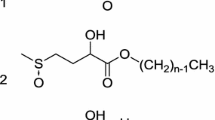
Abstract
2-Hydroxy-4-(methylthio)butanoic acid (HMTBA) is a commonly used animal feed additive available in large quantities. In this study, anionic surfactants were synthesized utilizing HMTBA as a starting material. Specifically, a straight-chain fatty acid containing 12 or 16 carbon atoms was attached to the hydroxyl group via esterification. After neutralization of the carboxylic acid with sodium, the molecules behave as anionic surfactants. Oxidation of the sulfur atom can be performed to further increase water solubility. The molecules exhibit critical micelle concentrations (CMCs), and lower the surface tension to 35–45 mN/m at the CMC. The derivatives have low Krafft points (<4 °C) and good wetting performance. The hardness tolerance of the ester made from dodecanoic acid is ~2.5–4 orders of magnitude higher than an analogous carboxylate surfactant, namely sodium dodecanoate. Foam created according to the Ross-Miles foam test is substantial, but dissipates quickly as compared to other anionic surfactants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anionic surfactants are essential in various applications, especially detergency [1, 2]. Anionic surfactants with carboxylic acid moieties have been used for thousands of years; soap was first made from the hydrolysis of naturally-occurring oils/fats. Fatty-acid surfactants have an excellent combination of properties including low CMC, low surface tension at the CMC, good foaming characteristics and mildness when applied to the skin. However, the 20th century saw the use of these surfactants relative to other anionic surfactants decrease significantly. The most significant disadvantage of these surfactants as opposed to other anionic surfactants is their propensity to precipitate when divalent cations, e.g., calcium and magnesium, are present; in other words fatty-acid surfactants have inferior hardness tolerance. Numerous efforts have been made to understand how to formulate hardness tolerant detergents: for example hardness tolerant anionic surfactants [3–15], mixed surfactant systems [16–19], builders which capture hardness ions [2, 20, 21], and microemulsion based formulations insensitive to water hardness [2, 22].
One well-utilized strategy to make hardness tolerant anionic surfactants is to add branches to the hydrophobic chain [3, 13]. However, early attempts to produce hardness tolerant soaps such as highly-branched alkylbenzene sulfonates made from polypropylene failed because of poor biodegradability [23, 24]. Less recalcitrant branching in the surfactants was then investigated, for example, a lower degree of branching in the hydrophobic group. Laurent et al. [7–9] developed high-solubility surfactants with mid-chain branched hydrophobic groups with low number of methyl groups. Another strategy to make hardness tolerant anionic surfactants is to use a hardness tolerant hydrophilic group, or multiple hydrophilic groups. Stirton et al. [12] investigated the high calcium stability of the sodium salts of alpha-sulfonated fatty acid methyl esters. El-Sukkary et al. [4] synthesized and tested N-acylethylenediamine triacetic surfactants with ability to chelate multivalent ions. Hong et al. [6] studied the low precipitation tendency of trisodium salt of 2-(2-carboxyethyl)-3-decyl maleic anhydride in the presence of various multivalent ions. 2-hydroxy-4-(methylthio)butanoic acid (HMTBA) has the potential to combine both the aforementioned structural strategies in one molecule, because this molecule contains both hydroxyl and carboxylic acid functionalities where there is a sulfur-containing branch between the two (see far left structure of Scheme1). This particular compound is a hydroxyl analog of methionine which converts to l-methionine in the animal and thus it is produced commercially in large quantities to be used as supplement in animal feed [25]. By esterifying the alcohol and deprotonating the acid group, a branched sodium carboxylate surfactant was generated. The hydrophobic chain is a straight chain, and hence has the highest possible aerobic biodegradability. Furthermore, the ester linkage is labile via chemical or enzymatic hydrolysis.
