Abstract
The perioperative management of a cardiac-patient candidate to non-cardiac surgery (NCS) remains a topic of considerable debate. In recent years, the overall tendency from professional societies has been to delineate how to identify and manage high-risk patients following the best evidence. However, significant concerns persist, especially in the care of intermediate-risk patients (also labeled at “acceptable” risk), who may not fit into the categories of “completely healthy” or “critically ill”, but that might still encounter dramatic (and unexpected) perioperative events. The specific interest and main goal of this expert viewpoint pertains to the care of cardiac patients scheduled for NCS, addressing central questions of real-life clinical care that practicing anesthesiologists and cardiologists face daily, discussing recent American College of Cardiology/American Heart Association (ACC/AHA), European Society of Cardiology/European Society of Anaesthesiology (ESC/ESA), and Canadian Cardiovascular Society (CCS) guidelines. The viewpoint aims to discuss few of the important topics pertaining perioperative assessment and management: type of NCS and perioperative cardiac events, risk prediction including testing, and perioperative management of cardiac therapy. The fact that cardiac adverse events have reduced in number mostly due to better preoperative management and prevention should not prompt a reduction in clinical evaluations. While debate remains pertaining the most appropriate way to evaluate patients for NCS within international societies, a comprehensive approach-evaluation best recognized to assess functional and heart status, should be maintained, keeping into consideration the surgical procedure and global health management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The perioperative management of a cardiac patient’s candidate to non-cardiac surgery (NCS) remains a topic of considerable debate [1]. In recent years, the overall tendency from professional societies has been to delineate how to identify and manage high-risk patients following the best evidence [2,3,4]. However, significant concerns persist, especially in the care of intermediate-risk patients (also labeled at “acceptable” risk), who may not fit into extreme categories of “completely healthy” or “critically ill,” but might encounter dramatic (and unexpected) perioperative events actually. The modern clinical approach based on precision medicine, combined with population-based management and large-scale databases analysis (i.e., large hospitals health networks), recently provides more efficient care (at reasonable cost) [5], and replaces the previous models based on cohort databases (i.e., Framingham model) [6, 7]. While this approach undoubtedly offers advantages (i.e., identification of rare events and actual incidence of moderate outcomes), its value primarily depends on which indicators are evaluated and how accurately they are assessed, results in potentially biased by under-reporting [2, 6]. In this context of uncertainty, expert consensus documents and international guidelines [2,3,4] are welcome to practically summarize evolving evidence into handy recommendations suggesting what to do (or not) in clinical practice; however, recent recommendations endorsed by different scientific societies do not concur on many topics of discussion, making the picture quite intricate [2,3,4]. The specific interest of this viewpoint pertains to the care of cardiac patients scheduled for NCS, addressing central questions of real-life clinical care that practicing anesthesiologists and cardiologists face daily, discussing recent American College of Cardiology/American Heart Association (ACC/AHA), European Society of Cardiology/European Society of Anaesthesiology (ESC/ESA), and Canadian Cardiovascular Society (CCS) guidelines [2,3,4].
An epidemiological perspective
Epidemiological “crude” data give an idea of the magnitude of the problem. Worldwide, cardiovascular and cerebrovascular complications due to NCS represent a potential source of morbidity and mortality for the about 300 million patients annually [3, 4]. The rate of major complications (including cardiac death, non-fatal MI, or ischemic stroke) approximates to 1 every 33 hospitalizations for NCS [5]. Moreover, these data mostly express short-term events and oversee sub-clinical cardiovascular deterioration that can negatively impact long-term outcomes [5]. Common cardiovascular risk factors (i.e., hypertension, diabetes mellitus, and obesity) are highly frequent (and often present in combination), in subjects referred for NCS [5]. One in every four patients (20–25%) undergoing NCS has known cardiovascular disease (CVD), and in 5% of cases, atherosclerosis involves two or more vascular beds [5]. Recently, an adverse trend in the prevalence of cardiovascular risk factors and diseases in NCS patients (about 1% per year) has also been reported. Unexpectedly, in contrast to the steady increase in cardiovascular risk burden of the last few decades, the frequency of perioperative cardiac complications over the same period declined [5]. This outcome paradox is likely to derive from multiple factors, such as the improvement in medical management of cardiac patients, advances in surgical and anesthesiology techniques, and progress in post-operative care (i.e., early mobilization) [5]. Thus, continuous implementation of perioperative strategies is needed to maintain this favorable trend in outcomes, considering the announced aging and increasing comorbidities of our patients in the next future [5].
