Abstract
Patients with chronic obstructive pulmonary disease (COPD) have an increased cardiovascular morbidity and mortality. Flow-mediated (FMD) and nitrate-mediated dilatation (NMD) are considered non-invasive methods to assess endothelial function and surrogate markers of subclinical atherosclerosis. We performed a systematic review with meta-analysis and meta-regression to evaluate the impact of COPD on FMD and NMD. Studies were systematically searched in the PubMed, Web of Science, Scopus and EMBASE databases. The random-effect method was used to take into account the variability among included studies. A total of eight studies were included in the final analysis, eight with data on FMD (334 COPD patients) and two on NMD (104 COPD patients). Compared to controls, COPD patients show a significantly lower FMD (MD −3.15%; 95% CI −4.89, −1.40; P < 0.001) and NMD (MD −3.53%; 95% CI −7.04, −0.02; P = 0.049). Sensitivity analyses substantially confirms the results. Meta-regression models show that a more severe degree of airway obstruction is associated with a more severe FMD impairment in COPD patients than in controls. Regression analyses confirm that the association between COPD and endothelial dysfunction is independent of baseline smoking status and most traditional cardiovascular risk factors. In conclusion, COPD is significantly and independently associated with endothelial dysfunction. These findings may be useful to plan adequate cardiovascular prevention strategies in this clinical setting, with particular regard to patients with a more severe disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic, progressive lung disease and an increasing cause of global morbidity and mortality [1]. In contrast to the trend for cancer and cardiovascular diseases, death rates from COPD have been progressively rising over the last decade, [2] and, according to estimates of the World Health Organization (WHO), by 2030 this condition may become the third leading cause of death worldwide [3].
Over the last few years, there has been an increased awareness about the extrapulmonary manifestations of COPD, [4] and the frequent and important chronic comorbidities that may contribute significantly to its severity and mortality [5]. In particular, there is a growing body of literature indicating that cardiovascular disease is more frequent in COPD patients than in the general population, representing a burden even greater than that of the lung disease itself in this clinical setting [6]. It has been estimated that for every 10% decrease in lung function [as expressed by forced expiratory volume in 1 s (FEV1)], cardiovascular mortality increases by nearly 30% [7].
COPD and cardiovascular disease share some major risk factors such as smoking habit, diabetes mellitus, hypertension and obesity [8, 9]. However, some evidence confirms that COPD is associated with cardiovascular manifestations independently from concomitant risk factors [7, 10], thus suggesting that COPD itself may be considered an independent cardiovascular risk factor. To further address this issue, a growing attention has been given to the assessment of the association between COPD and subclinical atherosclerosis, a recognized marker of cardiovascular disease [11].
Endothelial dysfunction is the earliest stage of the atherosclerotic process and even a trigger of cardiovascular events [12]. Flow-mediated dilatation (FMD) and nitrate-mediated dilatation (NMD) are widely accepted as accurate and non-invasive methods to assess endothelial function in humans [13], and in turn, as surrogate markers of subclinical atherosclerosis [14]. Moreover, FMD is currently considered an independent predictor of cardiovascular events [15], thus providing important prognostic data over and above traditional cardiovascular risk factors.
Some prospective studies [16, 17] document impaired endothelial function in COPD patients. However, these data have been challenged in recent studies, [18, 19] and no meta-analytical data providing an overall information about this issue are currently available.
The aim of the present study is to perform a systematic review with meta-analysis of all studies evaluating the impact of COPD on FMD and NMD. Moreover, we implemented some meta-regression models to evaluate the effect of some clinical and demographic variables on these outcomes.
Methods
Search strategy
To identify all available studies, a detailed search pertaining to COPD and the markers of cardiovascular risk (i.e., FMD and NMD) was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines [20]. A systematic search was performed in the electronic databases (PubMed, Web of Science, Scopus, EMBASE), using the following search terms in all possible combinations: chronic obstructive pulmonary disease, flow-mediated dilatation, nitrate-mediated dilatation, endothelium-dependent dilatation, endothelium-independent dilatation, endothelial function, and endothelial dysfunction. The last search was performed on 20th of December 2016. The search strategy was developed without any language restriction.
