Abstract
Self-piercing riveting is an established joining technique for lightweight materials. To increase the sustainability of the rivet manufacturing process, the authors of the present paper have developed an approach for shortening the process chain by omitting the heat treatment and rivet coating. To do this, use is made of high nitrogen steel as the rivet material. Successful joining with these rivets has already been proven, and it has also been shown that a competitive joint strength can be achieved with these rivets. Up until now, no studies have been conducted of the corrosion behaviour of uncoated rivets in high nitrogen steel compared to conventional rivets made of heat-treatable steel with a coating of Almac® or zinc-nickel with topcoat, and the corrosion behaviour of joints manufactured with these rivets has also not been investigated. Furthermore, the suitability of rivets in high nitrogen steel for structures undergoing cathodic dip painting has not been evaluated to date. These are therefore the aims of the research work presented in this paper. Corrosion behaviour is tested by exposing rivets and joints to a salt spray atmosphere. Cross-cut tests are conducted in order to classify the adhesion of cathodic dip paint to the different rivet surfaces and materials. The results of the experimental test show that the cathodic dip paint has sufficient adhesion to the uncoated rivets in high nitrogen steel and that these rivets can therefore be used in the manufacture of car bodies. Due to the stainless properties of the high nitrogen steel, better corrosion resistance is seen by comparison to the commonly used coatings of Almac® and zinc-nickel with topcoat. A study of the corrosion behaviour of the joints shows that the rivet head diameter and rivet head position, in particular, are decisive for preventing crevice corrosion under the rivet head and contact corrosion within the joint.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
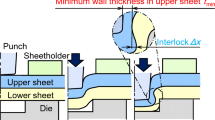
The objective of reducing greenhouse gas emissions in the transport sector is promoting the lightweight design of car bodies and the use of high-strength steel and aluminium in multi-material structures. To ensure the reliable joining of these sheet materials, mechanical joining techniques like self-piercing riveting are now well established in car body production [1]. In self-piercing riveting, two sheets are clamped between a blank holder and a die. A semitubular rivet is pressed into the sheets by a punch, see Fig. 1. The punch-sided sheet is pierced. The rivet then starts to flare, creating an interlock within the die-sided sheet [2]. The interlock is the most important joint parameter since it determines the mechanical strength of the joint. A minimum die-sided material thickness is required in order to ensure an intact closing head. Another parameter that characterises the joint quality is the rivet head position. This parameter defines how far the rivet head is pressed into the punch-sided sheet [3].
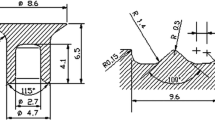
Characteristics of the self-piercing riveting and the riveted joint in cross-section according to [4]
With the self-piercing riveting low-strength sheet materials like aluminium as well as ultrahighstrength sheet materials like press-hardened steel 22MnB5 can be joined reliably [3]. Main advantages of the self-piercing riveting are the capability to join dissimilar materials and the tightness against media on the die side. In addition, the strength of self-piercing riveted joints is normally higher than the strength of welded joints [5]. The most important parts for the self-piercing riveting are the rivet and the die. They are selected in accordance with the properties of the sheets to be joined [1].
Conventional rivets are normally coated to counteract corrosion issues. Coatings of Almac® or zinc-nickel with topcoat are commonly applied to this end. Almac® consists of two layers of aluminum, zinc and tin and one sublayer of copper. The coating is applied mechanically. In a rotating drum, a metal powder, carried by glass beads, is applied to the rivets as they strike one another. There is no risk of hydrogen embrittlement with this technology. It is, however, possible to perform additional tempering [6]. Zinc-nickel with topcoat is applied in an alkaline zinc-nickel electrolyte. The galvanic process causes hydrogen to diffuse into the rivet material. The rivets are thus tempered before the topcoat is applied in a dip-spin process. The topcoat is an organic coat intended to reduce the amount of friction during the joining process. It is pre-dried at 80–100 °C and hardens at approx. 200 °C. The zinc-nickel-coating is tin-free. This reduces the danger of liquid metal embrittlement, especially compared to Almac® [7]. The main functions of the rivet coating are to protect the rivet against corrosion up to the time of cathodic dip painting and, after that, to protect the joined parts and the rivet against contact corrosion. Nevertheless, the surface coating not only affects the corrosion behaviour of the rivet and the joint. The rivet coating also influences the friction that prevails during the joining process and hence the joint parameters of interlock and minimum die-side material thickness [8]. The effects of an uncoated rivet surface on the joining process were thus studied by the authors in [9] with the finding that the friction behaviour of uncoated rivet surfaces and rivets with the commonly used Almac® coating are very similar and that reliable joining is not impeded by omitting the coating.