In the current study, three anionic surfactants were synthesized from HMTBA. Their purity was characterized with high performance liquid chromatography (HPLC), and structure with 1H NMR. Their surfactant properties, such as water solubility, CMC, surface tension at CMC (γ CMC), Krafft temperature, calcium tolerance, foaming ability and wetting performance were investigated and compared with anionic surfactants having similar structures. The effect of the branching and the oxidation state of the sulfide on the performance of the molecules as surfactants is explored by comparing the results to alkylcarboxylate salts.
Experimental
Materials
HMTBA was manufactured by Novus International. Lauroyl chloride (>98 %), palmitoyl chloride (98 %), hydrogen peroxide (30 %), hydrochloride acid (37 %), sodium chloride (ACS reagent grade, ≥99 %), meta-chloroperoxybenzoic acid (m-CPBA, 77 %), 4-dimethylaminopyridine (DMAP, 99 %), triethylamine (Et3N, >99 %), hexanes (HPLC grade), dichloromethane (DCM, HPLC grade), weak acid ion-exchange resin Dowex MAC-3, sodium hydroxide (>97 %), sodium bisulfite (ACS reagent grade), ethanol (>99 %) and methanol (>99 %) were purchased from Sigma-Aldrich. Ethyl acetate (EA, 99.6 %), acetonitrile (HPLC grade, ≥99 %) and Heptane (HPLC grade) were purchased from J. T. Baker. Glacial acetic acid (AcOH, Certified ACS grade), sulfuric acid (Certified ACS Plus grade) and magnesium sulfate (anhydrous, 97 %) were purchased from Fisher Scientific. SiliaPrep SPE carbonate cartridges were purchased from Silicycle. All materials were used without further purification.
Synthesis of 4-(Methylsulfinyl)-2-(Palmitoyloxy)Butanoic Acid (C16ESOCOOH)
Step 1: Synthesis of 4-(Methylthio)-2-(Palmitoyloxy)Butanoic Acid (C16ESCOOH)
To 2-hydroxy-4-(methylthio)butanoic acid (10.08 g, 67.1 mmol) in DCM (200 ml) at 0 °C was added DMAP (cat.), Et3N (18.7 ml, 134 mmol) followed by palmitoyl chloride (10.2 ml, 33.6 mmol) dropwise over ~1 h. The reaction was allowed to warm to room temperature with stirring overnight. The solution was concentrated to a small volume and re-dissolved in heptane (300 ml). The organic layer was washed with 1 N HCl (3 × 100 ml), water (2 × 100 ml) and brine (1 × 100 ml), then dried over magnesium sulfate, filtered and evaporated to give 14.4 g of a white solid. A portion (7 g) of this solid was purified by silica gel chromatography with 0–25 % EA/Heptane and 1 % AcOH additive to give a white solid (5.8 g, 45 %).
Step 2: Synthesis of 4-(Methylsulfinyl)-2-(Palmitoyloxy)Butanoic Acid (C16ESOCOOH)
To a solution of 4-(methylthio)-2-(palmitoyloxy)butanoic acid (5.8 g, 14.9 mmol) in methanol (30 ml) at 0 °C was added hydrogen peroxide (30 %, 4.57 ml, 44.8 mmol). The reaction was warmed to room temperature and stirred for ~5.5 h. The reaction was diluted with 200 ml of DCM and washed with water (1 × 100 ml). An emulsion formed which was allowed to separate overnight. The organic layer was washed with 10 % sodium bisulfite (1 × 100 ml), dried over magnesium sulfate, filtered and evaporated to give a white solid. The solid was purified by silica gel chromatography with 2–10 % MeOH/DCM containing 1 % AcOH additive to give a white solid (4.38 g, 73 %). 1H NMR (500 MHz, DMSO-d 6) δ ppm 0.85 (t, J = 6.83 Hz, 3 H) 1.09–1.35 (m, 24 H) 1.44–1.61 (m, 2 H) 2.01–2.25 (m, 2 H) 2.29–2.40 (m, 2 H) 2.54 (s, 3 H) 2.62–2.94 (m, 2 H) 4.96 (dd, J = 8.27, 4.45 Hz, 1 H) 13.06–13.36 (m, 1 H).