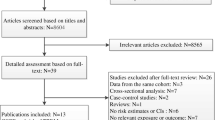
In addition, the reference lists of all retrieved articles were manually reviewed. In case of missing data, study Authors were contacted by e-mail to try to retrieve original data. Two independent Authors (PA and MNDDM) analyzed each article, and performed the data extraction independently. In case of disagreement, a third investigator was consulted (RL). Discrepancies were resolved by consensus. Selection results showed a high inter-reader agreement (κ = 1.00), and have been reported according to PRISMA flowchart (Fig. 1).
Data extraction and quality assessment
All studies evaluating the impact of COPD on the markers of cardiovascular risk were included. Case reports, case series without a control group, reviews and animal studies were excluded. To be included in the analysis, a study had to provide values (means with standard deviation or standard error) of brachial artery FMD or NMD among COPD patients and controls. The included studies were classified as having a prospective or a retrospective design.
In each study, data regarding sample size, major clinical and demographic variables, values of FMD and NMD in COPD patients and controls were extracted. Data on patients with acute exacerbation of COPD were excluded.
Given the characteristics of the included studies, the evaluation of methodological quality of each study was performed with the Newcastle–Ottawa Scale (NOS), which is specifically developed to assess quality of non-randomized observational studies [21]. The scoring system encompasses three major domains (selection, comparability, exposure) and a resulting score range between 0 and 8, a higher score representing a better methodological quality. Results of the NOS quality assessment are reported in Supplemental Table 1.
Statistical analysis and risk of bias assessment
Statistical analysis was carried out using the Review Manager software (Version 5.2, The Cochrane Collaboration, Copenhagen, Denmark).
Differences among cases and controls are expressed as mean difference (MD) with pertinent 95% confidence intervals (95% CI).
Endothelial function has been expressed as the percentage change (%) in the brachial artery diameter from baseline to maximal dilatation during reactive hyperemia (FMD) or after sublingual nitrate administration (NMD).
The overall effect is tested using Z scores and significance is set at P < 0.05. Statistical heterogeneity between studies is assessed with Chi-square Cochran’s Q test and with I 2 statistic, which measures the inconsistency across study results, and describes the proportion of total variation in study estimates, that is due to heterogeneity rather than sampling error. In detail, I 2 values of 0% indicate no heterogeneity, 25% low, 25–50% moderate, and 50% high heterogeneity [22].
Publication bias is represented graphically by funnel plots of the effect size (mean difference) versus precision (1/standard error of the mean difference) for studies evaluating flow-mediated dilatation and nitrate-mediated dilatation in patients with COPD, and in control subjects using both fixed and random effect. Visual inspection of funnel plot asymmetry is performed to address the possible small-study effect, and Egger’s and Begg and Mazumdar tests are used to assess publication bias, over and above any subjective evaluation. A P < 0.10 is considered statistically significant [23]. Moreover, the Duval and Tweedie’s trim and fill analysis is used to allow for the estimation of an adjusted effect size after trimming and imputing studies [24].
To be as conservative as possible, the random-effect method is used to take into account the variability among included studies.
Sensitivity analyses
We repeated analyses by including only the studies judged as “high quality” according to NOS (i.e., NOS ≥ to the median value found among included studies). Moreover, to avoid the risk of data overlap, a sensitivity analysis was performed after excluding studies involving the same recruitment Centres and enrolling patients in the same period time as other included studies. We also plan to perform a further sensitivity analysis after stratifying studies according to design (prospective and retrospective).
Meta-regression analyses
We hypothesize that differences among included studies may be affected by demographic variables [mean age, male gender] and clinical data related to disease severity [FEV1, forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC), Global Initiative for Chronic Obstructive Lung Disease (GOLD) class, Modified Medical Research Council (MMRC) class], disease activity [C-reactive protein (CRP), interleukin-6 (IL6) levels], COPD treatment [therapy with β-agonists, anticholinergic agents, corticosteroids (CCS), supplemental oxygen], concomitant use of cardiovascular medications [aspirin, statins, angiotensin converting enzyme-inhibitors (ACE-I), angiotensin receptor blockers (ARBs), β-blockers, calcium channel blockers (CCB)], and coexistence of traditional cardiovascular risk factors (hypertension, smoking habit, smoking pack-years, diabetes mellitus, obesity, hyperlipidemia). To assess the possible effect of such variables in explaining different results observed across studies, we planned to perform meta-regression analyses after implementing a regression model with changes in FMD or NMD as dependent variables (y) and the above mentioned covariates as independent variables (x). This analysis was performed with Comprehensive Meta-analysis [Version 2, Biostat, Englewood NJ (2005)].
