Abstract
The mechanisms of tolerance to desiccation are one of the main factors related to the ability to survive the conditions of water deficit imposed by abiotic stress. Understanding the limits of desiccation tolerance in species and environmental factors promotes this capacity, which is of great ecological importance since it can help in the choice of species used for ecological recovery. In this study, we analyze tolerance limits, and physiological and biochemical parameters in desiccation tolerance (DT) of Tabebuia aurea (Silva Manso) Benth. & Hook.f. ex S. Moore (Bignoniaceae) seeds and germinating seeds. First, we analyzed the DT in seeds under different free water contents (0.25, 0.75, 1.5, 2.25, and 3% of free water content) in silica gel at 25 °C and forced air circulation oven at 40 °C. During development, we evaluated the ability of germinating seeds with different root sizes (0 to 2, 2 to 5, and 5 to 10 mm) to tolerate desiccation. We quantified reducing sugars and total proteins in all evaluated treatments. Seeds and seedlings of T. aurea showed large DT to both types of desiccation. The concentration of reducing sugars increased with decreasing seed-free water contents. The germinating seed also contents of reducing sugars reduced. We conclude that the large DT before and after germination of T. aurea with roots of up to 10 mm is related to changes in biochemical mechanisms that are important to maintaining this tolerance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Desiccation tolerance is the ability to survive extreme water deficits without accumulating lethal damage after tissue rehydration (Alpert 2005; Oliver et al. 2020). This ability is acquired during seed formation, and tolerance activation occurs at the end of the maturation phase, which is followed by a marked decrease in the moisture content of the seeds and characterizes the desiccation phase (Pereira et al. 2017). After the acquisition of desiccation tolerance, seeds with low moisture content are dispersed in the environment and can withstand soil water deficit conditions after dispersion (Thompson 2017).
Desiccation tolerance is a relatively rare trait among flowering plants, with only a small percentage of species possessing this adaptation (Wyse and Dickie 2017). Desiccation tolerance in angiosperm seeds is achieved through a complex interplay of physiological and biochemical mechanisms that enable the embryo to survive complete dehydration (Oliver et al. 2020). Proteins, enzymes, and carbohydrates are produced at the end of the maturation phase during seed development, being important for the response to desiccation. Among the proteins produced are abundant late embryogenesis (LEA) proteins and thermal shock proteins involved with the protection of cell membranes and renaturation of proteins, promoting a vitreous state. In addition to proteins, oligosaccharides act by preventing the disintegration of membranes (Oliver et al. 2020; Matilla 2022).
Desiccation-tolerant organisms do not prevent water loss but, alternatively, use physiological mechanisms to minimize the damage desiccation causes (Matilla 2022). However, the desiccation rate can affect the survival of these organisms. A rapid desiccation may not allow the necessary time to induce the physiological mechanisms important to minimize the damage water loss causes (Clegg 2005). On the other hand, a slow desiccation prolongs the time of low water content and reduced metabolism, which can be physiologically harmful to the embryo (Walters et al. 2005).
Tolerance to desiccation in angiosperms is usually lost after seed germination (Daws et al. 2007; Maia et al., 2011). One of the sources of mortality is the loss of the ability to tolerate seedling desiccation (Daws et al. 2007). The period of loss of tolerance to desiccation may vary between seeds and seedlings of different species. The tolerance to desiccation may be lost soon after the protrusion of the radicle or when it reaches 2 mm in length (Pereira et al. 2018). Irregularities of rainy events, such as short periods of drought during the rainy season, can cause the death of seedlings if subjected to desiccation in the environment where they were established (Tweddle et al. 2003).
Tabebuia aurea (Silva Manso) Benth. & Hook.f. ex S. Moore (Bignoniaceae) is a species with wide distribution in Brazil occurring from humid environments to places with low availability of water in the soil such as the Caatinga. Its seeds do not present dormancy and have a tegument that allows the loss of water to the medium being subject to desiccation in the medium. In environments where water evaporates rapidly from the soil and presents a low humidity this species is forming a seedling bank (Cabral et al. 2003). The characteristics of T. aurea differ from angiosperm plants that support extreme and prolonged desiccation events since they do not present a preserved quiescent state protected against water loss to the medium (Vanburen et al. 2018). The presence of desiccation tolerance in seeds and seedlings of this species may bring a new response to the mechanisms of tolerance to desiccation in seeds and seedlings of angiosperms.