Synthesis of 4-(Methylsulfinyl)-2-(Dodecanoyloxy)Butanoic Acid (C12ESOCOOH)
Step 1: Synthesis of 4-(Methylthio)-2-(Dodecanoyloxy)Butanoic Acid (C12ESCOOH)
Prepared in a similar manner as C16ESCOOH to give a white solid (1.22 g, 11 %). 1H NMR (500 MHz, DMSO-d 6) δ ppm 1.83 (t, J = 6.83 Hz, 3 H) 2.13–2.30 (m, 16 H) 2.49 (d, J = 6.99 Hz, 2 H) 2.90–3.00 (m, 2 H) 3.02 (s, 3 H) 3.32 (d, J = 5.72 Hz, 2 H) 3.51 (d, J = 7.95 Hz, 2 H) 5.87–5.95 (m, 1 H).
Step 2: Synthesis of 4-(Methylsulfinyl)-2-(Dodecanoyloxy)Butanoic Acid (C12ESOCOOH)
This material was prepared via an alternate synthesis (see supplemental materials) with the desired product produced as a white solid (2.78 g), although it is assumed that this material could also be synthesized in a similar manner as C16ESOCOOH. 1H NMR (500 MHz, DMSO-d 6) δ ppm 0.86 (t, J = 6.68 Hz, 3 H) 1.25 (s, 17 H) 1.48–1.61 (m, 2 H) 2.02–2.25 (m, 2 H) 2.30–2.42 (m, 2 H) 2.56 (s, 3 H) 2.62–2.93 (m, 2 H) 4.97 (dd, J = 8.11, 4.29 Hz, 1 H).
Preparation of the Sodium Carboxylate Salts
Dowex MAC-3 resin (proton form) was swollen and neutralized with excess NaOH/water solution, and then assembled into a column. After the resin was balanced with 2 column volumes of 30 vol% water in ethanol, 1 column volume of a 0.1 g/ml acid in 30 vol% water in ethanol solution was loaded and incubated in the column for 10 min. Subsequently, the resin was rinsed with 2 column volumes of 30 vol% water in ethanol, and the eluent was collected. The solvent was then evaporated under a vacuum with mild heat (40 °C) to provide the sodium carboxylate salts.
Characterization
1H-nuclear magnetic resonance (1H-NMR) spectra were recorded on a Bruker 500 MHz NMR instrument with DMSO-d6 as solvent.
HPLC was performed in an Agilent 1260 system equipped with a diode array detector monitoring 210 nm using acetonitrile/2.5 mmol H2SO4 solvent and Dionex Acclaim® Organic Acid (OA 5 µm, 120 Å, 4 × 150 mm) column.
Surfactant Characterization
Solubility
Solubility of the surfactants in water was assessed visually at room temperature. The mixture at each concentration was examined after at least 10 min of mild shaking and ultrasonic bath agitation to see if the surfactant had completely dissolved. Solutions were observed for 2 h after mixing to ascertain the presence of supersaturation.
Surface Tension
Surface tension was determined with a Wilhelmy Plate tensiometer (Cahn DCA-322) at room temperature. Glass slides manufactured by Corning with a width of 22 mm and thickness of 0.1 mm were used as probes. The motor speed was set to be 100 µm/s. The CMC was determined from the break point of γ vs log C diagram with a custom Microsoft Visual Basic program.
Krafft Temperature
The Krafft temperature of the surfactant with the concentration at the room temperature CMC was determined by cooling the solution to 4 °C and then raising the temperature by 1 °C steps and visually inspecting its phase behavior after the solution reached thermal equilibrium [26]. For hardness tolerance, a known amount of calcium chloride was added to the solution prior to cooling to 4 °C. The solution was then heated to 25 °C and the solubility was assessed visually.
Foaming
The Ross-Miles foam test was performed according to the test protocol described in ASTM D1173-07 [27]. First, 50 ml of surfactant solution was carefully poured into the 1-m glass column, without creating any foam, which is called the receiver. A 200-ml pipette with the surfactant solution was placed 90 cm above the receiver and the solution was allowed to drop into the foam receiver. The height of the foam produced was measured immediately and after 5 min.