Results
After excluding duplicate results, the search retrieved 512 articles. Of these studies, 385 were excluded because they were off the topic after scanning the title or the abstract, 115 because they were reviews/comments/case reports or they lacked data of interest. Another four studies were excluded after full-length paper evaluation.
Thus, eight articles were included in the final analysis [16,17,18,19, 25,26,27,28] (Fig. 1). In detail, eight studies (334 COPD patients and 281 controls) report data on FMD and two studies (104 COPD patients and 88 controls) evaluate NMD.
Study characteristics
All included studies are observational and have a prospective design. Major characteristics of study populations are shown in Table 1 and further data about disease activity, disease severity and treatment are reported in Supplemental Tables 2, 3.
The number of patients varies from 10 to 96, the mean age from 62 to 76.7 years, and the prevalence of male gender from 40 to 100%.
The presence of diabetes mellitus is reported by 0–43.3% of patients, obesity by 0%, hypertension by 0–86.7%, smoking history by 70.5–100%, and hyperlipidemia by 0–56.7%.
Mean body mass index (BMI) varies from 25 to 29.1 kg/m2 and mean number of smoking pack-years from 24.7 to 66. Mean values of total cholesterol (TC) ranged from 4.83 to 5.04 mmol/l, of LDL-cholesterol (LDLc) from 2.76 to 3.13 mmol/l, of HDL-cholesterol (HDLc) from 1.30 to 1.99 mmol/l, and of triglycerides (TGs) from 1.16 to 1.52 mmol/l.
Mean values of FEV1 vary from 41 to 61.9% predicted, of FEV1/FVC from 43 to 59, of CRP from 2.4 to 4 mg/l, and of IL6 from 2.5 to 4.1 pg/ml. Only one study [27] provides information on GOLD class (class I in 10%, class II in 43.3%, class III in 26.7%, class IV in 20%) and no study reports data on MMRC class.
An ongoing treatment with aspirin is reported by 31.3–52.3%, with statins by 16.7–20.4%, with ACE-I by 33.3–54%, with ARBs by 6.7–21.9%, with β-blockers by 11.4–18.8%, and with CCB by 28.1–30%.
The use of β-agonists is documented in 46.6–68% of patients, of anticholinergics in 23.3–44%, of CCS in 36.6–75%, and of supplemental oxygen in 0–66.7%.
Endothelial function assessment
In eight studies [16,17,18,19, 25,26,27,28], we find that the 334 COPD patients show a significantly lower FMD than the 281 controls (MD −3.15%; 95% CI −4.89, −1.40; P < 0.001, Fig. 2). Heterogeneity among these studies is statistically significant (I 2 = 96%; P < 0.001). Moreover, a significantly lower NMD is found in two studies [25, 28] evaluating a total of 104 COPD patients and 88 controls (MD −3.53%; 95% CI −7.04, −0.02; P = 0.049, I 2 = 83.1%; P = 0.015).
Forest plot of the difference in flow-mediated dilatation (FMD) among chronic obstructive pulmonary disease (COPD) patients and controls. 95% CI 95% confidence interval, COPD chronic obstructive pulmonary disease. Gray squares represent the mean difference in FMD values among COPD patients and controls in each study. Lines are the standard deviation from the mean difference. The black diamond represents the cumulative mean difference for all included studies
Sensitivity analyses
The median value of NOS quality assessment is 7. Thus, the analyses were repeated by including only the five studies classified as “high quality” (NOS ≥7) [19, 25,26,27,28] (Supplemental Table 1). Of interest, after excluding studies classified as “low quality,” [16,17,18] our results are substantially confirmed both for FMD and NMD (Table 2).
Similarly, after excluding studies [28] potentially reporting on the same population as other included studies [18], the significant difference in FMD between COPD patients and controls is confirmed while results on NMD are unchanged (Table 2).
