Abstract
The surface tension of disodium hexadecyl diphenyl ether disulfonate (C16-MADS) was measured at different NaCl concentrations (0.00–0.50 mol L−1) and temperatures (298.0–318.0 K) using the drop-volume method. The results show that, with increasing temperature, the critical micelle concentration (CMC) of C16-MADS increases slightly, but the maximum surface adsorption capacity (Γ max) at the air–water interface decreases. When the concentration of NaCl was increased from 0.00 to 0.50 mol L−1, the CMC of C16-MADS decreased from 1.45 × 10−4 to 4.10 × 10−5 mol L−1, but the surface tension at the CMC (γ cmc) was not affected. When the concentration of NaCl was increased at 298.0 and 303.0 K, the Γ max of C16-MADS increased. When the temperature was increased from 308.0 to 318.0 K, the surface excess concentration (Γ max) of C16-MADS abnormally decreased from 2.26 to 1.41 μmol m−2 with increasing NaCl concentration. The micellization free energy (\(\Delta G_{\text{m}}^{ \circ }\)) decreased from −63.98 to −76.20 kJ mol−1 with increase of temperature and NaCl concentration. The micellar aggregation number (N m) of disodium hexadecyl diphenyl ether disulfonate (C16-MADS) was determined using the molecule fluorescence probe method with pyrene as probe and benzophenone as quencher. The results show that an appropriate N m could be measured only at surfactant concentration above the CMC. The N m increased with an increase in C16-MADS concentration, but the micropolarity in the micelle nucleus decreased. The temperature had little effect on N m. Compared with typical single hydrophilic headgroup surfactants, aggregates of C16-MADS exhibit different properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Disodium hexadecyl diphenyl ether disulfonate (C16-MADS) is a highly efficient and multifunctional anionic surfactant with two hydrophilic headgroups. The structure of disodium hexadecyl diphenyl ether disulfonate is shown in Fig. 1. It has unique double sulfonate hydrophilic groups, which are linked by a rigid diphenyl ether group which produces intramolecular hyperconjugation. C16-MADS has a variety of advantages when compared with traditional surfactants: excellent water solubility and coupling properties [1–3], extremely low Krafft point, excellent dispersion capacity [4–6], good hard water and bleach tolerance [7], and good stability in strong acid, strong alkali, and concentrated electrolyte solution [1, 8]. C16-MADS has been used in high inorganic salt systems, but the effects of inorganic salt on the product have not been reported. This paper reports on the surface properties and thermodynamics of micellization of C16-MADS solution using surface tension at different NaCl concentrations and temperatures. The micellar aggregation number was measured using the molecular fluorescent probe method, and changes of concentration, temperature, inorganic salt effect, and micropolarity were investigated. The results show that C16-MADS exhibits obvious differences compared with traditional surfactants [9–11].
Experimental
Materials
NaCl (AR, Shanghai Chemical Reagent Co., Ltd., used after calcining at 500 °C for 5 h), C16-MADS [homemade, high-performance liquid chromatography (HPLC) and mass spectrometry assay, content 99.8 %] [12], ultrapure water (Wuxi New Central Asia Institute of Microelectronics, conductivity 7.8 × 10−7 S cm−1), anhydrous methanol, ethanol, petroleum ether, pyrene (Sigma-Aldrich, 99.0 %), and benzophenone (CP, Shanghai Qunli Chemical Co., Ltd.) were used.
Measurements of Surface Tension
Solutions of C16-MADS were prepared at different concentrations using ultrapure water. The surface tension was measured with a DCA-315 automatic interfacial tensiometer (Thermo Cahn Co., USA) by pendant drop method at 298.0 ± 0.1, 303.0 ± 0.1, 308.0 ± 0.1, 313.0 ± 0.1, and 318.0 ± 0.1 K.
Measurement of Micellar Aggregation Number
The micellar aggregation number of C16-MADS was determined using the molecule fluorescence probe method with pyrene (Py) as probe (P) and benzophenone (DPK) as quencher (Q), with an RF-5301PC spectrofluorophotometer (Shimadzu Scientific Instruments). The fine structure of the steady-state fluorescence spectrum of pyrene has five vibronic peaks at wavelengths of 373, 379, 384, 390, and 393 nm [13]. In aqueous solution, pyrene and benzophenone can be closely integrated within surfactant micelles, and residence time in micelles is much longer than the probe’s fluorescence lifetime. If (1) the quenching of Q for P is static quenching, (2) the kinetic coefficient R = (K f + K D)/K q ≈ 0 (where K f, K D, and K q are constants of stimulated emission fluorescence, nonradiative decay, and quenching, respectively), and (3) Q follows the Poisson distribution between micelles, the following formula can be deduced [14]:
where I 1 is the fluorescence intensity when the concentration of quencher is [Q] and the wavelength is 373 nm, I 0 is the fluorescence intensity without the quencher, C Q is the concentration of the quencher, S T is the total concentration of surfactant, and cmc is the critical micelle concentration of the surfactant. Equation (1) can be converted to read
As can be seen from the above equations, when C Q is fixed, N m can be deduced from the relationship between [ln(I 0/I 1)]−1 and S T.