Wetting
The Draves wetting test was performed according to ASTM D2281-68 [28]. First, 500 ml of surfactant solution was poured into a 500 ml graduated cylinder (38 cm in height), and a 5.0 g standard skein hooked with a lead anchor was dropped into the solution. The skein floats in the solution initially and sinks when wetted, and the time taken was recorded as the time of wetting.
Results and Discussion
Synthesis
The synthetic scheme is shown in Scheme 1. Surfactants were prepared by a two-step process comprising an esterification reaction followed by oxidation. Initially, the oxidation was performed using m-CPBA as the oxidant. This reagent necessitated the use of a protection/deprotection strategy to achieve the removal of by-products from m-CPBA and resulted in a more lengthy synthesis. It was found that hydrogen peroxide was effective at converting the sulfide to the sulfoxide as shown in Scheme 1. This oxidant eliminates the need for a protection/deprotection strategy resulting in the two step synthesis shown. In this manner, C12ESCOOH, C12ESOCOOH and C16ESOCOOH can be prepared. According to the 1H NMR, the impurity based on unreacted fatty acids are less than 5 mol%, assuming unreacted HMTBA is fully removed in the wash.
Following the preparation of the acetylated HMTBA derivatives, the carboxylic acids were converted to sodium salts using ion-exchange chromatography using the general method outlined in the experimental section. The pH of the 1 wt% solutions/suspensions of the solids increased from 4 to 8 after passing through the column. Additionally, the sodium salts were fully soluble in water (deionized with 18 MΩ resistivity), as seen in Table 1, while the carboxylic acids were insoluble. Both of the above results indicate that full conversion to the sodium carboxylate salt was achieved. The molecular structures of the synthesized salts are presented in Fig. 2. In this manner, sodium 2-dodecanoloxy-4-(methylthio)butanoate (C12ESCOONa), sodium 2-dodecanoloxy-4-(methylsulfinyl)butanoate (C12ESOCOONa) and 2-hexadecanoloxy-4-(methylsulfinyl)butanoate (C16ESOCOONa) were synthesized.
To ensure that ester hydrolysis had not taken place during the formation of sodium salts using ion exchange, the sodium salts were analyzed by HPLC. Figure 1 shows the HPLC of C12ESOCOOH and C12ESOCOONa with H2SO4 present in the HPLC solvent. Evidence of hydrolysis is characterized by the appearance of the sulfoxide of 2-hydroxy-4-(methylsulfinyl)butanoic acid which has a retention time of 1.6 min. As can be seen from Fig. 1, only a small amount of 2-hydroxy-4-(methylsulfinyl)butanoic acid was present after ion exchange; i.e., ion exchange chromatography had little effect on the integrity of the ester. Also of note are that the sulfone and the sulfide did not elute at the same time as the sulfoxide, and hence Fig. 1 also shows that the conversion to sulfoxide was essentially complete and over-oxidation did not occur.
Surface Chemical Properties
Water/air interfacial properties of C12ESCOONa, sodium 2-dodecanoloxy-4-(methylsulfinyl)butanoate (C12ESOCOONa) and 2-hexadecanoloxy-4-(methylsulfinyl)butanoate (C16ESOCOONa) are presented in Table 1 in comparison to anionic surfactants with similar structures, including the linear carboxylates, sodium dodecyl sulfate (SDS) and sodium dodecylbenzene sulfonate (SDBS). The surface chemical properties of C12ESCOONa, C12ESOCOONa and C16ESOCOONa were computed from the surface tension data in Fig. 3. Undershoots near the CMC values are attributed to the presence of a small amount of impurity, likely carboxylic acids and their sodium salts from the residue of the esterification reaction and/or hydrolysis during neutralization. The magnitudes of the dip are large for the C12 species, especially for the C12ESOCOONa. However, the surface tension value at the dip, 30 mN/m, roughly matches the value for sodium dodecanoate at the CMC (see Table 1) as one would expect. Because of the large deviation from a regular surface tension isotherm, the discussion on surface adsorption profile of C12ESOCOONa should be limited to qualitatively instead of quantitatively. In particular, the values in Table 1 are suspect with respect to this molecule.