Normally, the joint is sealed on the die side against corrosive media by the closing head. Hence, the joint is only vulnerable on the punch side. Li et al. [10] were able to show that corrosion within the joint is commensurate with the size of cracks in the closing head. Crevice corrosion under the rivet head and contact corrosion between the rivet and the sheets inside the joint are of particular relevance [11]. Several research studies have looked into the corrosion of self-piercing riveted joints. Ioannou [12] investigated joint strength after exposure of the joint to a salt spray atmosphere. Under both quasistatic loading and cyclic loading, the joint strength was unaffected, or increased slightly, after a short exposure to salt spray. The reasons for this are a higher friction and additional locking of the joint. After longer periods of exposure to a salt spray atmosphere, the joint strength is greatly reduced. This perception is confirmed in a study conducted by Chrysanthou et al. [13]. By measuring the weight of the specimens over the exposure time, it was shown that the weight increases at the beginning of exposure to a salt spray atmosphere due to the deposition of corrosion products. Over the longer term, the weight decreases due to the degradation of the base material. Furthermore, it was shown that the weight of the specimens correlates with the joint strength. Li et al. [14] found that, even after a corrosion time of 1000 h, there was an increase in the joint strength under quasistatic load. In this case, a joint with aluminium sheets was examined. Calabrese et al., by contrast, showed in [15] and [16] that, even for pure aluminium joints, the mechanical joint strength falls in accordance with the duration of exposure in a salt spray test. Similar findings are reported by Lai et al. [17] for joints of titanium and aluminium. Abe et Mori [18] saw a decrease in mechanical joint strength for steel parts in particular. The decline was most pronounced for uncoated steel, less for zinc-plated steel and least of all for aluminium. Neugebauer et al. [19]. studied the influence of exposure of the joint to a salt spray atmosphere under cyclic loading. They found that crevice corrosion is promoted by the mechanical loading. No influence on the mechanical joint strength was found.
So far, the production of self-piercing rivets is a time-consuming and energy-intensive process. The authors have thus developed an approach for the sustainable manufacture of these rivets. Normally, the rivet manufacturing process consists of the three steps of forming, heat treatment and coating [20]. By using high nitrogen steel 1.3815 as a rivet material, a sufficient material strength can be achieved in the forming process through strain hardening. Additionally, it can be assumed that high nitrogen steel is resistant to corrosion on account of its stainless properties. By eliminating the heat treatment and the coating, it is possible to boost the efficiency of rivet production. Joining with rivets made of high nitrogen steel was studied by the authors in [21], see Fig. 2.
The rivets in high nitrogen steel 1.3815 are very similar to conventional rivets in 38B2 H4 as far as the joining process is concerned. However, the manufacturing process is very challenging due to the high forming forces required to deform the high strain hardening steel despite its elevated material strength. A two-stage forming process was developed and presented in [22]. During the first stage, the shank and foot are formed. In the second stage, the rivet head is formed. Even though the highest-performance tool materials are already being used, the forming stroke has to be reduced to prevent the forming tools from fracturing. The manufactured rivet geometry thus deviates from the intended geometry. In particular, the required rivet head diameter is not achieved. Instead of the desired head diameter of 7.75 mm, a diameter of approximately 7 mm is obtained. Further deviations, caused by a sporadic tilting of the counter punch, are seen in the foot and shank of the rivet. These deviations have a negative impact on the joint strength as was shown in [23].
