Abstract
Pulmonary edema and its association with low flow times has been observed in postcardiac arrest patients. However, diagnosis of distinct types of lung pathology is difficult.The aim of this study was to investigate pulmonary edema by transpulmonary thermodilution (TPTD) after out-of-hospital cardiac arrest (OHCA), and the correlation to downtimes. In this retrospective single-center study consecutive patients with return of spontaneous circulation (ROSC) following OHCA, age ≥ 18, and applied TPTD were enrolled. According to downtimes, patients were divided into a short and a long no-flow-time group, and data of TPTD were analysed. We identified 45 patients (n = 25 short no-flow time; n = 20 long no-flow time) who met the inclusion criteria. 24 h after ROSC, the extra vascular lung water index (EVLWI) was found to be lower in the group with short no-flow time compared to the group with long no-flow time (10.7 ± 3.5 ml/kg vs. 12.8 ± 3.9 ml/kg; p = 0.08) and remained at a similar level 48 h (10.9 ± 4.3 ml/kg vs. 12.9 ± 4.9 ml/kg; p = 0.25) and 72 h (11.1 ± 5.0 ml/kg vs. 13.9 ± 7.7 ml/kg; p = 0.27) post-ROSC. We found a statistically significant and moderate correlation between no-flow duration and EVLWI 48 h (r = 0.51; p = 0.002) and 72 h (r = 0.54; p = 0.004) post-ROSC. Pulmonary vascular permeability index (PVPI) was not correlated with downtimes. Our observation underlines the presence of cardiac arrest-related lung edema by determination of EVLWI. The duration of no-flow times is a relevant factor for increased extravascular lung water index.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sudden cardiac arrest is a major issue in modern emergency and intensive care medicine and one of the leading causes of death worldwide [1]. Although programs to improve survival rates have been implemented, outcomes remain poor [2]. One of the most important factors with a beneficial influence on survival is the early recognition of cardiac arrest and call for help, followed by early cardiopulmonary resuscitation, especially high-quality chest compressions. As long as no restoration of spontaneous circulation (ROSC) is obtained, chest compressions should be continued [3]. After ROSC is established, proper postcardiac arrest care is recommended. In addition to postresuscitation myocardial depression, postcardiac arrest syndrome (PCAS) is an independent factor for mortality [4, 5]. This postreperfusion state is characterized by systemic inflammation leading to multiorgan dysfunction or even failure, which are associated with the survival rate [5,6,7]. However, many studies in this field have focused on myocardial and brain injuries and dysfunctions [8,9,10]. We found a paucity of clinical studies regarding pulmonary damage and dysfunction in the postcardiac arrest phase.
Pulmonary edema has often been observed in the early assessment of postcardiac arrest patients, and it was associated with hospital mortalitiy and poor neurological outcomes [5, 11,12,13,14,15]. However, distinct types of lung pathology with similar appearances on chest radiographs and clinical presentations make a definite diagnosis difficult. Especially in the peri-arrest setting, a multitude of factors can precipitate and contribute to the development of cardiogenic lung edema, contusion, or ARDS [7, 11, 12, 16,17,18]. Furthermore, elevated administration of packed red blood cells and coagulation preparations in cases of circulatory arrest caused by hemorrhagic shock can increase the risk of transfusion-related acute lung injury (TRALI) [19].
Pulmonary edema, whether cardiogenic or noncardiac, is characterized by excessive accumulation of extravascular lung water (EVLW); an increase in pulmonary vascular permeability (PVPI) is a typical finding of ARDS [20]. However, it is difficult to evaluate it quantitively by radiographic findings [14, 15].
As such, the European Society of Intensive Care Medicine (ESICM) recommends the use of cardiac output monitoring (e.g., transpulmonary thermodilution) in patients with severe shock not responding to initial treatment [21]. However, EVLWI and PVPI data from patients after cardiac arrest are sparse, and target values have not been described. Furthermore, the influence of no-flow and low-flow times in the clinical setting has not been investigated thus far.
The aim of this study was to investigate pulmonary edema by extravascular water index (EVLWI) and pulmonary vascular permeability index (PVPI) after out-of-hospital cardiac arrest (OHCA) and their correlation to no-flow and low-flow times.