Relative to sodium laurate (NaL) (0.02 g/ml) and sodium myristate (NaMy) (0.0015 g/ml), the solubility of C12ESCOONa is much higher (0.1 g/ml). Comparing the molecular structure of C12ESCOONa and NaMy in Fig. 2, C12ESCOONa has an additional ester group on the main chain and a methyl-sulfur-ethyl group as a branch. This combination increased the solubility by ~2 orders of magnitude while lowering the CMC by a factor of ~4. The sulfur-containing branch interferes with intermolecular arrangement and hence considerably increases the solubility. At the same time, the three additional hydrocarbon units reduce the CMC significantly.
All critical micelle concentrations of the sulfoxide/sulfide carboxylate surfactants based on HMTBA are lower than that of SDS, SDBS, NaL, and NaMy measured at the same temperature, or sodium palmitate (NaPa) at higher temperatures. Sulfoxide/sulfide carboxylate surfactants possess relatively high pC20, indicating high efficiency in lowering the surface tension, even though their surface tensions at the CMC and surface excess concentrations are not superior to other anionic surfactants, suggesting comparable effectiveness of surface adsorption. One important parameter is CMC/C20, which is an indicator of the tendency of liquid–air adsorption versus micelle formation (the higher the value, the more favored is surface adsorption). The reason that sulfoxide/sulfide carboxylate surfactants favor micelle formation more than its carboxylate counterpart is likely a result of the packing factor of sulfoxide/sulfide carboxylate surfactants being smaller than molecules with a straight-chain hydrophobe. The anomaly is C16ESOCOONa, which has a high surface area per molecule (i.e., low effectiveness), and a very high pC20 (i.e., high efficiency). The latter was expected since the carbon chain is longer, but the former is less likely since a longer hydrophobe usually increases the adsorption effectiveness. Our suspicion is that surface active impurities in this sample interfered with surface arrangement of C16ESOCOONa although the dip in surface tension at the CMC is not as large for this surfactant as for the other surfactants.
The comparison between C12ESCOONa and C12ESOCOONa represents the difference between sulfide and sulfoxide functional groups. Sulfur oxidation increased the water solubility and CMC, which was expected. Although molecular adsorption below the CMC is potentially disturbed by surface-active impurities, the CMC of C12ESOCOONa is still lower than the suspected impurity, sodium dodecanoate.
Precipitation Tendencies
The precipitation tendencies of sulfoxide/sulfide carboxylate surfactants are presented in Table 2, in comparison with common anionic surfactants. Within 48 h, 1 wt% solutions of C12ESCOONa, C12ESOCOONa or C16ESOCOONa did not show precipitation in the 4 °C water bath, and hence the Krafft points of all three sulfoxide/sulfide carboxylate surfactants were lower than 4 °C. Based on the reported data, the sulfoxide/sulfide carboxylate surfactants have better low temperature operability than SDS (Krafft point at 15 °C). In terms of hardness tolerance, the C12ESCOONa solution showed precipitation with CaCl2 only above 100 µM. NaL and SDS were also tested; the results show that the C12ESCOONa has approximately 2.5 orders of magnitude better calcium tolerance than the linear carboxylate (which agrees with the water solubility data) and about half an order of magnitude better than the linear sulfate, SDS. This improved calcium tolerance is a combined effect of the increased hydrogen bonding due to the ester group, and the branching in the head group. Oxidation of the sulfur atom further improved its hardness tolerance. C12ESOCOONa has a hardness tolerance of 5000 mM of CaCl2, which is 50 times higher than the unoxidized form. In fact, the increased hardness tolerance from sulfur oxidation is exactly offset by 4 additional hydrocarbon units, which is observed by comparing the data of C12ESCOONa and C16ESOCOONa. Due to its good water solubility, low CMC and high hardness tolerance, C12ESOCOONa is a potential calcium tolerant anionic surfactant, and even a candidate as a builder in hard-water formulations.