The impact on the corrosion behaviour of the rivet and joint of using high nitrogen steel as a rivet material and omitting the surface coating has not, however, been examined to date. And, as the joints produced by self-piercing riveting – especially those in car bodies – are normally coated by cathodic dip painting, paint adhesion has to be ensured for conventional rivets as well as for uncoated rivets in stainless steel. The aim of the present paper is therefore the evaluation of the corrosion behaviour as well as the paintability of rivets and joints as a function of the rivet properties.
2 Materials and methods
2.1 Joining operation and experimental plan
For the examination of the corrosion behaviour and the paintability of rivets and joints, two different combinations of sheet materials are selected, see Table 1. Material combination 1 (MC 1) consists of high-strength steel HCT780X on both sides. Material combination 2 (MC 2) consists of aluminium EN AW-5083 on the punch side and steel HCT780X on the die side. All the sheets have a thickness of 1.5 mm. The steel sheets are galvanised. The surfaces of the aluminium sheets are uncoated.
In [4], commercially available rivets and dies were selected for joining these two material combinations. Furthermore, an improved rivet geometry has been developed for joining both material combinations with just a single rivet geometry. For the studies described in this paper, use was made of commercially available rivets in heat-treatable steel coated with Almac® or with zinc-nickel with a topcoat respectively, as well as uncoated rivets in high-nitrogen steel 1.3815. The commercially available rivets are made of 38B2 H4, which is a conventional heat-treatable steel that is quenched and tempered to a hardness of 480 ± 30 HV10. The high-nitrogen steel 1.3815 is a stainless steel which is known for its high strain hardening and its high corrosion resistance. The special characteristic of this material is achieved through its high nitrogen content, which is introduced into the alloy by electroslag pressure remelting [24].
2.2 Neutral salt spray test
To characterise the corrosion behaviour of the rivets and the riveted joints, the Neutral Salt Spray (NSS) test to DIN EN ISO 9227 [25] is conducted. The solution for the salt spray in the testing cabinet consists of distilled water with the properties listed in Table 2.
The specimens are placed in plastic holders where they are angled at 20 ± 5°. In this way, the corrosion products can flow off. In DIN EN ISO 9227, exposure times of up to 1008 h are recommended and the testing cabinet should only be opened for brief visual inspections. After testing, the specimens are taken out of the cabinet and dried so that the corrosion products are not removed. The spray solution residue is then removed through gentle rinsing. During testing, the condition of the specimens is visually inspected every 240 h. The time up to the first appearance of corrosion traces is documented in particular. A testing cabinet of type SKB 1000 ATR from Gebr. Liebisch GmbH & Co., Germany, is used for the study.
2.3 Cross-cut test
The paint adhesion is examined in cross-cut tests. With this test method, which is standardised in DIN EN ISO 2409 [26], it is possible to study paint adhesion as a function of the different surface and material properties of the rivets. For the tests, a grid is produced on the end face of the rivet head. This grid consists of six cuts in both directions so that 25 squares are obtained. The grid is made by a cutting edge. Paint adhesion can be classified by evaluating the delamination of the cathodic dip paint. Depending on the degree of delamination, a cross-cut rating Gt of between 0 and 5 can be achieved, where a value of 0 represents strong paint adhesion and a value of 5 fully delaminated paint.
3 Results and discussion
To evaluate the protective effectiveness of the two commonly used coatings compared to the corrosion properties of stainless steel, rivets in 38B2 H4 coated with Almac® and zinc-nickel with topcoat respectively, and also uncoated rivets in high-nitrogen steel 1.3815, are exposed to a salt spray atmosphere for 1008 h. The end face of the rivet head is chosen as a reference surface for comparing the corrosion behaviour. Due to the challenges involved in forming rivets made of high-nitrogen steel 1.3815, parts with the head geometry of conventional rivets are manufactured by turning. The end face of these parts is ground so that the surface properties are the same as for the formed rivets in high nitrogen steel. This is ensured by examining the surfaces with a confocal microscope. Thirty parts are tested for each of the three surface conditions, see Fig. 3.