A sensitivity analysis according to study design (prospective or retrospective) was not performed because all included studies were prospective.
Publication bias
Because it is recognized that publication bias can affect the results of meta-analyses, we attempted to assess this potential bias using funnel plots analysis (Supplemental Fig. 1).
Funnel plots of effect size versus precision for studies evaluating FMD are rather symmetrical, suggesting the absence of publication bias and small-study effect. Egger’s and Begg and Mazumdar tests consistently confirm the absence of significant publication bias. Moreover, the Duval and Tweedie’s trim and fill analysis shows that all results are confirmed after trimming and imputing studies. Because of the low number of studies on NMD, publication bias was not assessed for this outcome.
Meta-regression analyses
Regression models for studies comparing COPD patients and healthy controls showed that age significantly impacts upon FMD (Z = 3.51; P < 0.001, Supplemental Fig. 2A).
Moreover, we find that a more severe disease (as expressed by lower FEV1 values) is associated with a more severe FMD impairment in COPD patients than in controls (Z = 3.76; P < 0.001, Supplemental Fig. 2B).
All the other demographic and clinical variables do not impact upon the observed results (Supplemental Table 4).
Because of the low number of studies, no meta-regression analysis was performed for NMD.
Discussion
Results of the present meta-analysis consistently show that COPD is associated with endothelial dysfunction. In particular, we demonstrate impaired FMD and NMD in COPD patients, confirmed by appropriate sensitivity analyses. Moreover, regression models are able to further refine results showing that a more severe disease (as expressed by lower FEV1 values) is associated with a higher difference in FMD between COPD patients and controls.
Endothelial dysfunction is the earliest stage of the atherosclerotic process, and a trigger of cardiovascular events [12]. Our findings extend results of previous studies [29], showing an increased carotid intima-media thickness (IMT) accompanied by higher prevalence of carotid plaques in patients with COPD.
Thus, the assessment of these markers consistently suggests an increased cardiovascular risk in COPD patients, which is widely confirmed by some epidemiological studies showing an increased incidence of major cardiovascular events in this clinical setting [6]. This supports the need for a strict monitoring of cardiovascular risk factors and signs of subclinical atherosclerosis in this clinical setting.
Many cardiovascular risk factors are thought to have a causal role in the atherosclerotic process [30]. Although most COPD patients have a smoking history, the relationship between subclinical atherosclerosis and COPD seems to be more complex, and the smoking habit might not entirely explain the accelerated atherosclerotic process in this clinical setting [10]. Accordingly, our regression models show that the association between COPD and endothelial dysfunction is independent of baseline smoking status and most traditional cardiovascular risk factors. Thus, other mechanisms should be considered to explain such an association.
Inflammatory and immunological mechanisms have been suggested to independently impact upon atherosclerosis progression in COPD patients [31]. Immune-mediated inflammation seems to play a crucial role in the pathogenesis of the atherosclerotic process [32,33,34], being involved in endothelial dysfunction, plaque rupture and thrombosis [35, 36]. COPD patients have elevated levels of CRP [37], a marker of inflammation associated with the atherosclerotic burden in the general population, [38, 39] and poor prognosis in COPD patients [40]. The reduction in the rate of decline of lung function with inhaled corticosteroid further confirms the role of inflammation in the pathogenesis of COPD [41]. More recently, the presence of systemic inflammation has also been documented in moderate COPD [31]. This may explain why even a modest decline in FEV1 (from a mean of 109% of predicted to 88% of predicted) is associated with a fivefold increase in cardiovascular morbidity and mortality [6]. However, a pooled analysis of large cohort studies (>500 patients) reporting on the relationship between FEV1 and cardiovascular mortality demonstrates that individuals in the lowest FEV1 quintile have a 75% increase in the risk for cardiovascular mortality compared with those in the highest FEV1 quintile [31]. Overall, these data suggest that cardiovascular risk progressively increases as the lung function decreases. This is consistent with the results of our regression analyses, showing that a more severe disease (as expressed by lower FEV1 values) is associated with a higher difference in FMD between COPD patients and controls.