Patients and methods
In this retrospective longitudinal single-center study, we identified patients after OHCA who were treated between August 2019 and August 2022 in the intensive care unit (ICU) of a tertiary teaching hospital in Germany. Patients were enrolled in the study if they fulfilled the inclusion criteria (OHCA, ROSC, ≥ 18 years, TPTD). Patients receiving advanced mechanical cardiac support (VA-ECMO) were excluded because the determination via thermodilution methods during VA-ECMO has not been validated. Patients with occlusion of large pulmonary arteries were also excluded due to the assumed underestimation of EVLWI in this patient group [20]. As patients with active SARS-CoV-2 infection showed ARDS-like clinical pictures, we did not include patients with positive SARS-CoV-2-PCR in this observation.
Details of the patients’ inclusion/exclusion are presented in Fig. 1. Institutional medical records and charts were reviewed to evaluate the in-hospital management in the first 72 h after admission. Data related to prehospital emergency care were obtained from emergency medical service records and Utstein reports. The following data were collected for further analysis: demographic data (age, sex, and comorbidities) and clinical, laboratory, and echocardiography data. Patient’s continuous ICU monitoring was complemented by invasive hemodynamic monitoring using pulse indicator continuous cardiac output system (PICCO, Pulsion Medical Systems AG, Munich, Germany). The PICCO system was routinely calibrated by thermodilution with 20 ml ice cold saline (4 °C) every 8 h. A minimum of three consecutive measurements was obtained and a difference of < 10% between the results was accepted for data collection. Mean value of these consecutive measurements was used for analysis. EVLWI indexation was done according to the predicted body weight of the patients.
Ethical approval was waived by the Ethics Committee of the regional Medical Association (North-Rhine; Nr. 20210506) because of the retrospective nature of the study, and all the procedures performed were part of routine care.
Measures and outcome
The primary evaluation criterion was the comparison of EVLWI and PVPI between a group of post-cardiac arrest patients who experienced prolonged no-flow times due to delayed chest compressions and a control group with minimal no-flow times as a result of bystander chest compressions that were immediately started after the cardiac arrest was witnessed.
The second question was to evaluate whether the duration until ROSC was obtained correlates with EVLWI and whether the pulmonary permeability index (PVPI) varies in both groups to determine the pulmonary edema origin.
Statistical analysis
Count and percentage summarize distributions of categorical data, and continuous data are reported as mean ± standard deviation (SD). Since we found indications for nonnormality (using the Shapiro–Wilk test), we thoroughly applied nonparametric rank-based methods for comparisons of groups (Mann–Whitney U test), and the chi-square test or (in case of small sample sizes) Fischer’s exact test was applied for categorical variables. Correlations between continuous variables were evaluated by the Pearson correlation coefficient. A p value < 0.05 was considered statistically significant. All analyses were performed using MedCalc® Statistical Software version 19.5.3 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2020).
Results
Baseline characteristics
During the observation time, 154 OHCA patients were identified. Patients who expired within the first 24 h after ICU admission, received VV/VA-ECMO therapy, or had not undergone an invasive hemodynamic measurement were excluded, leaving a total of 45 patients available for analysis (Fig. 1). The mean age of the cohort was 63.8 ± 11.2 years, and 88.9% were male. Acute myocardial infarction was the most common cause of cardiac arrest (66.6%), followed by primary arrhythmia. Almost 68.9% of patients exhibited ventricular fibrillation or pulseless ventricular tachycardia as the first recorded rhythm. The remaining patients showed an initial non-shockable rhythm (asystole/PEA).
In 20 patients (45%) representing the long no-flow-time group, resuscitation attempts were delayed because cardiac arrest was not witnessed or bystanders did not apply chest compressions. These patients were older than the control group (67.0 ± 9.6 vs. 60.6 ± 11.4; p = 0.05). When comparing data from EMS records, the no-flow time (1.9 ± 1.9 min vs. 7.0 ± 3.7 min; p < 0.0001) and the time to ROSC (22.6 ± 13.8 min vs. 33.1 ± 18.7 min; p = 0.03) were significantly longer in the long no-flow-time cohort. Additionally, in this group, there were more defibrillations performed (2.4 [1.1–3.8] vs. 3.5 [2.0–5.0]; p = 0.25), and a higher dosage of adrenaline (2.4 mg [1.4–3.4] vs. 4.8 mg [3.3–6.3]; p = 0.005) and amiodarone (78.0 [9–148] mg vs. 142.5 [62–223] mg; p = 0.21) was administered.There was no significant difference in baseline parameters between the groups concerning age, sex, or comorbidities. The clinical characteristics of all patients are summarized in Table 1, and prehospital and intrahospital procedural data are presented in Table 2.