Draves Wetting Test
For the Draves wetting test, 0.1 wt% solutions were used for all the surfactants. As presented in Table 3, C12ESCOONa outperforms NaL in wetting time with a comparable performance to SDS. C12ESCOONa is a larger molecule than NaL and SDS, and should have a smaller diffusion coefficient and hence a higher wetting time, but since C12ESCOONa has lower CMC, this surfactant should be less likely to experience monomer depletion in the Draves wetting test than the other two surfactants.
Ross-Miles Foam Test
For the Ross-Miles foam test, all surfactants were used at 1 wt% concentration, which is above all CMC. The results in Fig. 4 show that the C12ESCOONa generates foam comparable to the reference anionic surfactants (measured in our laboratory using the identical equipment and procedure), but dissipates much faster. Fast dissipation indicates lack of surface cohesiveness. Judging from its structure, the branching could have disturbed the surface arrangement of surfactant molecules. Quick dissipation of foams is a desirable property in low-foaming applications, such as automatic dishwashing and laundry.
Conclusions
A novel family of anionic surfactants, sodium 2-alkanoloxy-4-(methylthio)butanoate or sodium 2-alkanoloxy-4-(methylsulfinyl)butanoate, was successfully synthesized. These surfactants have good water solubility, low Krafft point, high surface tension reduction efficiency, low critical micelle concentrations and improved calcium tolerance compared to sodium laurate and sodium dodecyl sulfate. The solutions of the surfactants have comparable wetting performance as sodium dodecyl sulfate, which exceeds that of sodium laurate. The surfactant generates foam volume roughly equivalent to similar anionic surfactants, but the foam dissipates much more quickly. The combination of the improved critical micelle concentration, Krafft point, calcium tolerance, wetting time and foam dissipation show that the new surfactants have some promise in detergency formulations, especially in hard water and cold water formulations.
Abbreviations
- HMTBA:
-
2-Hydroxy-4-(methylthio)butanoic acid
- AES:
-
Alkyl ether sulfate
- SDS:
-
Sodium dodecyl sulfate
- SDBS:
-
Sodium dodecylbenzene sulfonate
- CMC:
-
Critical micelle concentration
- γ CMC :
-
Surface tension at the CMC
- Γm :
-
Maximum surface excess concentration/maximum adsorption density
- C20:
-
Surfactant concentration that lowers the surface tension by 20 mN/m
- a min :
-
Minimum area per molecule at the interface
References
Tsoler U (1999) Handbook of detergents. M. Dekker, New York
Smulders E, von Rybinski W, Sung E, Rähse W, Steber J, Wiebel F, Nordskog A (2000) Laundry detergents. Wiley, Germany
Atwood JL, Steed JW (2004) Encyclopedia of supramolecular chemistry. Marcel Dekker, New York
El-Sukkary MMA, Soliman EA, Ismail DA, El Rayes SM, Saad MA (2011) Synthesis and properties of some N-acylethylenediamine triacetic acid chelating surfactants. Tenside Surfactants Deterg 48:82–86
Fujiwara M, Miyake M, Abe Y (1993) Colloidal properties of alpha-sulfonated fatty acid methyl esters and their applicability in hard water. Colloid Polym Sci 271:780–785
Hong J-J, Yang S-M, Choi Y-K, Lee C-H (1995) Precipitation of tricarboxylic acid biosurfactant derived from spiculisporic acid with metal ions in aqueous solution. J Colloid Interface Sci 173:92–103
Laurent JCTRBS, Connor DS, Cripe TA, Dupont JS, Scheibel JJ, Stidham RE, Vinson PK, Willman KW (2000) Mid-chain branched surfactants. US Patent 6,020,303