The evaluation is based on the findings of Möhring who has studied the corrosion behaviour of different coatings for fasteners [6]. White deposits on the surface indicate zinc corrosion. This is possible for both coatings studied, as Almac® also contains a proportion of zinc. In the case of the red deposits, it is the base material that is affected. The protection provided by the Almac® coating only lasts 240 h. After that short time, red deposits are already visible on the surface of 28 out of 30 rivets. With zinc-nickel with topcoat, however, only slight white deposits are visible. Indeed, the proportion of rivets with this deposits is increasing in dependence with the time of exposure in salt spray atmosphere, see Fig. 4. Red rust, however, cannot be seen with rivets with zinc-nickel-coating, even after an exposure time of 1008 h. These results are in accordance with the findings of [27], who mentioned the difference between mechanically applied coatings and galvanic coatings when it comes to corrosion protection. With uncoated high nitrogen steel, no signs of corrosion are evident even after an exposure time of 1008 h. In the case of 1.3815 as rivet material no deposits can be found on the surface, evan after a exposure for 1008 h in salt spray atmosphere.
Although the general corrosion behaviour of the rivetsurfaces can be classified using the test described, the corrosion behaviour of joints also has to be studied. Due to the properties of the cathodic dip paint, painted joints are not expected to corrode. Because of that, uncoated joints are exposed to a salt spray atmosphere. In the case of material combination 1 with galvanised steel on the punch side, corrosion of the sheet material is visible after 240 h (see Fig. 5). The corrosion phenomena increase continuously during the exposure time. After 1008 h in a salt spray atmosphere, the joint is covered with corrosion deposits. The aluminium of material combination 2, however, is still intact at the end of the exposure period. As demonstrated in studies on the corrosion behaviour of rivets, it is assumed that, with the exception of rivets that are coated with Almac®, the visible corrosion of the joint is caused by the sheet material and not by corrosion of the rivet.
Rivets are extracted from the joint for further analysis of the corrosion behaviour, especially in respect of crevice and contact corrosion. With material combination 2 no corrosion is found under the rivet head, see Fig. 6. With material combination 1, by contrast, crevice corrosion may have occurred under the rivet head. There are also indications of corrosion phenomena in the area of the rivet shank. Corrosion in this area is mainly facilitated by corrosion of the galvanised steel sheet. In addition, the crevice between the rivet head and the punch-sided sheet is very large with material combination 1. The rivet cannot be pressed further into the sheets due to the rising joining force and loading of the rivet, which increases the danger of cracks within the rivet.
With material combination 2, the rivet head can be pressed slightly into the punch-sided sheet due to the lower sheet material strength. The reduced rivet head position will seal the joint so that the salt spray cannot infiltrate the joint. Another influencing factor is the rivet head diameter. Compared to conventional rivets made of 38B2 H4, the rivet head diameter of the high nitrogen steel rivet is reduced from 7.75 mm to approximately 7.0 mm. This deviation is caused by the challenges experienced during forming, as stated at the beginning. Corrosive media can infiltrate the joint more easily through the reduced rivet head diameter. This explains the slightly stronger corrosion deposits on the rivet in high nitrogen steel. Due to the stainless nature of the high nitrogen steel, as confirmed by the results shown above, these deposits constitute slight corrosion of the steel sheet material.
The purpose of the cathodic dip paint is to protect the joined parts against corrosion. This requires permanent adhesion of the cathodic dip paint to the rivet and the sheet surfaces so as to ensure protection against corrosion on the one hand and a good optical appearance on the other. The paint adhesion is thus examined in cross-cut tests following 1008 h exposure to a salt spray atmosphere. As the cathodic dip paint is adapted primarily to the surface properties of the sheets, the paint adhesion to the galvanised steel HCT780X and the uncoated aluminium serves as a reference. As can be seen in Fig. 7, the paint adhesion with the sheets under consideration is not affected by either the sheet properties or the salt spray test. The best cross-cut test rating of 0 is achieved with both sheet materials. No comparable result can be achieved with any of the rivets examined. In the case of Almac®-coated rivets and uncoated rivets in high nitrogen steel 1.3815, a cross-cut test rating of 2 can be achieved. For rivets with zinc-nickel-coating, however, the worst rating of 5 is measured, which means that there is no paint adhesion. One reason for the decreased paint adhesion could be the topcoat of the zinc-nickel-coating. The main function of the topcoat is to reduce friction. But, as can be seen from the results, the adhesion of the cathodic dip paint is also impaired by this kind of coating. With uncoated rivets in stainless steel, by contrast, the adhesion of the cathodic dip paint is similar to that with Almac®. It can thus be assumed that the application of the cathodic dip paint, which is a fundamental step in car body manufacturing, is not impeded by the use of stainless steel as a rivet material.