Fluid therapy
During the first 24 h in the ICU, patients receiving immediate CPR received an average fluid volume of 3496 ± 1556 ml. Patients in the long no-flow-time group were infused with a cumulative volume of 4196 ± 2501 ml within the same period (p = 0.64). Furthermore, the amount of fluid was not statistically significantly lower in patients with short compared with long no-flow times 48 h (5271 ± 1775 ml vs.6007 ± 3748 ml, p = 0.82) and 72 h (6730 ± 2855 ml vs. 7400 ± 4365 ml, p = 0.13) after ROSC.
Respiratory data following OHCA and the effect of downtimes
The PaO2/FiO2 ratio to evaluate lung function and oxygenation was markedly reduced in both groups at ICU admission. 62.2% of all patients had a ratio below 300 mmHg, and 57.7% even had a ratio below 200 mmHg. Although a lower PaO2/FiO2 ratio was observed 24 h, 48 h, and 72 h after ROSC in the long no-flow time group, no significant difference between the groups was shown (Table 3).
The inflammatory response following OHCA and the effect of downtimes
Standard inflammatory biomarkers (CRP, WBC) showed a profound increase during the first 72 h after ROSC in both cohorts. No overall differences in CRP and leucocytes between the groups in the study period were found (Online Resource 1). In addition, pneumonia was diagnosed equally in both groups (32% vs. 40%; p = 0.58; Table 2).
EVLWI and PVPI following OHCA and effect of downtimes
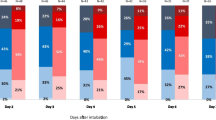
Twenty-four hours post-ROSC, the ELWI was found to be lower in the group with short no-flow times compared to the group with long no-flow times (10.7 ± 3.5 ml/kg vs 12.8 ± 3.9 ml/kg; p = 0.08). This pattern persisted at 48 h (10.9 ± 4.3 ml/kg vs 12.9 ± 4.9 ml/kg; p = 0.25) and 72 h (11.1 ± 5.0 ml/kg vs 13.9 ± 7.7 ml/kg; p = 0.27) post-ROSC. Statistical significance was lacking at all time points (Fig. 2).
At 24, 48 and 72 h after return of spontaneous circulation (ROSC), the PVPI values for the group with prolonged no-flow-times were 2.7 ± 0.6, 2.5 ± 0.8, and 3.7 ± 2.1 respectively, and for the control group 2.8 ± 1.5, 3.1 ± 1.5, and 2.7 ± 0.9 respectively, showing no statistically significant difference between the groups.
Analysis for EVLWI and PVPI 24 h post-ROSC are illustrated in Table 4. Our detailed results reveal a statistically significant and moderate correlation between no-flow duration and EVLWI 48 h (r = 0.51; p = 0.002) and 72 h (r = 0.54; p = 0.004) post-ROSC. Our analysis found a weak but still significant association between downtimes and EVLWI in the early post-resuscitation phase (r = 0.48; p = 0.001). We found no correlation between EVLWI and total time until ROSC. Furthermore, PVPI was not correlated with no-flow times or time until ROSC.
Discussion
Prior work has documented the influence of resuscitation times on radiological, clinical, and laboratory results in patients suffering from OHCA [6, 12,13,14,15,16]. However, there are no published studies regarding the association between no-flow and low-flow times on the extravascular lung water index (EVLWI) and pulmonary vascular permeability index (PVPI) in these patients. Our data present real-world findings about patients after OHCA matching with data from the German resuscitation register concerning no-flow times in patients without immediate bystander CPR (7:00 ± 3:42 vs. 7:04 ± 3:49 min) [22]. Our study revealed that EVLWI was substantially higher in the group with prolonged no-flow times compared to the group with minimal no-flow times. However, we found no substantial correlation between the overall duration until ROSC and EVLWI.
EVLWI remained stable during the first 72 h post-cardiac arrest. Moreover, we found that even after immediate bystander CPR, elevated EVLWI levels were measured and that prolonged no-flow times were positively correlated with higher EVLWI but not PVPI. Based on these findings, our research contributes to the current literature by providing more detailed insights of cardiac arrest related lung edema.