Laurent JCTRBS, Connor DS, Cripe TA, Dupont JS, Vinson PK, Willman KW (2000) Mid-chain branched alkyl sulfate surfactants. US patent 6,060,443
Laurent JCTRBS, Connor DS, Cripe TA, Vinson PK, Willman KW (1999) Mid-chain branched alkoxylated sulfate surfactants. US Patent 6,008,181
Scheibel J (2004) The evolution of anionic surfactant technology to meet the requirements of the laundry detergent industry. J Surfactants Deterg 7:319–328
Shi J, Kloepper-Sams P, Giolando S, Federle T, Versteeg D, Belanger S (2000) Biodegradable high solubility alkyl sulfate surfactants: environmental safety profiles, in 5th World Surfactant Congress. Federchimica, Milan, pp 1525–1531
Stirton AJ, Bistline RG, Maurer EW, Weil JK, Ault WC (1962) Sodium salts of alkyl esters of alpha-sulfo fatty acids–wetting, lime soap dispersion, and related properties. J Am Oil Chem Soc 39:128–139
van Duynhoven J, Leika A, van der Hoeven R (2005) Quantitative assessment of alkyl chain branching in alcohol-based surfactants by nuclear magnetic resonance. J Surfactants Deterg 8:73–82
Xing F, Niu J, Liu X, Wang X (2014) Effect of a spacer group on surface activity, salinity and hardness tolerance, mimic oil washing efficiency of monododecyl diaryl disulfonate. J Surfactants Deterg 17:95–100
Yu D, Wang Y, Zhang J, Tian M, Han Y, Wang Y (2012) Effects of calcium ions on solubility and aggregation behavior of an anionic sulfonate gemini surfactant in aqueous solutions. J Colloid Interface Sci 381:83–88
Dawe B, Oswald T (1991) Reduced adsorption and separation of blended surfactants on sand and clay. J Can Pet Technol 30:133–137
Lad K, Bahadur A, Pandya K, Bahadur P (1995) Clouding and aggregation behavior of ethylene-oxide propylene-oxide ethylene-oxide block-copolymers in aqueous-media in the presence of sodium dodecyl-sulfate. Indian J Chem Sec a-Inorg Bio-Inorg Physical Theor Anal Chem 34:938–945
Rodriguez CH, Scamehorn JF (1999) Modification of Krafft temperature or solubility of surfactants using surfactant mixtures. J Surfactants Deterg 2:17–28
Sharma R, Desai A, Bahadur P (2003) Hardness tolerance of anionic surfactants in the presence of nonionic surfactants. Tenside Surfactants Deterg 40:31–34
Satsuki T, Nagoh Y, Yoshimura H (1998) Effect of calcium ions on detergency—part 2: interactions between a surfactant, a calcium-sequestering builder and calcium ions. Tenside Surfactants Deterg 35:112–118
Yu YX, Zhao J, Bayly AE (2008) Development of surfactants and builders in detergent formulations. Chin J Chem Eng 16:517–527
Tanthakit P, Nakrachata-Amorn A, Scamehorn JF, Sabatini DA, Tongcumpou C, Chavadej S (2009) Microemulsion formation and detergency with oily soil: V. Effects of water hardness and builder. J Surfactants Deterg 12:173–183
Sweeney W, Anderson R (1989) Biodegradability of alkylbenzene sulfonates. J Am Oil Chem Soc 66:1844–1849
Anonymous (1965) Testing for surfactant biodegradability industrial and engineering chemistry 57: 45–6
Dibner JJ, Knight CD (1984) Conversion of 2-hydroxy-4-(methylthio)butanoic acid to l-methionine in the chick—a stereospecific pathway. J Nutr 114:1716–1723
Schott H, Han SK (1976) Effect of inorganic additives on solutions of nonionic surfactants 4: Krafft points. J Pharm Sci 65:979–981
International ASTM (2007) Standard test method for foaming properties of surface-active agents. West Conshohocken, Pennsylvania
International ASTM (2010) Standard test method for evaluation of wetting agents by the Skein test. West Conshohocken, Pennsylvania