Overall, our findings extend some previous evidence supporting the hypothesis that premature atherosclerosis may be one of the main features of COPD. The clinical relevance of our results can be better understood when we consider that each 1% decrease in FMD is associated with a 12% increase of cardiovascular events [42]. In contrast, few literature data are currently available on NMD, and its role as a predictor of cardiovascular events has not been properly studied [43]. In some studies on FMD, NMD is also assessed as a measure of smooth muscle function (endothelium-independent dilation). Interestingly, in the present meta-analysis, we find an impairment of both FMD and NMD, thus suggesting an impairment of both NO-dependent and NO-independent endothelial function in COPD patients. However, the very limited number of studies evaluating NMD (n = 2) should be taken into account. In addition, with the less used technique of forearm plethysmography, no significant impairment in endothelium-dependent or -independent vasomotor function has been previously documented in COPD patients [44].
Our results suggest the need for a strict monitoring of cardiovascular risk factors in COPD patients, with particular regard to those with severe disease. Careful risk stratification may orientate the choice on which COPD patients might benefit from a very strict monitoring of cardiovascular risk factors and of subclinical signs of atherosclerosis. Another issue is the possibility that COPD treatment may somehow have an impact upon the cardiovascular risk profile of these patients. The SUMMIT study [41] recently demonstrates that treatment with a combination of an inhaled corticosteroid and long-acting β-agonists reduces the rate of decline of lung function in COPD patients, but these benefits do not lead to reduction in overall mortality or cardiovascular events. However, this trial was limited to patients with moderate COPD. In the previous TORCH trial [45], inhaled combination therapy with salmeterol and fluticasone propionate seem to be associated with a 2.6% absolute reduction (17.5% relative risk reduction) in all-cause mortality in patients with moderate-to-severe COPD, and a post hoc analysis suggests that the combination treatment is also able to reduce cardiovascular mortality [46]. In keeping with this, a pilot study demonstrates that salmeterol has positive effects on alveolar fluid clearance in COPD patients, thus potentially reducing both cardiogenic and non-cardiogenic pulmonary oedema [47].
Some potential limitations of our study need to be discussed. First, studies included in our meta-analysis have different inclusion and exclusion criteria, although most of patients included in the analysis have concomitant cardiovascular risk factors (hypertension, smoking, obesity, diabetes mellitus, hyperlipidemia) and different disease activity status. Since meta-analysis is performed on aggregate data and some missing information is present in each study, the multivariate approach allows for the adjustment for some (but not all) potential confounders. Thus, although results of meta-regression analyses are able to refine analyses by assessing the influence of most clinical and demographic variables on the observed results, caution is necessary in overall results interpretation.
As a further limitation of our meta-analysis, heterogeneity among the studies is generally significant, and it is not possible to conclusively ascertain sources of heterogeneity. However, the impact of clinical and demographic data on the outcome has been evaluated by means of meta-regression models, thus partially offsetting the above limitation.
Finally, we have to consider that FMD measurement may be influenced by many confounding factors [48], significantly limiting reproducibility of FMD assessment, and in turn, the relevance of results. In particular, some studies indicate that cuff placement [49] or duration of cuff occlusion [50] may affect results.
In conclusion, COPD is significantly associated with endothelial dysfunction, and in turn, with cardiovascular morbidity and mortality. Thus, patients with severe COPD and lower FEV1 values may benefit from a more meticulous screening for cardiovascular risk factors and more specific cardiovascular prevention strategies.