In a retrospective study analyzing plain chest X-rays of one hundred and seven patients resuscitated in an emergency department, the duration of CPR was described as an independent predictor for developing severe pulmonary edema [19]. This observation is in line with recent data investigating lung density evaluated by CT scans in postcardiac arrest patients [13]. This translational study provides a comprehensive depiction of lung abnormalities, comparing mechanical chest compressions (mCPR) to manual chest compressions. Notably, the mCPR group exhibited more pronounced lung abnormalities, including increased lung weight, decreased lung aeration, and impaired oxygenation and respiratory system compliance.Furthermore, their results indicate that patients with shorter low-flow times had lower mean lung density compared to those with low-flow times exceeding 26 min. This effect was statistically significant among patients who received mCPR. It is important to note that neither of the studies differentiated patient groups based on no-flow times.
Currently, the influence of different downtimes on EVLWI has only been studied in porcine animal models.Yang et al. showed in their animal model that longer periods of untreated VF cause more pronounced changes in gas exchange, respiratory, and histopathology in the early stages after ROSC [23]. They proposed that prolonged VF is associated with more severe ischemia–reperfusion injury and chest compression injury. Compared with the sham group, both intervention groups showed a relevant increase in EVLWI. The difference of approximately 2 ml/kg/m2 between the 5 min and 10 min no-flow groups diminished 4 h post ROSC. Both groups showed stable elevated values for EVLWI compared with the sham group.
Although this animal model gives some insights into the trend of EVLWI after cardiac arrest, the result cannot be transferred to typical situations in human cohorts.
All animals were in ventricular fibrillation compared with only 68.9% in our human cohort. The presence of ongoing cardiac ischemia by a culprit lesion in our study and the physiological effect of analgosedation and targeted temperature management could be a reason for the slower increase in the cardiac index in our cohort. Furthermore, the animal study gives no data about the prognostically relevant early 72 h-period after the restoration of cardiac circulation.
While cardiac output is an established parameter of cardiac function, EVLWI is often used to reflect the severity of pulmonary edema [20, 24, 25]. Although we observed an increase in EVLWI in both groups, the values remained stable even when cardiac output seemed to normalize. This finding indicated that the postcardiac arrest myocardial dysfunction caused by myocardial stunning might recover more quickly, as described in recent literature [8, 26]. Moreover, pulmonary edema after resuscitation might be associated not only with cardiac dysfunction but also with lung injuries. Lung injury and ARDS are increasingly reported, and a new definition of cardiopulmonary resuscitation related lung edema (CRALE) has been proposed [12, 13, 16, 27].
In our study population, more than 50% of all patients had a calculated PaO2/FiO2 ratio resonating with the diagnosis of mild to moderate ARDS. A retrospective study by Johnson et al. found an incidence of 48% and a mean PaO2/FiO2 ratio of 155 mmHg in mechanically ventilated OHCA patients [6]. Analysing data of in-hospital-cardiac-arrest (IHCA) patients, Shi et al. reported an even higher incidence of 72% [12]. The authors concluded that the higher incidence in IHCA patients could be due to other acute illnesses (e.g., sepsis) having a higher likelihood of ARDS. Both studies looked at results after 48 h or 72 h but did not reveal invasive hemodynamic data such as EVLWI or PVPI to evaluate for possible cofounders such as hypostatic cardiac edema.
However, it is difficult to evaluate hypostatic cardiac edema quantitively by radiographic findings [14, 15]. As found in previous studies, there is not a good correlation between CT scans and EVLWI measured with the thermodilution technique [28, 29].
Actual recommendations suggest that EVLWI above 10 ml/kg is a reasonable criterion for pulmonary edema. EVLWI above 12–15 ml/kg has been shown to have a high degree of severe morbidity and mortality in different critical care illnesses (e.g., sepsis, pancreatitis, ARDS). Furthermore, a PVPI greater than 3.0 suggests increased vascular permeability, as in ARDS, and a PVPI less than 2.0 represents normal vascular permeability, as present in cardiogenic pulmonary edema.
While we observed a significant positive correlation of EVLWI to no-flow times in our study, PVPI did not correlate with the duration of no-flow time. In a porcine model, histopathological changes of lung injury, such as neutrophils and erythrocytes in the alveolar space and alveolar walls, exudate and fibrin-like fillers in the alveolar space, and formation of hyaline membranes, were noted [11]. Although these inflammatory responses after OHCA have often been described, no routinely obtained inflammatory biomarkers have been shown to differentiate between high and low inflammation at this stage [30, 31]. This is in line with the results of our study of white blood cell count and CRP plasma levels after ROSC.