Measurement Methods for Micellar Aggregation Number
Solutions of C16-MADS at different concentrations were prepared using water saturated with pyrene as solvent. Benzophenone of a certain concentration was prepared using anhydrous methanol as solvent. A certain amount of methanol solution of benzophenone was accurately weighed into a clean, dry 100-mL flask with stopper, and the methanol was dried under pure nitrogen. The solutions of prepared surfactant and pyrene were accurately transferred to flask with a pipette. After agitation in an ultrasonic bath for 10 min, solutions were equilibrated in a constant-temperature water bath for 12 h. Binary solutions of the same probe and surfactant concentration but without quencher were prepared as reference reagent. The fluorescence intensity of the solutions was measured by fluorescence spectrometer with excitation wavelength of 335 nm. I 1 and I 0 were measured at 373 nm using slit width of 1.5 nm.
Results and Discussion
Effect of NaCl Concentration on Surface Tension
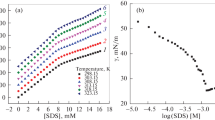
The surface tension of C16-MADS solutions at different concentrations was measured for different NaCl concentrations (0.00, 0.05, 0.10, 0.20, and 0.50 mol L−1) and temperatures (298.0, 303.0, 308.0, 313.0, and 318.0 K). The surface tension isotherms are shown in Fig. 2.
The critical micelle concentration (CMC) and the surface tension at the critical micelle concentration (γ cmc) are important parameters to assess the surface activity of surfactants [15]. For conventional ionic surfactants, when the inorganic salt added is the same as the surfactant counterion, the surface activity is improved while the CMC and γ cmc decrease. This is mainly because the hydration film is damaged with addition of inorganic salt and the diffuse double layer is compressed around the ionic headgroups. When the electrostatic repulsion is shielded, and surfactant molecules in the surface layer of the micelle can arrange more closely, micelles can form more easily [16]. The CMC of C16-MADS solution significantly decreased with addition of NaCl, but γ cmc remained essentially unchanged because the two hydrophilic groups are linked with a rigid diphenyl ether group which lacks flexibility.
Surface Chemical Properties of Disodium Hexadecyl Diphenyl Ether Disulfonate
According to the Gibbs adsorption equation, under excess counterion conditions, the change in surface tension (γ) is given by [16, 17]
where Γ s is the surface adsorption of the surfactant, R is the gas constant, T is the temperature in Kelvin, and C s is the concentration of surfactant ions in solution. Then, the maximum adsorption (Γ max) of the solution surface can be calculated from Eq. (5).
Γ max can be obtained from the slope of γ versus log C s curves [15, 18]. The average minimum area per molecule in a monolayer (A min) can be calculated from Eq. (6):
where N A is Avogadro’s constant. Table 1 shows that the CMC of C16-MADS increased gradually with increasing temperature at all NaCl concentrations. The hydration of the hydrophilic headgroup decreases with increasing temperature, so micelle formation is easier. On the other hand, the structure of water around the hydrophobic tail group is disrupted with increasing temperature, which is not conducive to micelle formation. Normally, the effect of temperature on the hydrophobic effect plays the dominant role, so the CMC of C16-MADS solution tends to increase with increasing temperature. Likewise, the Γ max value of the C16-MADS solution decreased slightly with increasing temperature at given NaCl concentration, and the average molecular area increased.
Increase in the NaCl concentration affects two aspects of C16-MADS at 298.0 and 303.0 K. On the one hand, the ionic strength of the solution is changed, so the activity of the surface-active ion is changed. Surface adsorption as a balance property must also change with the change in activity. On the other hand, it is conducive to the combination of the counterion and the surface-active ions due to the increase in counterion concentration, which weakens the electrical repulsion in the adsorption layer, so the adsorbed molecules can pack more closely, thus Γ max increases.