4 Summary and Outlook
The aim of this paper is to evaluate the corrosion behaviour of rivets and joints as a function of the rivet properties. It also sets out to determine the suitability of an uncoated rivet in high nitrogen steel for use in components that have to be painted. A study was conducted of the adhesion of cathodic dip paint to commercially available rivets in 38B2 H4 coated with Almac® and zinc-nickel with topcoat, as well as its adhesion to uncoated rivets in high nitrogen steel 1.3815. It was found that the adhesion of the cathodic dip paint is very low with the zinc-nickel coating. For the uncoated rivets in high nitrogen steel, however, paint adhesion comparable to that obtained with Almac®-coated rivets can be achieved. The use of uncoated rivets made of high nitrogen steel in car body production, where cathodic dip painting is mandatory, is thus feasible from this point of view. The stainless properties of the high nitrogen steel result in better corrosion resistance than the commonly used Almac® coating and zinc-nickel with topcoat, as is demonstrated when the coated rivets in 38B2 H4 and the uncoated rivets in high nitrogen steel are exposed to a salt spray atmosphere. With Almac®, in particular, the first signs of corrosion of the base material are already visible after 240 h, whereas with zinc-nickel with a topcoat only slight indications of zinc corrosion from the coating itself can be found after 1008 h in salt spray atmosphere. At the end of the test there are no signs of corrosion for the rivets in high nitrogen steel. By studying the corrosion behaviour of joints it can be seen that the rivet head diameter and rivet head position are especially decisive in preventing crevice corrosion under the rivet head and contact corrosion within the joint. Eliminating the geometric deviations of formed rivets in high nitrogen steel still remains an issue and will be the subject of future research.
References
Mori K, Abe Y (2018) A review on mechanical joining of aluminium and high strength steel sheets by plastic deformation. Int J Lightweight Mater Manuf 1:1–11
Ang H (2021) An overview of selfpiercing riveting process with Focus on Joint Failures, corrosion issues and optimisation techniques. Chin J Mech Eng 34:2
Technical Bulletin DVS (2019) Self-pierce riveting - overview, vol 3410. DVS Media, Düsseldorf
Uhe B, Kuball CM, Merklein M, Meschut G (2020) Improvement of a rivet geometry for the self-piercing riveting of high-strength steel and multi-material joints. Prod Eng 14:417–423
Briskham P, Blundell N, Han L, Hewitt R, Young K (2006) Comparison of self-pierce riveting, resistance spot welding and spot friction joining for aluminium automotive sheet. SAE Technical Papers 2034
Möhring J (2011) Qualifizierung von Fügeelementbeschichtungen für den Einsatz des druckluftbetriebenen Bolzensetzens im Karosserierohbau, PhD Thesis, Paderborn University
Barkhausen F, Hornborstel N (2015) Semi-Tubular Rivet Element, Patent WO2015/ 106779A1
Karim A, Jeong T, Noh W, Kim C, Kam D (2020) Joint quality of self-piercing riveting (SPR) and mechanical behavior under the frictional effect of various rivet coatings. J Manuf Process 58:466–477
Uhe B, Kuball CM, Merklein M, Meschut G (2023) Approach for a sustainable process chain in manufacturing of fasteners for mechanical joining. Mater Res Proc 25:397–404
Li D, Han L, Chryssanthou A, Shergold M (2018) Influence of corrosion of self-piercing riveted high strength Aluminium Alloy joints with Button cracks on the mechanical strength. J Mater Sci Technol 5:16–27
Regener D, Hahn O, Göllner J, Hußmann D (2007) Füge- Und Korrosionsuntersuchungen an Stanznietverbindungen Aus Chrom-Nickel-Stahl und oberflächenveredelten Feinblechen. European Research Association for Sheet Metal Working. Research report no. 263