Recent studies have reported that inflammatory cytokines in out-of-hospital cardiac arrest show a time-dependent profile in the early post-resuscitation period [30,31,32,33]. Furthermore, an animal study investigating rats found that the histopathological score and TNF-α immunoreactivity were significantly increased in the lung after cardiac arrest. These results indicate that inflammation triggered by ischemia–reperfusion damage after cardiac arrest leads to pulmonary injury/dysfunction and may contribute to a low survival rate [34]. Due to the retrospective design of our investigation, no TNF-alpha plasma levels could be included in our analysis.
The findings of this study hold significant clinical relevance and have implications for daily practice in the management of postcardiac arrest patients. Firstly, they confirm the presence of cardiac arrest-related lung edema by demonstrating elevated EVLWI levels. This supports the importance of monitoring and managing pulmonary edema in postcardiac arrest patients. The use of TPTD provides a valuable tool for assessing and quantifying the severity of lung edema. Moreover, the study highlights the relevance of the duration of no-flow times in relation to increased extravascular lung water index. This suggests that shorter no-flow times may contribute to less severe pulmonary edema in postcardiac arrest patients. These findings emphasize the significance of early initiation and prompt delivery of cardiopulmonary resuscitation (CPR) to minimize the duration of no-flow time and potentially mitigate lung edema. Our data support the assumption that the exact mechanism of cardiac arrest-related lung edema still has to be identified and that potential cofounders exist (e.g., ischemia/reperfusion, aspiration, hyperoxia-induced lung injury, chest compression-induced lung contusion, and atelectasis). These results have the potential to guide clinical decision-making and optimize the management of postcardiac arrest patients in daily practice. Particularly, regarding fluid management and respiratory settings in this vulnerable phase. Despite the complexity of postcardiac arrest syndrome and its implications for cardiac hemodynamics, there are no guideline recommendations of target goals for advanced hemodynamic values like EVLWI or PVPI during patient management, and which additional monitoring tools (e.g., CT-Scan, echocardiography) should be used in the post-resuscitation period [35, 36].
Limitations
Our study has several limitations, most of which are related to the retrospective monocentric design and the small sample size. Overestimation of EVLWI by positive-end expiratory pressure ventilation and lung resection or underestimation by positive end-expiratory pressure, pulmonary vascular occlusion, heterogeneous lung injury and pleural effusion or hemothorax could not be completely excluded. Moreover, data about ventilation parameters such as driving pressures or tidal volume, which may influence the development of ventilator-associated lung injury in this cohort, are missing.
Conclusion
Our observation underlines the presence of cardiac arrest related lung edema by determination of EVLWI and the heterogeneous origin of this pathology. The duration of no-flow times by delayed beginning of chest compressions is a relevant factor of high extravascular lung water index and consecutive lung injury. Further prospective studies are needed to verify these observations and to evaluate whether protective ventilation strategies or a targeted fluid management may have a beneficial effect on outcome.
Data availability
Data will be available for reasonable requests.
References
Benjamin EJ, Muntner P, Alonso A et al (2019) Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 139:e56–e528. https://doi.org/10.1161/CIR.0000000000000659
Gräsner J-T, Wnent J, Herlitz J et al (2020) Survival after out-of-hospital cardiac arrest in Europe—results of the EuReCa TWO study. Resuscitation 148:218–226. https://doi.org/10.1016/j.resuscitation.2019.12.042
Nolan JP, Sandroni C, Böttiger BW et al (2021) European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: post-resuscitation care. Resuscitation 161:220–269. https://doi.org/10.1016/j.resuscitation.2021.02.012
Mongardon N, Dumas F, Ricome S et al (2011) Postcardiac arrest syndrome: from immediate resuscitation to long-term outcome. Ann Intensive Care 1:45. https://doi.org/10.1186/2110-5820-1-45