The Γ max value of C16-MADS decreased with increase of the NaCl concentration at 308.0, 313.0, and 318.0 K. This occurs not only because the molecular thermal motion is strengthened and the tendency for protonation of C16-MADS ions is weakened, but because the combined action of temperature and NaCl is also a major factor. According to Bjerrum theory [19], when the NaCl concentration and temperature increase, ion hydration is weakened in solution. The distance between the positive and negative ions is shortened, and the electronegativity of Cl− is greater than that of the C16-MADS ionic headgroup. When the electrostatic attraction energy between Na+ and Cl− is larger than the thermal motion energy, a relatively stable ion association solution can be formed, called an ion pair, reducing the number of counterions in the C16-MADS ionic headgroup, increasing the electronegativity and electrical repulsion of the C16-MADS ionic headgroup above that of traditional anionic surfactants, so Γ max decreases. This is also consistent with decreasing detergency with increase of temperature [20].
Thermodynamic Parameters of Micellization
For 2–1-type ionic surfactants with an excess of inorganic salt, the free energy of micellization (\(\Delta G_{\text{m}}^{ \circ }\)), enthalpy (\(\Delta H_{\text{m}}^{ \circ }\)), and entropy (\(\Delta S_{\text{m}}^{ \circ }\)) can be calculated by the thermodynamic functions [15, 16]
The micellization thermodynamics of C16-MADS are given in Table 2. \(\Delta G_{\text{m}}^{ \circ }\) is negative at all temperatures, which suggests that micellization can occur spontaneously. \(\Delta H_{\text{m}}^{ \circ }\) is the micelle formation heat, which is an important thermodynamic parameter for the micelle formation process [21, 22]. \(\Delta H_{\text{m}}^{ \circ } < 0\) suggests that micelle formation is an exothermic process, because the C16-MADS molecule loses translational energy when micelles form, and the heat released from the interaction of the hydrocarbon chains exceeds that from the “iceberg structure.” The decrease of \(\Delta H_{\text{m}}^{ \circ }\) indicates that C16-MADS molecules have a stronger tendency to form micelles spontaneously in solution [23]. The \(\Delta H_{\text{m}}^{ \circ }\) of C16-MADS solution is small when C NaCl = 0.50 mol L−1, so its CMC is small. The entropy (\(\Delta S_{\text{m}}^{ \circ }\)) reflects the changes of disorder in the process of transition state formation. All the \(\Delta S_{\text{m}}^{ \circ }\) values are positive, which means that it is easy to carry out the process in which C16-MADS molecules are added to the micelles. In aqueous solution, water molecules around C16-MADS molecules can form an ordered region, which is called the “iceberg structure.” After micelles are formed, the “iceberg structure” around the molecules disintegrates, and the disorder of the system is increased, so the \(\Delta S_{\text{m}}^{ \circ }\) value becomes positive. It can also be seen from Table 2 that \(\Delta S_{\text{m}}^{ \circ }\) decreases with increasing temperature. This is because the micellization tendency of C16-MADS weakens when the temperature increases. Meanwhile, the absolute value of \(\Delta H_{\text{m}}^{ \circ }\) is smaller than the absolute value of \(- T\Delta S_{\text{m}}^{ \circ }\), so the formation process of C16-MADS micelles is a mainly entropy-driven process.
Measurements of Micellar Aggregation Number
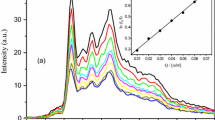
Solutions of C16-MADS at different concentrations were prepared using pyrene-saturated water solution as solvent with quencher concentration of 1.0 mmol L−1, and the micellar aggregation number (N m) was determined by the molecular fluorescent probe method. The results are shown in Table 3.
For traditional surfactants, the appropriate N m of spherical micelles can be measured when the surfactant concentration is within 10 times of the CMC. For C16-MADS, the measured N m was very small when the concentration was low. There are two kinds of forces that control surfactant aggregation. One is the hydrophobic interaction between alkane chains, which is the force that drives surfactant molecules to spontaneously form orderly aggregates. The other is the repulsive force between ion headgroups, which is caused by electrostatic repulsion or hydration layer resistance. For C16-MADS, the force of the latter is larger than that of the former, and the two hydrophilic groups linked by a rigid group have no bending flexibility. The cross-sectional area of each molecule is large, which hinders the formation of close aggregation. So C16-MADS tends to be adsorbed at the interface (surface) when the concentration is low, and the tendency to form micelles is relatively weak. The arrangement of surfactant molecules which contribute to micelle formation is loose, so it cannot solubilize enough probe and quencher. This is also the main reason why C16-MADS behaves differently from conventional surfactants. It is also different from other gemini surfactants, which exhibit a strong tendency for micellization in aqueous solution [24]. For this reason, N m had to be measured at a higher concentration range.