Guzman A, Bueno A, Carbognani L (2009) Molecular weight determination of asphaltenes from Colombian crudes by size exclusion chromatography (SEC) and vapor pressure osmometry (VPO). Pet Sci Technol 27:801–816
Dahanayake M, Cohen AW, Rosen MJ (1986) Relationship of structure to properties of surfactants. 13. Surface and thermodynamic properties of some oxyethylenated sulfates and sulfonates. J Phys Chem 90:2413–2418
Glukhareva NA, Pletnev MY (1995) Krafft points of some mixtures based on individual sodium soaps. Colloid J 57:253–255
Elworthy PH, Mysels KJ (1966) Surface tension of sodium dodecylsulfate solutions and phase separation model of micelle formation. J Colloid Interface Sci 21:331–347
Acevedo S, Gutierrez LB, Negrin G, Pereira JC, Mendez B, Delolme F, Dessalces G, Broseta D (2005) Molecular weight of petroleum asphaltenes: a comparison between mass spectrometry and vapor pressure osmometry. Energy Fuels 19:1548–1560
van Voorst Vader F (1960) Adsorption of detergents at the liquid-liquid interface. Part 1. Trans Faraday Soc 56:1067–1077
DeLisi R, Inglese A, Milioto S, Pellerito A (1997) Demixing of mixed micelles Thermodynamics of sodium perfluorooctanoate sodium dodecanoate mixtures in water. Langmuir 13:192–202
Ingram T, Jones MN (1969) Membrane Potential Studies on Surfactant Solutions. Trans Faraday Soc 65:297–304
Akhter MS (1997) Effect of acetamide on the critical micelle concentration of aqueous solutions of some surfactants. Colloids Surf A Physicochem Eng Asp 121:103–109
Kralchevsky PA, Danov KD, Pishmanova CI, Kralchevska SD, Christov NC, Ananthapadmanabhan KP, Lips A (2007) Effect of the precipitation of neutral-soap, acid-soap, and alkanoic acid crystallites on the bulk pH and surface tension of soap solutions. Langmuir 23:3538–3553
Jackson LP, Townsend C, Grady BP (2013) Mixtures of nonionic surfactants made from renewable resources with alkyl sulfates and sodium n-alkanecarboxylates: comparison of mixing behavior using Rubingh’s treatment. J Surfactants Deterg 16:893–902
Wen X, Franses EI (2000) Effect of protonation on the solution and phase behavior of aqueous sodium myristate. J Colloid Interface Sci 231:42–51
Campbell AN, Lakshmin GR (1965) Conductances and surface tensions of aqueous solutions of sodium decanoate sodium laurate and sodium myristate at 25° and 35°. Can J Chem 43:1729–1737
Tanaka S, Kawasaki H, Maeda H (2005) Complex formation in alkyldimethylamine oxide/sodium palmitate/water mixtures. J Colloid Interface Sci 283:238–244
Blanco E, González-Pérez A, Ruso JM, Pedrido R, Prieto G, Sarmiento F (2005) A comparative study of the physicochemical properties of perfluorinated and hydrogenated amphiphiles. J Colloid Interface Sci 288:247–260
Weil JK, Smith FD, Bistline RG, Stirton AJ (1963) Long chain alkanesulfonates and 1-hydroxy-2-alkanesulfonates—structure and property relations. J Am Oil Chem Soc 40:538–541
Stellner KL, Scamehorn JF (1989) Hardness tolerance of anionic surfactant solutions. 1. Anionic surfactant with added mono-valent electrolyte. Langmuir 5:70–77
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Yu, G., Long, S.A., Karinshak, K.A. et al. Synthesis and Characterization of Novel Surfactants Based on 2-Hydroxy-4-(Methylthio)Butanoic Acid: 1. Anionic Surfactants. J Surfact Deterg 18, 895–903 (2015). https://doi.org/10.1007/s11743-015-1690-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1690-x