mol Liq 135:128–134
Tardajos G, Junquera E, Aicart E (1994) Isothermal compressibility and isobaric thermal expansivity of linear and branched hexanols at 298.15 K. J Chem Eng Data 39:349–350
Manabe M, Tokunaga A, Kawamura H, Shiomi M, Hiramatsu K (2002) The counterion releasing effect and the partition coefficient of branched alkanols in ionic micellar solution. Colloid Polym Sci 280:929–935
Zana R (1995) Aqueous surfactant-alcohol systems: a review. Adv Colloid Interface Sci 57:1–64
Bhattarai A, Nandi P, Das B (2006) The effects of concentration, relative permittivity and temperature on the transport properties of sodium polystyrenesulfonate in methanol-water mixed solvent media. J Polym Res 13:475–482
Das S, Mondal S, Ghosh S (2013) Physicochemical studies on the micellization of cationic, anionic, and nonionic surfactants in water-polar organic solvent mixtures. J Chem Eng Data 58:2586–2595
Lind JE Jr, Zwolenik JJ, Fuoss RM (1959) Calibration of conductance cells at 25 °C with aqueous Solutions of potassium chloride. J Am Chem Soc 81:1557–1559
Singh M (2006) Survismeter type I and II for surface tension, viscosity measurements of liquids for academic, research and development studies. J Biochem Biophys Methods 67:151–161
Kumar D, Chandra A, Singh M (2014) Influence of urea on shifting hydrophilic to hydrophobic interactions of Pr(NO3)3, Sm(NO3)3, and Gd(NO3)3 with BSA in aqueous citric acid: a volumetric, viscometric, and surface tension study. J Chem Eng Data 59:3643–3651
Bhattarai A (2015) Studies of micellization of cationic-anionic surfactant system in water and methanol-water mixed solvents. J Solution Chem 44:2090–2105
Moumouzias GD, Panopoulos K, Ritzoulis G (1991) Excess properties of the binary liquid system propylene carbonate + acetonitrile. J Chem Eng Data 36:20–23
Naorem H, Devi SD (2006) Conductrometric and surface tension studies on the micellization of some cationic surfactants in water-organic solvent mixed media. J Surface Sci Technol 22:89–100
Manna K, Panda AK (2011) Physicochemical studies on the interfacial and micellization behavior of CTAB in aqueous polyethylene glycol media. J Surfact Deterg 14:563–576
Zana R (1980) Ionization of cationic micelles: effect of the detergent structure. J Colloid Interface Sci 78:330–337
Lianos P, Zana R (1980) Surfactant-alcohol mixed-micelle formation: cetyltrimethylammonium bromide-1 butanol system. Chem Phys Lett 72:171–175
Zana R, Yiv S, Strazielle C, Lianos P (1981) Effect of alcohol on the properties of micellar systems. J Colloid Interface Sci 80:208–223
Di Michele A, Brinch L, Di Profio P, Germani R, Savelli G, Onori G (2011) Effect of head group size, temperature and counterion specificity on cationic micelles. J Colloid Interface Sci 358:160–166
Kabir-ud-Din N, Rub MA, Naqvi AZ (2011) Self-association behavior of amitriptyline hydrochloride as a function of temperature and additive (inorganic salts and urea) concentration. Colloid Surf B 82:87–94
Yilmaz H (2002) Excess properties of alcohol-water system at 298.15 K. Turk J Phys 26:243–246
Bakshi MS, Kaur G (2000) Effects of glycol additives on the mixed micelle formation by hexadecyltrimethylammonium bromide + dodecylpyridinium chloride mixtures. J Mol Liq 88:15–32
Bakshi MS, Doe H (2000) Hydrophobic hydration of cationic mixed micelles by alkoxyethanols in aqueous media. J Surfact Deterg 3:497–504
Kim H-U, Lim K-H (2004) A model on the temperature dependence of critical micelle concentration. Colloids Surf A 235:121–128
Ruiz CC (1999) Thermodynamics of micellization of tetradecyltrimethylammonium bromide in ethylene glycol-water binary mixtures. Colloid Polym Sci 277:701–707
Ali A, Uzair S, Malik NA, Ali M (2014) Study of interaction between cationic surfactants and cresol red dye by electrical conductivity and spectroscopy methods. J Mol Liq 196:395–403
Nagarajan R, Wang C-C (2000) Theory of surfactant aggregation in water/ethylene glycol mixed solvents. Langmuir 16:5242–5251
Chung JJ, Lee SW, Kim YC (1992) Solubilization of alcohols in aqueous solution of cetylpyridinium chloride. Bull Korean Chem Soc 13:647–649
Islam MN, Kato T (2003) Thermodynamic study on surface adsorption and micelle formation of poly(ethylene-glycol) mono-n-tetradecyl ethers. Langmuir 19:7201–7205
Rosen MJ (2004) Surfactants and interfacial phenomenon, 3rd edn. Wiley, New York
Mukharjee I, Moulik SP, Rakshit K (2013) Tensiometric determination of Gibbs surface excess and micelle point: a critical revisit. J Colloid Interface Sci 394:329–336
Sugihara G, Miyazono A, Nagadome S, Oda T, Hayasi Y, Ko JS (2003) Adsorption and micelle formation of mixed surfactant systems in water II: a combination of cationic gemini-type surfactant with MEGA-10. J Oleo Sci 52:449–461
Israelachivili JN, Mitchell DJ, Ninham BW (1976) Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J Chem Soc, Faraday Trans 2(72):1525–1568
Pan A, Naskar B, Prameela GKS, Phani KBVN, Mandal AB, Bhattacharya SC, Moulik SP (2012) Amphiphile behavior in mixed solvent media I: self-aggregation and ion association of sodium dodecylsulfate in 1,4-dioxane-water and methanol-water media. Langmuir 28:13830–13843
Acknowledgments
Sujit Kumar Shah is thankful to the University Grants Commission (UGC), Government of Nepal, for his Ph.D. fellowship. Thanks also goes to the Head of the Department of Chemistry, M.M.A.M.C., Tribhuvan University, Biratnagar, Nepal for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Shah, S.K., Chatterjee, S.K. & Bhattarai, A. The Effect of Methanol on the Micellar Properties of Dodecyltrimethylammonium Bromide (DTAB) in Aqueous Medium at Different Temperatures. J Surfact Deterg 19, 201–207 (2016). https://doi.org/10.1007/s11743-015-1755-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1755-x