Type of NCS and cardiac complications
The perioperative risk for adverse cardiac events significantly depends on the type of surgery, including urgency and duration of the procedure. Elective endoscopic, laparoscopic, and endovascular procedures are generally considered at lower risk of perioperative complications, as compared with open techniques. To give examples, major surgeries addressing aorta, lungs, duodeno-pancreas, and liver are usually considered at high risk of short-term complications (more than 5%), and in these cases, a careful perioperative assessment is needed. Of note, currently adopted models used to classify different types of surgery according to their level of risk generally focus on 30-day outcomes, resulting not entirely applicable to the strictly perioperative period [3].
Prediction and prevention of perioperative risk
The prediction and prevention of cardiovascular complications in NCS remain a primary concern in perioperative care. Physicians daily estimate perioperative risk integrating different strategies, such as risk scores and biomarkers, mainly focusing on the dreadful cardiac conditions they are concerned with [i.e., myocardial infarction (MI), dysrhythmias, heart failure (HF), and valvular heart disease (VHD)]. Cardiac risk is complex and polyhedral (i.e., ischemic, dysrhythmic, hypotensive/shock, and chronic condition exacerbations), and an evidence-based and objective approach is firmly required in the perioperative setting, although clinical decisions rely too often on unreliable and subjective health indicators yet.
Risk scores For surgeons, cardiologists, and anesthesiologists, it is important to quantify in numbers patients’ risks. To carry out this delicate task, current guidelines recommend the use of clinical risk prediction models (i.e., risk scores and indices) to define patient’s risk profile objectively. The 2014 ESC/ESA and AHA/ACC guidelines [3, 4] recommend using (without preference) different tools, such as the Revised Cardiac Risk Index (RCRI) [8], the American College of Surgeons National Surgery Quality Improvement Program (ACS NSQIP) Surgical Risk Calculator [6], and the myocardial infarction or cardiac arrest (MICA) calculator [7]. Differently, the 2017 CCS guidelines recommend the use of the RCRI over the others, despite this index lack of external validation and objective assessment of functional status. Moreover, Canadian guidelines raise concern about the possible risk underestimation using the ACS NSQIP and MICA indices due to the absence of systematic measurement of perioperative troponin [2]. To date, the reliability of different scores is still debated. The point is still that recognition of falsely low-risk (concealed high risk) patients remains inaccurate (more or less) for all. Cardiovascular risk factors are scarcely reported (and often with low significance) into risk scores, and varies largely amongst them. Moreover, the problem of acute perioperative exacerbations or worsening of chronic cardiac conditions is not actually taken into account and remains largely unaddressed.
Authors’ viewpoint: the use of risk indices may be implemented in clinical practice to assess perioperative risk objectively; however, their reliability in non-high-risk patients needs further validation.
Biomarkers International guidelines differ substantially regarding their attitude towards biomarkers monitoring. Regarding natriuretic peptides, the ACC/AHA position is prudent, considering the lack of strong evidence. Indeed, while acknowledging their incremental value in predicting cardiac events (especially for patients with or at high risk for HF), they do not indicate any perioperative timing or setting for their measurement. Opposing this waiting position, CCS guidelines state that BNP or NT-proBNP should be pre-operatively measured in patients considered at high risk for clinical characteristics, presence of CVD, and risk indices. Canadian recommendations also note the importance of age in the decision-making based on the VISION study, which showed how patients with ≥ 65 years or 45–64 years of age with known CVD had a baseline risk > 5% for 30-day cardiovascular complications, whereas those without had less than 2.0% of risk [2, 9]. Similar to Canadian, the ESC/ESA recommendations suggest to limit pre-operative or post-operative BNP/NT-proBNP measurement in high-risk patients, like those with known or suspected HF or coronary artery disease (CAD), poor functional capacity (METs ≤ 4), or abnormal risk indices (RCRI value > 1, Apgar score < 7), discouraging their use in stable cardiac conditions.