In this sense, the present study aimed to analyze the limits, physiological parameters, and biochemical mechanisms of desiccation tolerance in T. aurea. Four questions led to this study: (1) What is the level of desiccation tolerated by T. aurea seeds in the germination period? (2) Is desiccation tolerance lost after seed germination? (3) Is there a tolerance desiccation limit for T. aurea germinating seeds? (4) What are some of the biochemical mechanisms involved in allowing the seed, embryo, or seedling to survive desiccation at each stage? (5) Does drying methods affect TD? To answer these questions, we analyzed the physiological and biochemical parameters in three experiments that evaluated the effects of desiccation during and after germination.
Materials and methods
Characterization of the seed collection area
The Caatinga is a Brazilian Tropical Dry Forest, a typical vegetation formation in semiarid climates. According to the Köppen–Geiger climate classification system, the climate of this ecosystem is BSh and varies from semiarid to tropical sub-humid. The annual rainfall is irregularly distributed in time and space. The average temperatures vary from 26 to 29 °C. The combination of higher temperatures, sandy soils, and rains concentrated over a short period of time results in the rainwater quickly evaporating from the ground (Alves 2009). In the state of Sergipe, this ecosystem covers an area that extends from the municipality of Canindé de São Francisco, in the extreme northwest of the state, to the municipality of Tobias Barreto in the southwest of the state.
We collected seeds of T. aurea from twenty matrices located in the Caatinga areas of the municipality of Canindé de São Francisco. The climate in the region is characterized by low rainfalls during the year (~ 520 mm yr−1) and an average annual temperature of 25.3 °C (Climate Data 2018).
Experiment I: influence of desiccation on the pre-germination period
After procuring the seeds, we determined their initial free water content using the standard oven method at a temperature of 105 °C for 24 h (ISTA 1993). The initial water content of the seeds was found to be 3%.
We determined the seed desiccation curve to assess the influence of desiccation on the seeds and seedlings’ behavior of T. aurea. To determine the desiccation curve, we used 200 seeds of T. aurea divided into eight replications of 25 seeds each. After separating the samples, we weighed the seeds of each replication on an analytical balance and used them to execute two types of desiccation. We placed four replications in a forced air circulation oven at 40 °C, and the other four replications remained inside a Gearbox with a separation screen over 40 g of silica gel at 25 °C for 72 h. We weighed the seeds at one-hour intervals to monitor desiccation. We repeated this procedure until there was no more variation in seed weight for three consecutive weighing procedures. From this desiccation curve, it was possible to determine how long the seeds would take to reach a certain percentage of their free water content during the phase of total drying of the seeds.
Based on the desiccation curves, ten treatments were selected (3, 2.25, 1.5, 0.75, and 0.25%), corresponding to the varying degrees of water loss induced by the two desiccation methods used. We subjected T. aurea seeds to these desiccation treatments using the same procedure as for determining the desiccation curve (Fig. 1). After desiccation, we placed the seeds to germinate in 15 cm diameter Petri dishes lined with a double layer of filter paper moistened with 25 mL of distilled water. The seeds were placed in BOD at a temperature of 25 °C and a photoperiod of 12 h. Each treatment comprised 100 seeds and divided into four replications of 25 seeds each. We monitored germination completion daily and evaluated the seeds ten days after the start of observations. The criterion for considering a seed as germinated was the protrusion of the root.
Experiment II: influence of seed desiccation on germinating seeds
The determination of the initial content of germinating seeds was carried out by the standard oven method at a temperature of 105 °C for 24 h (ISTA 1993). The initial water content of the seeds was 30%. To evaluate DT in the germination period, we placed the seeds to germinate in Petri dishes 15 cm in diameter lined with a double layer of filter paper and moistened with 25 mL of distilled water. After root protrusion, we separated the germinating seeds according to root size into three groups: < 2 mm, 2–5 mm, and 5–10 mm. Each group consisted of one treatment. We submitted the germinating seeds of each treatment to maximum desiccation using the two types of desiccation as evaluated in Experiment I. For the germinated seeds, the desiccation times were recalculated using as a basis the free water content and the desiccation time determined by the curves of Experiment I. After total desiccation, we placed the germinated and again desiccated seeds in Petri dishes 15 cm in diameter lined with a double layer of filter paper and moistened with 25 mL of distilled water to evaluate resumption of development. The seeds were placed in biochemical oxygen demand (BOD) at a temperature of 25 °C and a photoperiod of 12 h. Each treatment consisted of 100 seedlings divided into four replications of 25 seedlings each. We evaluated the continuation of establishment daily, and evaluations finished ten days after the beginning of observations. The criterion for considering the resumption of development was the growth or the production of new roots after total drying of seedlings.