The micellar aggregation number (N m) measured at different temperatures versus concentration is shown in Fig. 3. The micellar aggregation number increases with increasing concentration. N m is not sensitive to temperature variations when the surfactant concentration is from 1.32 × 10−2 to 6.68 × 10−2 mol L−1, which shows that temperature has little influence on the surfactant. However, N m changes significantly with temperature when the concentration reaches 1.10 × 10−1 mol L−1. This is probably due to a sphere to rod transition.
Critical Micellar Aggregation Number
In this work, the critical micellar aggregation number [N m] of C16-MADS was obtained by extrapolating the aggregation number to the CMC. As shown in Table 4, the [N m] of C16-MADS is much lower than that of sodium dodecyl sulfate (SDS). This also shows that the force between headgroups of the double hydrophilic group surfactant is strong. The micellization tendency is relatively weak when the solution concentration is low. At the same time, the effect of temperature on the [N m] of C16-MADS is also less than the effect on the [N m] of SDS. So, the C16-MADS solution is stable at low concentration (Table 5).
Relationship Between Micellar Aggregation Number and NaCl Concentration
For traditional ionic surfactants with a single hydrophilic group, there is an electrical double layer around the micelle with a hydration film formed by water molecules. The hydration film is destroyed with addition of NaCl, and the diffuse double layer around the ionic groups is compressed, which increases N m significantly. Chen Jingyuan [26–28] showed that the N m of SDS increases sharply from 63 to 176 when the concentration of NaCl is 0.6 mol L−1. Figure 4 shows that the N m value of C16-MADS is not significantly affected by addition of NaCl. The double hydrophilic group of C16-MADS and the hyperconjugation make the interaction between molecular electric charges larger, and steric effects also prevent molecules outside the micelles from entering. So NaCl has little effect on the N m, which illustrates that C16-MADS has excellent salt resistance properties. This is a significant feature of the surfactant, which also explains why it can be applied in special areas.
Changes of I 1/I 3
I 1 and I 3 were obtained from the pyrene fluorescence spectra. The micropolarity of micelle nucleus around the pyrene probe can be reflected by the ratio of I 1 and I 3 [29, 30]. Pyrene should be solubilized in the palisade layers near the surfactant polar head. Increase of the concentration of C16-MADS and the micellar aggregation number is likely to cause the surfactant molecules to pack more closely, which excludes water from the palisade layer. Meanwhile, pyrene molecules can transfer to the micelle interior, making its microenvironment less polar, so the value of I 1/I 3 decreases with increasing concentration. Temperature has almost no influence on the I 1/I 3 ratio of C16-MADS. The micropolarity of micelle nucleus of C16-MADS is stronger than SDS, which shows that the polarity of the former is greater than that of the latter, and this also agrees with the molecular structure of C16-MADS.
Conclusions
For certain NaCl concentrations, the CMC of C16-MADS solution increases slightly with increasing temperature, while Γ max decreases. The CMC decreases significantly with increasing NaCl concentration while γ cmc remains essentially unaffected. At 298.0 and 303.0 K, the Γ max of C16-MADS solution increases with increasing NaCl concentration. When the temperature is 308.0, 313.0, and 318.0 K, the Γ max of C16-MADS solution shows a surprising decrease with increasing NaCl concentration. The thermodynamic parameters of micellization of C16-MADS have been calculated, showing that the process of micellization of C16-MADS is a spontaneous entropy-driven process.
C16-MADS has a strong tendency to adsorb at the air–water interface, and the tendency for forming micelles is relatively weak. The micellar aggregation number is about half those of traditional anionic surfactants, while the micropolarity of the micellar nucleus is larger. Inorganic salt has little effect on N m. When the surfactant concentration is in the range from 1.32 × 10−2 to 1.10 × 10−1mol L−1, the micellar aggregation number increases linearly with increasing concentration, and the micropolarity of micelle nucleus decreases. In addition, temperature has little effect on N m in this range.