As for natriuretic peptides, the benefits of perioperative troponin monitoring are not well established, as excessive concerns in the case of weakly positive results might lead to inappropriate coronary angiography and hospital stay prolongation. The occurrence of perioperative MI, both type 1 (due to stress-induced rupture of vulnerable plaques) and type 2 (due to perioperative demand–supply ischemia), represents one of the principals responsible for poor cardiac outcome after NCS. Dosing troponins might be useful to recognize early perioperative MI (occurring in about 1% of NCS patients, associated with a fivefold increase in the risk of mortality) [10], and myocardial injury after cardiac surgery (MINS) (reported in about 20% of patients, associated with two-to-fourfolds increase in 30-day mortality) [11]. As perioperative MIs fulfill the definition of MI, in this case, invasive management is expected to reduce adverse events [10, 12]. Differently, MINS is strictly an enzymatic diagnosis (post-operative hsTnT ≥ 65 ng/L or between 20 and 65 ng/L with an absolute change ≥ 5 ng/L), and its treatment remains controversial, although recent data suggest a net benefit of Dabigatran therapy in these patients [11]. Discordance amongst guidelines on troponin measurement reflects the sparsity of evidence. CCS guidelines stress the importance of MINS in predicting adverse outcome, and recommend monitoring troponin for 48–72 h after surgery in patients considered at higher risk for perioperative events, as those with elevated NT-proBNP/BNP, RCRI score ≥ 1, known CVD, or ≥ 65 years. While the ESC/ESA guidelines substantially agree with this position, the ACC/AHA guidelines consider troponin measurement appropriate only for patients suspected of having ongoing myocardial ischemia, giving less importance to troponin rise in the absence of symptoms to simplify (maybe oversimplifying) the burden of perioperative tests.
Authors’ viewpoint: while perioperative biomarkers monitoring, including natriuretic peptides and troponin, is recommended in patients considered at higher risk for myocardial ischemia and dysfunction, current evidence does not support their routine measurement in unselected NCS patients.
Perioperative non-invasive testing
Non-invasive tests assessing myocardial dysfunction or ischemia have a key role in perioperative risk assessment, as they can indicate the need for medical therapy optimization, and, in proper cases, of coronary angiography. However, strict criteria should be applied to avoid their inappropriate use before surgery. The battery of cardiovascular non-invasive tests is wide, and mainly includes resting ECG, cardiac, and vascular ultrasound (US) (for the neck, the abdominal aorta, and legs), and stress tests [13].
-
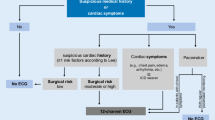
ECG 12-lead ECG is a widely available and inexpensive test able to identify cardiac diseases and influence anesthesia. Hence, it should be recorded in nearly all patients, becoming mandatory in all symptomatic patients (i.e., for CAD, dysrhythmias, VHD, and HF), and not strictly necessary only in asymptomatic low-risk patients [3, 13].
-
Cardiac US The benefit of pre-operative US examination is not well established. Heart US allows the exclusion of major cardiac conditions, evaluating systolic/diastolic function and valvular status. This test should be considered pre-operatively only in the case of new or worsening signs or symptoms suggestive of cardiovascular deterioration, whereas it is usually unnecessary in patients with known and stable cardiac conditions not contributing to reducing the risk of major complications [14].
-
Vascular US This test can be considered appropriate in patients with known neurologic or vascular disease (i.e., previous stroke and peripheral atherosclerosis). Regarding the use of carotid US for lowering the risk of perioperative stroke, definitive evidence is lacking [13].