Biochemical analyses
For biochemical analyses, we used embryos with roots of 5–10 mm from all treatments evaluated in experiments I and II. We performed an extraction using centrifugation (4000 rpm for 60 min) of 0.1 g of macerated sample in 5 ml of 0.1 M phosphate buffer solution, pH 7.5, with 1 mM ethylenediaminetetraacetic acid (EDTA), 3 mM dithiothreitol (DTT), and 5% polyvinylpolypyrrolidone(PVPP) (Gomes-Junior et al 2006). After centrifugation, we discarded the pellet and removed the supernatant using an automatic pipette. We stored the aliquots in 1.5 ml Eppendorf and kept them in the freezer at –5 °C until the quantification of macromolecules. We quantified reducing sugars (RS) from the reaction of 250 μL of the aliquot with 3.5 dinitrosalicylic acid (DNS) using glucose solution as blank and performing spectrophotometer readings at 545 nm wavelength (Miller 1959). We quantified soluble proteins (PT) from the reaction of 100 μL of the aliquot with Coomassie Blue using BSA-Casein solution as blank and performing spectrophotometer readings at 595 nm wavelength (Bradford 1976).
Statistical analyses
At the end of observations, we calculated germinability (G = %), and T50 by the formula T50 = ti + (N/2–ni) (tj–ti) / nj–ni, where N is the total number of seeds that have completed germination, ni and nj are the number of seeds that had completed germination according to the following structure: ni < N/2 < nj, and ti and tj are the days when ni and nj occurred (Farooq et al. 2005) The normality of data residuals and the homogeneity of variances were verified using the Shapiro–Wilk and Levene’s tests, respectively. We submitted the results of experiments I and II to two-way ANOVA (type and degree of desiccation) in Experiment I, and two-way (size root and desiccation type) in Experiment II. We compared the averages a posteriori by Tukey’s test (Ranal and Santana 2006). The biochemical analyses followed the same experimental design, and we analyzed reducing sugars and total proteins in all experiments in the same way as described for germinative parameters. We performed all statistical analyses using the software STATISTICA 13 at α = 5% (StatSof 2016).
Results
Experiment I: influence of desiccation on the pre-germination period
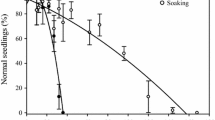
Four hours of drying on silica gel at 25 °C and six hours in the forced air circulation were necessary to achieve maximum desiccation of seeds (Fig. 1). The seeds are tolerant of desiccation during the quiescence period, since they subsequently complete germination up to a percentage of more than 80% when placed on water even with a reduction to free water content of 0.25% in both desiccation treatments (Fig. 2). In addition, we observed 100% of normal seedling formation from seeds that completed germination in the treatments evaluated.
Germinability (G -%) and T50 (days) of Tabebuia aurea (Silva Manso) Benth seeds. & Hook.f. ex S. Moore (Bignoniaceae) submitted to 0.25, 0.75, 1.50, 2.25, and 3% of the free water content in the two types of desiccation. Data shown represent means ± standard error. Different letters indicate significant differences between free water content (upper case) and type of desiccation (lower case) according to Tukey’s test at P < 0.05
For seeds dried to 0.75% of free water content, seeds subsequently completed germination to a greater percentage if the seeds were dried over silica gel than those dried using forced air circulation (Table 1). The T50 differed between free water content treatments and types of desiccation (Table 1). The T50 increased as free water content decreased regardless of how the seeds were dried. This increase was significantly greater in seeds that underwent silica gel compared to those submitted to forced air circulation in the treatments 0.75, 1.50, and 2.25% of free water content (Fig. 2).