References
Global Initiative for Chronic Obstructive Lung Disease (GOLD) (2009) Global strategy for the diagnosis, management and prevention of COPD. http://www.goldcopd.org. Accessed 2 June 2017
Hurd SS, Lenfant C (2005) COPD: good lung health is the key. Lancet 366(9500):1832–1834
Murray CJ, Lopez AD (1997) Alternative projection of mortality by cause 1990–2020: global burden of disease study. Lancet 349:1498–1504
Shapiro SD, Ingenito EP (1997) The pathogenesis of chronic obstructive pulmonary disease: advances in the past 100 years. Am J Respir Cell Mol Biol 32:367–372
Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW (2005) Clinical practice guidelines and quality of care for older patients with multiple comorbid disease: implications for pay for performance. JAMA 294:716–724
Sin DD, Wu L, Man SF (2005) The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 127:1952e9
Sin DD, Man SF (2005) Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc 2:8e11
Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF (2007) Systemic effects of smoking. Chest 131:1557–1566
Cebron Lipovec N, Beijers RJ, van den Borst B, Doehner W, Lainscak M, Schols AM (2016) The prevalence of metabolic syndrome in chronic obstructive pulmonary disease: a systematic review. COPD 13:399–406
Stone IS, Barnes NC, Petersen SE (2012) Chronic obstructive pulmonary disease: a modifiable risk factor for cardiovascular disease? Heart 98:1055–1062
Simon A, Chironi G, Levenson J (2006) The performance of subclinical arterial disease detection as screening test for coronary heart disease. Hypertension 48:392–396
Vaudo G, Marchesi S, Gerli R, Allegrucci R, Giordano A, Siepi D, Pirro M, Shoenfeld Y, Schillaci G, Mannarino E (2004) Endothelial dysfunction in young patients with rheumatoid arthritis and low disease activity. Ann Rheum Dis 63:31–35
Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R, International Brachial Artery Reactivity Task Force (2002) Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39:257–265
Khan F, Galarraga B, Belch JJ (2010) The role of endothelial function and its assessment in rheumatoid arthritis. Nat Rev Rheumatol 6:253–261
Ras RT, Streppel MT, Draijer R, Zock PL (2013) Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol 168:344–351
Blum A, Simsolo C, Sirchan R (2014) Vascular responsiveness in patients with Chronic Obstructive Pulmonary Disease (COPD). Eur J Intern Med 25:370–373
de Matthaeis A, Greco A, Dagostino MP, Paroni G, Fontana A, Vinciguerra M, Mazzoccoli G, Seripa D, Vendemiale G (2014) Effects of hypercapnia on peripheral vascular reactivity in elderly patients with acute exacerbation of chronic obstructive pulmonary disease. Clin Interv Aging 9:871–878
Costanzo L, Pedone C, Battistoni F, Chiurco D, Santangelo S, Antonelli-Incalzi R (2017) Relationship between FEV1 and arterial stiffness in elderly people with chronic obstructive pulmonary disease. Aging Clin Exp Res 29(2):157–164
Ozben B, Eryüksel E, Tanrikulu AM, Papila-Topal N, Celikel T, Başaran Y (2010) Acute exacerbation impairs endothelial function in patients with chronic obstructive pulmonary disease. Turk Kardiyol Dern Ars 38:1–7
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 6(7):e1000097
Juni P, Witschi A, Bloch R, Egger M (1999) The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 282:1054–1060
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Sterne JA, Egger M, Smith GD (2001) Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ 323:101–105
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455–463
Eickhoff P, Valipour A, Kiss D, Schreder M, Cekici L, Geyer K, Kohansal R, Burghuber OC (2008) Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 178:1211–1218
Hartmann SE, Waltz X, Leigh R, Anderson TJ, Poulin MJ (2016) Blood flow during handgrip exercise in COPD: effect of vitamin C. Med Sci Sports Exerc 48:200–209
Ives SJ, Harris RA, Witman MA, Fjeldstad AS, Garten RS, McDaniel J, Wray DW, Richardson RS (2014) Vascular dysfunction and chronic obstructive pulmonary disease: the role of redox balance. Hypertension 63:459–467
Moro L, Pedone C, Scarlata S, Malafarina V, Fimognari F, Antonelli-Incalzi R (2008) Endothelial dysfunction in chronic obstructive pulmonary disease. Angiology 59:357–364
Ambrosino P, Lupoli R, Cafaro G, Iervolino S, Carone M, Pappone N, Di Minno MND (2017) Subclinical carotid atherosclerosis in patients with chronic obstructive pulmonary disease: a meta-analysis of literature studies. Ann Med 49:513–524
Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ (2014) Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes 5:444–470
Sin DD, Man SF (2003) Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 107:1514–1519