-
Stress tests Exercise or pharmacological stress tests should be recommended in patients with known CAD or at high risk for ischemia (i.e., multiple clinical risk factors and impaired functional capacity) [3]. The most used is currently the exercise ECG test that permits the evaluation of functional capacity, heart rate and blood pressure response, and ischemic ST-segment changes. In specific populations (i.e., older patients with insufficient physical fitness), a pharmacological stress test with echocardiography or myocardial scintigraphy is recommended alternatively. In a recent study investigating the clinical impact of routine pre-operative dobutamine stress echo in candidates for NCS [15], the low rate of major events among different risk groups (0% in low risk and 0.8% in high risk) invalidates the use of this test in unselected populations. Therefore, current risk categories are probably not suitable for the selection of which patients can actually benefit from stress tests. Accordingly, Canadian guidelines deemphasize the importance of stress testing, recommending more often the use of biomarkers for screening patients (potentially reducing costs and delays of surgery).
Authors’ viewpoint: while non-invasive tests may be performed if it is expected the results will influence the perioperative and operative management (i.e., medications optimization), the availability of objective measures and estimation of patient functional status and perioperative cardiac risk are lacking and often jeopardize the patient course in real-life clinical care.
Perioperative medications
The appropriate management of common perioperative medications also remains controversial.
Beta-blockers (BBs) A definite answer to the question of whether perioperative use of BBs is of benefit remains elusive. In a large registry including 136,745 propensity-matched patients, exposure to BBs on the day of or following surgery is associated with a lower mortality risk (relative risk [RR] 0.73; P < 0.001) and a lower rate of non-fatal MI or cardiac arrest (RR, 0.67; P < 0.001), with a crescent benefit with increasing risk [16]. However, a recent Cochrane meta-analysis of 35 randomized controlled trials reports a significant potential increase in the risk of all-cause mortality (RR 1.25) and cerebrovascular events (RR 1.59), only partially balanced by a reduction in MI (RR 0.73) [17]. Considering these data, in daily practice, the use of perioperative BBs should be managed with caution. Of importance, concomitant clinical conditions potentially at risk for BBs-related complications (i.e., pre-existing conduction disorders and medications lowering blood pressure) should be taken into account in the assessment of risk–benefit ratio.
Authors’ viewpoint: among patients taking BBs chronically (i.e., for CAD, tachydysrhythmia, stable HF), therapy should be continued (including on the day of surgery), and the optimal dosage of BBs should be selected to avoid hypotension and bradycardia. Among BBs naïve patients, therapy initiation should be considered for patients at higher risk for cardiac complications. In this case, the initial low dose should be prescribed at least 1 week before (but preferentially 4–6 weeks prior) the intervention and a careful up-titration of the drug must be pursued.
Statins Potential benefits deriving from statins in the perioperative period has been intensely discussed, as large cohort studies report an overall clinical benefit at the expense of a negligible increase in the risk of cardiac adverse events [18]. In the the Vascular Events in Non-cardiac Surgery Patients Cohort Evaluation (VISION) study [18], including a matched population of 2845 patients on statins and 4492 controls, pre-operative statins show a significant 17% reduction in the risk of the primary endpoint (a composite of all-cause mortality, MINS, or stroke at 30 days), approximately halving the risk of all-cause mortality (RR, 0.58; P = 0.003), and cardiovascular death (RR, 0.42; P = 0.004). The Authors’ conclusion rightly called for the need for confirmatory data from large randomized studies. However, the more recent lowering the risk of operative complications using atorvastatin loading dose (LOAD) trial [19] has not confirmed those results. In this study, randomizing to either atorvastatin or placebo, 648 statin-naïve patients scheduled for NCS and considered at high risk for cardiac complications, statins administration was completely neutral, not reducing cardiovascular complications [19]. An important limitation of the study was the inadequate sample size, considering that a number of patients tenfold higher (about 7000 subjects) should have been enrolled to show significant results. Current international guidelines do not actively support perioperative initiation of statins in naïve patients scheduled for NCS, but they all concur continue this therapy in patients already treated.