Dohi S (1983) Postcardiopulmonary resuscitation pulmonary edema. Crit Care Med 11:434–437. https://doi.org/10.1097/00003246-198306000-00008
Johnson NJ, Caldwell E, Carlbom DJ et al (2019) The acute respiratory distress syndrome after out-of-hospital cardiac arrest: Incidence, risk factors, and outcomes. Resuscitation 135:37–44. https://doi.org/10.1016/j.resuscitation.2019.01.009
Adrie C, Laurent I, Monchi M et al (2004) Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care 10:208–212. https://doi.org/10.1097/01.ccx.0000126090.06275.fe
Yao Y, Johnson NJ, Perman SM et al (2018) Myocardial dysfunction after out-of-hospital cardiac arrest: predictors and prognostic implications. Intern Emerg Med 13:765–772. https://doi.org/10.1007/s11739-017-1756-z
Jentzer JC, Anavekar NS, Mankad SV et al (2018) Echocardiographic left ventricular diastolic dysfunction predicts hospital mortality after out-of-hospital cardiac arrest. J Crit Care 47:114–120. https://doi.org/10.1016/j.jcrc.2018.06.016
Schick A, Prekker ME, Kempainen RR et al (2022) Association of hypoxic ischemic brain injury on early CT after out of hospital cardiac arrest with neurologic outcome. Am J Emerg Med 54:257–262. https://doi.org/10.1016/j.ajem.2022.02.003
Liu Z, Liu Q, Wu G et al (2018) Quantitative CT assessment of lung injury after successful cardiopulmonary resuscitation in a porcine cardiac arrest model of different downtimes. Quant Imaging Med Surg 8:946–956. https://doi.org/10.21037/qims.2018.10.04
Shih JA, Robertson HK, Issa MS et al (2022) Acute respiratory distress syndrome after in-hospital cardiac arrest. Resuscitation 177:78–84. https://doi.org/10.1016/j.resuscitation.2022.05.006
Magliocca A, Rezoagli E, Zani D et al (2021) Cardiopulmonary resuscitation-associated lung edema (CRALE). A translational study. Am J Respir Crit Care Med 203:447–457. https://doi.org/10.1164/rccm.201912-2454OC
Kang D-H, Kim J, Rhee JE et al (2015) The risk factors and prognostic implication of acute pulmonary edema in resuscitated cardiac arrest patients. Clin Exp Emerg Med 2:110–116. https://doi.org/10.15441/ceem.14.016
Kim J, Kim T, Kim K et al (2013) Risk factors and prognostic implication of acute pulmonary edema in resuscitated out-of-hospital cardiac arrest patients. Resuscitation 84:S78. https://doi.org/10.1016/j.resuscitation.2013.08.199
Johnson NJ, Town JA (2022) Don’t go breaking my…lungs? The acute respiratory distress syndrome is common, deadly, and probably underrecognized after cardiac arrest. Resuscitation 177:1–2. https://doi.org/10.1016/j.resuscitation.2022.06.002
Ar P, Ja B, Jg C et al (2020) Part 3: adult basic and advanced life support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. https://doi.org/10.1161/CIR.0000000000000916
Snashall PD, Keyes SJ, Morgan BM et al (1981) The radiographic detection of acute pulmonary oedema. A comparison of radiographic appearances, densitometry and lung water in dogs. Br J Radiol 54:277–288. https://doi.org/10.1259/0007-1285-54-640-277
Zeeuw van der Laan EAN, van der Velden S, Porcelijn L et al (2020) Update on the pathophysiology of transfusion-related acute lung injury. Curr Opin Hematol 27:386–391. https://doi.org/10.1097/MOH.0000000000000607
Jozwiak M, Teboul J-L, Monnet X (2015) Extravascular lung water in critical care: recent advances and clinical applications. Ann Intensive Care 5:38. https://doi.org/10.1186/s13613-015-0081-9
Cecconi M, De Backer D, Antonelli M et al (2014) Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 40:1795–1815. https://doi.org/10.1007/s00134-014-3525-z
Fischer M, Wnennt J, Gräsner J-T et al (2021) Öffentlicher Jahresbericht 2021 des Deutschen Reanimationsregisters: Außerklinische Reanimation
Yang Z, Zheng H, Lin L et al (2019) Alterations in respiratory mechanics and neural respiratory drive after restoration of spontaneous circulation in a porcine model subjected to different downtimes of cardiac arrest. J Am Heart Assoc. https://doi.org/10.1161/JAHA.119.012441
Bhattacharjee A, Pradhan D, Bhattacharyya P et al (2017) How useful is extravascular lung water measurement in managing lung injury in intensive care unit? Indian J Crit Care Med Peer-Rev 21:494. https://doi.org/10.4103/ijccm.IJCCM_40_17