Ioannou J (2009) Mechanical behaviour and corrosion of interstitial-free steel to aluminium alloy self-piercing riveted joints. PhD Thesis, University of Hertfordshire
Chrysanthou A (2014) Corrosion behaviour of self-piercing riveted joints. In Chrysanthou, A, Sun X (Eds.): Self-piercing riveting - Properties, processes and applications. Cambridge: Woodhead Publishing 41–55
Li D, Han L, Chryssanthou A, Shergold M, Williams G (2014) The Effect of Setting Velocity on the Static and Fatigue Strengths of Self-Piercing Riveted Joints for Automotive Applications. In TMS 2014: 143rd Annual Meeting & Exhibition
Calabrese L, Bonaccorsi L, Proverbio E, Di Bella G, Borsellino C (2013) Durability on alternate immersion test of self-piercing riveting aluminium joint. Mater Des 46:849–856
Calabrese L, Proverbio E, Pollicino E, Galtieri G, Borsellino C (2015) Effect of galvanic corrosion on durability of aluminium/steel self-piercing rivet joints. Corros Eng Sci Techn 50(1):10–17
Lai J, Huang Z, Tang N, Hu Z, Jiang Y (2022) Insight of Salt Spray Corrosion on Mechanical properties of TA1-Al5052 self-piercing riveted Joint. Materials 15:8643
Abe Y, Mori K (2021) Mechanical clinching and self-pierce riveting for sheet combination of 780-MPa high-strength steel and aluminium alloy A5052 sheets and durability on salt spray test of joints. Int J Adv Manuf Technol 113:59–72
Neugebauer R, Mauermann R, Grützner R (2012) Einfluss Von Kombinierter mechanisch-medialer Beanspruchung Auf die Schwingfestigkeit Von stanz- und blindgenieteten Mischverbindungen. European Research Association for Sheet Metal Working. Research report no. 348
Li D, Chrysanthou A, Patel I, Williams G (2017) Self-piercing riveting - a review. Int J Adv Manuf Tech 92:1777–1824
Uhe B, Kuball CM, Merklein M, Meschut G (2021) Self-Piercing Riveting Using Rivets Made of Stainless Steel with High Strain Hardening. In Daehn G, Cao J, Kinsey B, Tekkaya E, Vivek A, Yoshida Y (Eds) Forming the Future - Proceedings of the 13th International Conference on the Technology of Plasticity, The Minerals, Metals & Materials Series 1495–1506
Kuball CM, Uhe B, Merklein M, Meschut G (2020) Process design for the forming of semi-tubular self-piercing rivets made of high nitrogen steel. Procedia Manuf 50:280–285
Uhe B, Kuball CM, Merklein M, Meschut G (2021) Strength of Self-Piercing Riveted Joints with Conventional Rivets and Rivets Made of High Nitrogen Steel. In Proceedings of the ESAFORM 2021–24th International Conference on Material Forming. Liège, BE
Gavriljuk VG, Berns H (1999) High Nitrogen steels: structure, Properties, manufacture, applications. Springer, Berlin
DIN EN ISO 9227 (2017) Corrosion tests in artificial atmospheres - Salt spray tests. Beuth: Berlin
DIN EN ISO 2409 (2020) Paints and varnishes - Cross-cut test. Beuth: Berlin
Chung P, Wang J, Durandet Y (2019) Deposition processes and properties of coatings on steel fasteners - a review. Friction 7:389–416
Acknowledgements
The authors would like to thank the German Research Foundation (Deutsche Forschungsvereinigung, DFG) for funding the research project “Forming and joining of semi-tubular self-piercing rivets made of high-strength steel with adapted mechanical properties and numerical analysis of the process chain” under grant number 328853593, on which this paper is based.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Uhe, B., Kuball, CM., Merklein, M. et al. Corrosion behaviour of self-piercing riveted joints with uncoated rivets in high nitrogen steel. Prod. Eng. Res. Devel. 18, 475–482 (2024). https://doi.org/10.1007/s11740-024-01262-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11740-024-01262-6