Authors’ viewpoint: statins continuation up to the day of surgery should always be recommended (to be further investigated whether holding them on the day of surgery may significantly remove protection), as well as early resumption (a window for the cardiac protection not established) in patients chronically treated by taking into account potential benefits and the minimal risk of adverse events. In naïve patients, a common-sense recommendation could be to initiate statins in patients considered at high cardiovascular risk, in those who could benefit from lowering cholesterol levels independently (and beyond) perioperative care. In this regard, if we can suppose a protective effect on endothelium and cardiovascular system in the setting of surgery, statins initiation 4–6 weeks before the NCS might maximize their potential benefits.
Antiplatelet and anticoagulant medications Patients receiving antithrombotic therapy undergoing NCS are numerous, and they can require temporary treatment changes or withdraw [2, 3, 12, 20]. Thrombotic and hemorrhagic risk should be assessed carefully taking into account surgery type, potential consequence for thrombotic complications (especially if life-threatening, i.e., stent thrombosis), severity of bleedings (according to the likelihood of achieving effective hemostasis), and patient’s characteristics (i.e., kidney/liver disease, history of the previous spontaneous bleedings). In patients on dual antiplatelet therapy and recent stent implantation, NCS should ideally be delayed until the risks of stopping antiplatelets are acceptable. In the case of nondeferrable surgery, a general recommendation is to continue aspirin, and discontinue oral P2Y12 5–7 days before surgery (to resume with loading dose after 24–72 h) [12, 21]. The use of infusive bridging therapy with short half-life antiplatelet agents (i.e., cangrelor, tirofiban) has also been suggested to offer an additional stent safety perioperatively [12, 21]. For anticoagulants, the introduction and diffusion of direct oral anticoagulants (DOACs) simplified for several aspects the practical management of perioperative anticoagulation (i.e., not needing INR monitoring), but potentially added complexity by multiplying possible therapeutic schemes. In general, anticoagulants should be stopped pre-operatively (without bridging therapy with heparin) approximately 5 days before for warfarin (with a target INR of < 1.5), and 1–4 days before for DOACs (depending on creatinine clearance rate and bleeding risk). Practically, anticoagulants can usually be reintroduced after 24 h, although this decision should always be discussed with the surgeons, and postponed after 48 h in high-risk patients for post-operative bleedings [20].
Authors’ viewpoint: the introduction of novel anticoagulant and antithrombotic agents has provided practical advantages in the perioperative care of cardiac patients. Considering the variety of therapeutic options and schemes, modifications of perioperative therapy should be discussed by a multidisciplinary team to adapt treatment case by case.
Conclusions
Perioperative care of cardiac patient candidates for NCS surgery is an important aspect of clinical care. The fact that cardiac adverse events have reduced in number mostly due to better pre-operative management and prevention should not prompt a reduction in appropriate clinical evaluation. While debate remains pertaining the most appropriate way to evaluate patients for NCS within international societies, a common sense, but comprehensive approach–evaluation of cardiac risks, major and intermediate, as well as appropriate testing best recognized to assess functional and heart status, should be maintained, keeping into consideration the surgical procedure, yet maintaining a conservative disposition towards global health management.