Di Minno MN, Ambrosino P, Lupoli R, Di Minno A, Tasso M, Peluso R, Tremoli E (2015) Clinical assessment of endothelial function in patients with rheumatoid arthritis: a meta-analysis of literature studies. Eur J Intern Med 26:835–842
Ambrosino P, Lupoli R, Tarantino P, Di Minno A, Tarantino L, Di Minno MN (2015) Viral hepatitis and anti-phospholipid antibodies positivity: a systematic review and meta-analysis. Dig Liver Dis 47:478–487
Ambrosino P, Tasso M, Lupoli R, Di Minno A, Baldassarre D, Tremoli E, Di Minno MN (2015) Non-invasive assessment of arterial stiffness in patients with rheumatoid arthritis: a systematic review and meta-analysis of literature studies. Ann Med 47:457–467
van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC (2004) Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation 109:1089–1094
Ambrosino P, Tarantino L, Criscuolo L, Nasto A, Celentano A, Di Minno MN (2016) The risk of venous thromboembolism in patients with hepatitis C. A systematic review and meta-analysis. Thromb Haemost 116:958–966
Sin DD, Man SF (2007) Systemic inflammation and mortality in chronic obstructive pulmonary disease. Can J Physiol Pharmacol 85:141–147
Quaglia LA, Freitas W, Soares AA, Santos RA, Nadruz W Jr, Blaha M, Coelho OR, Blumenthal R, Agatston A, Nasir K, Sposito AC (2014) C-reactive protein is independently associated with coronary atherosclerosis burden among octogenarians. Aging Clin Exp Res 26:19–23
Ridker PM, Hennekens CH, Buring JE, Rifai N (2000) C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 342:836–843
Man SF, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD (2006) C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax 61:849–853
Vestbo J, Anderson JA, Brook RD, Calverley PM, Celli BR, Crim C, Martinez F, Yates J, Newby DE, SUMMIT Investigators (2016) Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet 30(387):1817–1826
Shimbo D, Grahame-Clarke C, Miyake Y, Rodriguez C, Sciacca R, Di Tullio M, Boden-Albala B, Sacco R, Homma S (2007) The association between endothelial dysfunction and cardiovascular outcomes in a population-based multi-ethnic cohort. Atherosclerosis 192:197–203
Raitakari OT, Seale JP, Celermajer DS (2001) Impaired vascular responses to nitroglycerin in subjects with coronary atherosclerosis. Am J Cardiol 87(217–219):A8
Maclay JD, McAllister DA, Mills NL, Paterson FP, Ludlam CA, Drost EM, Newby DE, Macnee W (2009) Vascular dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 180:513–520
Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J, TORCH investigators (2007) Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 356:775–789
Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Crim C, Willits LR, Yates JC, Vestbo J, Investigators TORCH (2010) Cardiovascular events in patients with COPD: TORCH study results. Thorax 65:719–725
Di Marco F, Guazzi M, Sferrazza Papa GF, Vicenzi M, Santus P, Busatto P, Piffer F, Blasi F, Centanni S (2012) Salmeterol improves fluid clearance from alveolar–capillary membrane in COPD patients: a pilot study. Pulm Pharmacol Ther 25:119–123
Soltész P, Erekes G, Dér H, Szücs G, Szántó S, Kiss E, Bodolay E, Zeher M, Timár O, Szodoray P, Szegedi G, Szekanecz Z (2011) Comparative assessment of vascular function in autoimmune rheumatic diseases: considerations of prevention and treatment. Autoimmun Rev 10:416–425
Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J (2001) Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci 101:629–635
Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ (2001) Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo. Circ Res 88:145–151
Author information
Authors and Affiliations
Contributions
AP, LR and DMMND conceived and designed the study, performed statistical analysis interpreted results and drafted the manuscript. IS, DFA, PN and SA acquired clinical data, drafted the manuscript and performed critical revisions. All authors read and approved the final version of the manuscript. AP had full access to all data in the study, and takes responsibility for the integrity of the data and the accuracy of data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Statement of human and animal rights
This article does not contain any studies with human participants or animals performed by the authors.
Informed consent
None.
Funding
No funding or economic support has been received for this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ambrosino, P., Lupoli, R., Iervolino, S. et al. Clinical assessment of endothelial function in patients with chronic obstructive pulmonary disease: a systematic review with meta-analysis. Intern Emerg Med 12, 877–885 (2017). https://doi.org/10.1007/s11739-017-1690-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-017-1690-0