References
Rosen MJ, Zhu ZH, Hua XY (1992) Relationship of structure to properties of surfactants. 16. Linear decyldiphenylether sulfonates. JAOCS 69(1):30–33
Liu J (1995) Alkyldiphenyl oxide disulfonates—a new type of high efficiency surfactants. Si Chuan Deterg Cosmet 1:33–38
Bob DT (1991) Reduced adsoption and separation of blended surfactants on sand and clay. J Can Pet Technol 30(2):133–137
Hongzoon KT (1985) Photographic developer composition. US Patent 4565776
Ishii TN, Sasaki K (1987) Alkyldiphenyl ether disufonic acid disodium salts as dispersing agents for wettable pesticides. JP Patent 6299356
Wada TY, Yonemura S (1987) Diphenyl ether sulfonate plant fungicides. JP Patent 62169704
Quencer LB (1996) The detergency properties of mono and disufonated diphenyl oxide surfactants. In: Proceedings of the 4th world surfactant congress. Barcelona, pp 192–201
Loughne TJ, Quencer L (1992) Surfactants for high-performance cleaning. SCCS 68(1):24–30
Doughty DA (1979) Isopiestic compositions of aqueous ionic surfactant systems as a measure of preferential interactions. Application to the determination of micelle aggregation numbers by equilibrium ultracentrifugation. J Phys Chem 83(20):2621–2628
Turro NJ, Yekta A (1978) Luminescent probes for detergent solutions. A simple procedure for determination of the mean aggregation number of micelles. J Am Chem Soc 100(18):5951–5952
Rosen MJ (2004) Surfactants and interfacial phenomena. Wiley, NewYork
Xu HJ, Lv CX, Ye ZW (2005) Preparation and properties of alkyldiphenyl ether disulfonate. Fine Chem 22(1):19–22
Kalyanasundaram K, Thomas JK (1977) Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc 99:2039–2044
Mats A, Shanti S (1983) Size of sodium dodecyl sulfate micelles in the presence of additives. J Colloid lnterface Sci 91(1):256–266
Zhao GX, Zhu BY (2003) Principles of surfactant action. China Light Industry Press, Beijing
Manilal DA, Rosen MJ (1986) Relationship of structure to properties of surfactants. 13. Surface and thermodynamic properties of some oxyethylenated sulfates and sulfonates. J Phys Chem 90:2413–2418
Yan Y, Huang JB, Li ZC (2002) Surface property and aggregation behavior of anionic bolaform surfactant/C12Et3 mixed system. Acta Chim Sin 60(7):1147–1150
Frumkin A (1925) Surface tension curves of the higher fatty acids and the equation of condition of the surface layer. Z Phys Chem 116:466–484
Liu YP (1979) Physical chemistry. China People Education Press, Beijing
Li GZ, Sui H, Zhu WZ (1999) Progress of surfactant study. Chin Surfact Deterg Cosmet 1:24–26
Rosen MJ, Zhu ZH, Gao T (1993) Synergism in binary mixture of surfactants. 11. Mixture containing mono-and disulfonated alkyl-and dialkyldiphenylethers. J Colloid Interface Sci 157(1):254–259
Li YH, Huang JB, Wang CZ (2002) The surface chemical properties of hydrolytic surfactants. In: Proceedings of 2002 international conference on surfactants and detergents. Shen Zhen, pp 195–199
Lana M, Nita M, Pranab KB (1997) Thermodynamics of formation of biological microemulsion (with cinnamic alcohol, Aerosol OT, Tween 20, and water) and kinetics of alkaline fading of crystal violet in them. J Colloid Interface Sci 186(1):1–8
Zhao JX (1999) A new generation of surfactants: geminis. Prog Chem 11(4):348–357
Fang Y, Liu XF, Xia YM (2011) Determination of critical micellar aggregation numbers by steady-state fluorescence probe method. Acta Physicochim Sin 17(9):828–832
Chen JY, Wang GT, Liu JZ (1993) Investigation on the determination of micellar aggregation number by steady-stable fluorescence quenching method. Acta Physicochim Sin 9(4):461–465
Kratohvil JP (1980) Comments on some novel approaches for the determination of micellar aggregation numbers. J Colloid Interface Sci 75(1):271–275
Chen JM, Su TM, Mou CY (1986) Size of sodium dodecyl sulfate micelle in concentrated salt solutions. J Phys Chem 90:2418–2421
Raoul Z, Martin I, Helene L (1997) Alkanediyl-α, ω-bis(dimethylalkylammonium bromide). T. Fluorescence probing studies of micelle micropolarity and microviscosity. Langmuir 13:5552–5557
Huang JB, Zhao GX, Jiang YC (1993) Fluorescence probe study on the mixed catanionic surfactants. Acta Physicochim Sin 9(5):577–580
Acknowledgments
We are grateful for the financial support from the Sinopec Group Jinlin Petrochemical Company and the Key Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Xu, H., Xu, K. & Wang, D. Surface Chemical Properties and Micellization of Disodium Hexadecyl Diphenyl Ether Disulfonate in Aqueous Solution. J Surfact Deterg 18, 1073–1080 (2015). https://doi.org/10.1007/s11743-015-1741-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1741-3