References
Weiser TG, Haynes AB, Molina G, Lipsitz SR, Esquivel MM, Uribe-Leitz T, Fu R, Azad T, Chao TE, Berry WR, Gawande AA (2015) Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet 385:S11. https://doi.org/10.1016/S0140-6736(15)60806-6
Duceppe E, Parlow J, MacDonald P, Lyons K, McMullen M, Srinathan S, Graham M, Tandon V, Styles K, Bessissow A, Sessler DI, Bryson G, Devereaux PJ (2017) Canadian cardiovascular society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 33:17–32. https://doi.org/10.1016/j.cjca.2016.09.008
Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, Hert SD, Ford I, Gonzalez-Juanatey JR, Gorenek B, Heyndrickx GR, Hoeft A, Huber K, Iung B, Kjeldsen KP, Longrois D, Lüscher TF, Pierard L, Pocock S, Price S, Roffi M, Sirnes PA, Sousa-Uva M, Voudris V, Funck-Brentano C, Authors/Task Force Members (2014) 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management. Eur Heart J 35:2383–2431. https://doi.org/10.1093/eurheartj/ehu282
Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, Davila-Roman VG, Gerhard-Herman MD, Holly TA, Kane GC, Marine JE, Nelson MT, Spencer CC, Thompson A, Ting HH, Uretsky BF, Wijeysundera DN (2014) 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation 130:e278–e333. https://doi.org/10.1161/CIR.0000000000000106
Smilowitz NR, Gupta N, Guo Y, Beckman JA, Bangalore S, Berger JS (2018) Trends in cardiovascular risk factor and disease prevalence in patients undergoing non-cardiac surgery. Heart. https://doi.org/10.1136/heartjnl-2017-312391
Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME (2013) Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 217(833–842):e3. https://doi.org/10.1016/j.jamcollsurg.2013.07.385
Gupta PK, Gupta H, Sundaram A, Kaushik M, Fang X, Miller WJ, Esterbrooks DJ, Hunter CB, Pipinos II, Johanning JM, Lynch TG, Forse RA, Mohiuddin SM, Mooss AN (2011) Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 124:381–387. https://doi.org/10.1161/CIRCULATIONAHA.110.015701
Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KKL, Ludwig LE, Pedan A, Goldman L (1999) Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 100:1043–1049. https://doi.org/10.1161/01.CIR.100.10.1043
Botto F, Alonso-Coello P, Chan MTV, Villar JC, Xavier D, Srinathan S, Guyatt G, Cruz P, Graham M, Wang CY, Berwanger O, Pearse RM, Biccard BM, Abraham V, Malaga G, Hillis GS, Rodseth RN, Cook D, Polanczyk CA, Szczeklik W, Sessler DI, Sheth T et al (2014) Myocardial injury after noncardiac surgery. Anesthesiology 120:564–578. https://doi.org/10.1097/ALN.0000000000000113
Smilowitz NR, Gupta N, Guo Y, Berger JS, Bangalore S (2017) Perioperative acute myocardial infarction associated with non-cardiac surgery. Eur Heart J 38:2409–2417. https://doi.org/10.1093/eurheartj/ehx313
Devereaux PJ, Duceppe E, Guyatt G, Tandon V, Rodseth R, Biccard BM, Xavier D, Szczeklik W, Meyhoff CS, Vincent J, Franzosi MG, Srinathan SK, Erb J, Magloire P, Neary J, Rao M, Rahate PV, Chaudhry NK, Mayosi B, de Nadal M, Iglesias PP, Berwanger O, Villar JC, Botto F, Eikelboom JW et al (2018) Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 391:2325–2334. https://doi.org/10.1016/S0140-6736(18)30832-8
Rossini R, Tarantini G, Musumeci G, Masiero G, Barbato E, Calabrò P, Capodanno D, Leonardi S, Lettino M, Limbruno U, Menozzi A, Marchese UOA, Saia F, Valgimigli M, Ageno W, Falanga A, Corcione A, Locatelli A, Montorsi M, Piazza D, Stella A, Bozzani A, Parolari A, Carone R, Angiolillo DJ (2018) A multidisciplinary approach on the perioperative antithrombotic management of patients with coronary stents undergoing surgery. JACC Cardiovasc Interv 11:417–434. https://doi.org/10.1016/j.jcin.2017.10.051