Rasch S, Schmidle P, Sancak S et al (2021) Increased extravascular lung water index (EVLWI) reflects rapid non-cardiogenic oedema and mortality in COVID-19 associated ARDS. Sci Rep 11:11524. https://doi.org/10.1038/s41598-021-91043-3
Oksanen T, Skrifvars M, Wilkman E et al (2014) Postresuscitation hemodynamics during therapeutic hypothermia after out-of-hospital cardiac arrest with ventricular fibrillation: a retrospective study. Resuscitation 85:1018–1024. https://doi.org/10.1016/j.resuscitation.2014.04.026
Geri G, Richard J-C (2021) Cardiopulmonary resuscitation–associated lung edema: the price to pay to get the heartbeat? Am J Respir Crit Care Med 203:405–406. https://doi.org/10.1164/rccm.202009-3445ED
Saugel B, Wildgruber M, Staudt A et al (2019) Quantitative computed tomography in comparison with transpulmonary thermodilution for the estimation of pulmonary fluid status: a clinical study in critically ill patients. J Clin Monit Comput. https://doi.org/10.1007/s10877-018-0144-1
Saugel B, Holzapfel K, Stollfuss J et al (2011) Computed tomography to estimate cardiac preload and extravascular lung water. A retrospective analysis in critically ill patients. Scand J Trauma Resusc Emerg Med 19:31. https://doi.org/10.1186/1757-7241-19-31
Bro-Jeppesen J, Kjaergaard J, Wanscher M et al (2015) Systemic inflammatory response and potential prognostic implications after out-of-hospital cardiac arrest: a substudy of the target temperature management trial. Crit Care Med 43:1223–1232. https://doi.org/10.1097/CCM.0000000000000937
Langeland H, Damås JK, Mollnes TE et al (2022) The inflammatory response is related to circulatory failure after out-of-hospital cardiac arrest: a prospective cohort study. Resuscitation 170:115–125. https://doi.org/10.1016/j.resuscitation.2021.11.026
Bro-Jeppesen J, Kjaergaard J, Wanscher M et al (2014) The inflammatory response after out-of-hospital cardiac arrest is not modified by targeted temperature management at 33 °C or 36 °C. Resuscitation 85:1480–1487. https://doi.org/10.1016/j.resuscitation.2014.08.007
Braunstein M, Williamson M, Kusmenkov T et al (2017) Significant cytokine mRNA expression changes immediately after initiation of cardiopulmonary resuscitation. Mediat Inflamm 2017:1–10. https://doi.org/10.1155/2017/8473171
Park Y, Tae H-J, Cho JH et al (2018) The relationship between low survival and acute increase of tumor necrosis factor α expression in the lung in a rat model of asphyxial cardiac arrest. Anat Cell Biol 51:128–135. https://doi.org/10.5115/acb.2018.51.2.128
Kovács E, Pilecky D, Becker D et al (2012) Hemodynamic changes and invasive hemodynamic monitoring during post-resuscitation therapeutic hypothermia (preliminary data). Resuscitation 83:e96–e97. https://doi.org/10.1016/j.resuscitation.2012.08.250
Kovács E, Gyarmathy VA, Pilecky D et al (2021) An interaction effect analysis of thermodilution-guided hemodynamic optimization, patient condition, and mortality after successful cardiopulmonary resuscitation. Int J Environ Res Public Health 18:5223. https://doi.org/10.3390/ijerph18105223
Author information
Authors and Affiliations
Contributions
IV conceptualization, methodology, investigation, writing—original draft, writing—review and editing, visualization. MM investigation, writing—review and editing. HW, OB writing—review and editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Ethical Statement
Ethical approval was waived by the Ethics Committee of the regional Medical Association (North-Rhine; Nr. 20210506) because of the retrospective nature of the study, and all the procedures performed were part of routine care. Procedures were followed in accordance with the ethical and data protection standards of the approving committees and with the Helsinki Declaration of 1975.
Informed consent
For this study Informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11739_2023_3420_MOESM1_ESM.pdf
Supplementary file1 Online Resource 1 Inflammatory biomarkers post- ROSC : At admission (Leuko0,CRP0), 24h (Leuko24,CRP24), 48h (Leuko48,CRP48) and 72h(Leuko72,CRP72) post-ROSC (PDF 93 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Voigt, I., Mighali, M., Wieneke, H. et al. Cardiac arrest related lung edema: examining the role of downtimes in transpulmonary thermodilution analysis. Intern Emerg Med 19, 501–509 (2024). https://doi.org/10.1007/s11739-023-03420-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-023-03420-7