Zwissler B, Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin (DGAI), Deutsche Gesellschaft für Innere Medizin (DGIM), Deutsche Gesellschaft für Chirurgie (DGCH) (2017) Preoperative evaluation of adult patients before elective, noncardiothoracic surgery. Anaesthesist. https://doi.org/10.1007/s00101-017-0376-3
Halm EA, Browner WS, Tubau JF, Tateo IM, Mangano DT (1996) Echocardiography for assessing cardiac risk in patients having noncardiac surgery. Study of perioperative ischemia research group. Ann Intern Med 125:433–441. https://doi.org/10.7326/0003-4819-125-6-199609150-00001
Widmer RJ, Cullen MW, Salonen BR, Sundsted KK, Raslau D, Mohabbat AB, Dougan BM, Bierle DM, Lawson DK, Widmer AJ, Bundrick M, Gaba P, Tellez R, Schroeder DR, McCully RB, Mauck KF (2018) Cardiac events after noncardiac surgery in patients undergoing preoperative dobutamine stress echocardiography: findings from the mayo poce-dse investigators. Am J Med. https://doi.org/10.1016/j.amjmed.2017.12.025
London MJ, Hur K, Schwartz GG, Henderson WG (2013) Association of Perioperative β-Blockade With Mortality and Cardiovascular Morbidity Following Major Noncardiac Surgery. JAMA. https://doi.org/10.1001/jama.2013.4135
Blessberger H, Kammler J, Domanovits H, Schlager O, Wildner B, Azar D, Schillinger M, Wiesbauer F, Steinwender C (2018) Perioperative beta-blockers for preventing surgery-related mortality and morbidity. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004476.pub3
Berwanger O, Le Manach Y, Suzumura EA, Biccard B, Srinathan SK, Szczeklik W, Santo JAE, Santucci E, Cavalcanti AB, Archbold RA, Devereaux PJ (2016) Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the vision study. Eur Heart J 37:177–185. https://doi.org/10.1093/eurheartj/ehv456
Berwanger O, de Barros PGMS, Barbosa RR, Precoma DB, Figueiredo EL, Hajjar LA, Kruel CDP, Alboim C, Almeida AP, Dracoulakis MDA, Filho HV, Carmona MJC, Maia LN, de Oliveira JBF, Saraiva JFK, Soares RM, Damiani L, Paisani D, Kodama AA, Gonzales B, Ikeoka DT, Devereaux PJ, Lopes RD (2017) Atorvastatin for high-risk statin-naïve patients undergoing noncardiac surgery: the lowering the risk of operative complications using atorvastatin loading dose (LOAD) randomized trial. Am Heart J 184:88–96. https://doi.org/10.1016/j.ahj.2016.11.001
Vivas D, Roldán I, Ferrandis R, Marín F, Roldán V, Tello-Montoliu A, Ruiz-Nodar JM, Gómez-Doblas JJ, Martín A, Llau JV, Ramos-Gallo MJ, Muñoz R, Arcelus JI, Leyva F, Alberca F, Oliva R, Gómez AM, Montero C, Arikan F, Ley L, Santos-Bueso E, Figuero E, Bujaldón A, Urbano J, Otero R, Hermida JF, Egocheaga I et al (2018) Perioperative and periprocedural management of antithrombotic therapy: consensus document of SEC, SEDAR, SEACV, SECTCV, AEC, SECPRE, SEPD, SEGO, SEHH, SETH, SEMERGEN, SEMFYC, SEMG, SEMICYUC, SEMI, SEMES, SEPAR, SENEC, SEO, SEPA, SERVEI, SECOT and AEU. Rev Esp Cardiol 71:553–564. https://doi.org/10.1016/j.rec.2018.01.029
Di Minno MN, Prisco D, Ruocco AL, Mastronardi P, Massa S, Di Minno G (2009) Perioperative handling of patients on antiplatelet therapy with need for surgery. Intern Emerg Med 4:279–288. https://doi.org/10.1007/s11739-009-0265-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent is not required.
Rights and permissions
About this article
Cite this article
Gragnano, F., Cattano, D. & Calabrò, P. Perioperative care of cardiac patient’s candidate for non-cardiac surgery: a critical appraisal of emergent evidence and international guidelines. Intern Emerg Med 13, 1185–1190 (2018). https://doi.org/10.1007/s11739-018-1927-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-018-1927-6