Experiment II: influence of desiccation of germinating seeds
Germinating seeds were dried for 40 h in silica gel at 25 °C and sixty hours in forced air circulation were required to reach maximum desiccation. The high desiccation tolerance observed in the quiescent period was maintained during the post-germination period. More than 80% of germinating seeds of T. aurea resumed development after the establishment was interrupted by desiccation down to 0.25% of the free water content. We observed this result in all root-size treatments evaluated in this study, with no differences regarding the types of desiccation evaluated (Fig. 3). However, when assessing seedling desiccation tolerance after the completion of germination, there was a significant difference in how rapidly seedlings resumed development between different root size treatments for seedlings desiccated over silica gel, not for those desiccated using forced air circulation (Table 2). Seedlings with 5–10-mm roots took longer to resume development compared to seedlings with root lengths < 2 mm, and this was for seedlings desiccated over silica gel only. There were no differences apparent for seedlings, regardless of root size, desiccated using forced air circulation (Table 2; Fig. 3).
A Seeds growth recommencement (SGR-%) and B T50 (days) of Tabebuia aurea (Silva Manso) Benth seedlings. & Hook.f. ex S. Moore (Bignoniaceae) with different root sizes and subjected to two types of desiccation (0.25% of the initial water content). Data shown represent means ± standard error. Different letters indicate significant differences among root sizes (upper case) and type of desiccation (lower case) according to Tukey’s test at P < 0.05
In addition, we also observed three different strategies for resuming development after desiccating seedlings in the evaluated treatments. Some seedlings, after being desiccated and rehydrated, resumed growth with the emission of a new primary root through the meristematic region. On the other hand, some seedlings emitted adventitious roots before a new primary root formed and, finally, some seedlings expanded the cotyledons and invested in the development of shoots prior to the development of a new primary root (Fig. 4).
Resumption of growth A growth of adventitious roots; B new root formation, C cotyledon emergence and expansion from Tabebuia aurea (Silva Manso) seedlings Benth. & Hook.f. ex S. Moore (Bignoniaceae) with different root sizes subjected to two types of desiccation (0.25% of the free water content). Color green in the images corresponds to the area of the plant
Biochemical analyses
In experiment I, there was no significant difference in the amount of reducing sugars between types of desiccation (Table 1). However, the amount of these sugars doubled in seeds that were subjected to 0.25% treatment compared to the amount of reducing sugars in 3% treatment in both types of desiccation (Table 3). The protein content did not vary between treatments in that experiment.
The contents of reducing sugars in the root size treatments of experiment II significantly reduced compared to that of the control (quiescent seeds that underwent desiccation). We found the same response for the two types of desiccation evaluated. However, the amount of sugars did not differ between the largest and the smallest root size evaluated in both types of desiccation (Table 4). Regardless of the size of the root when the seedlings were desiccated to 0.25% of free water content, those desiccated over silica gel consistently had greater reducing sugar amounts than seedlings desiccated by forced air circulation (Table 4).
Discussion
The research involved the desiccation tolerance of seeds and seedlings of T. aurea, native to South America. Seed germination is an important topic in semi-arid and arid environments due to the necessity to get the timing and location propitious of germination to maintain populations over the longer term. The Caatinga is characterized by regular, long-lasting dry periods (i.e., months or more). Therefore, desiccation tolerance helps plants survive the establishment phase in this ecosystem.
The seeds of the studied population presented a low initial water content. The fruit collection area is located in Brazil’s region Polygon of Droughts and stands out for the low rainfall indices and their interannual irregularity, together with high evapotranspiration that configures high rates of aridity (Gois et al. 2022). In addition to the parental environment being drier, species of the same genus show a variation of up to 6% in water content in different populations. Tabebuia aurea seeds had water contents of 12.53% and 6.5% in batches from different populations (Cabral et al. 2003; Brito et al. 2020).
The ability to tolerate desiccation is important for maintaining the survival of seeds that are subjected to severe desiccation because of the stability of dry tissues (Smolikova et al. 2021). Other authors conducted evaluations of desiccation tolerance in the studied species in periods that reached up to 48 h of drying at 24 °C and reported that seeds maintained about 4% of water content. In an analysis conducted by other investigators, germination percentage was reduced when seeds were subjected to extreme desiccation (Nassari et al. 2014). This is attributed to the prolonged exposure time to desiccation conditions (Guimarães et al. 2016). However, in our current study, the seeds of T. aurea showed a high desiccation tolerance when subjected to extreme desiccation over a short period. The high temperatures in the soil of arid and semiarid environments promote rapid evaporation of water from the superficial layers, and the desiccation of seeds also occurs in a short period, especially in species with thinner integuments, as is the case of T aurea seeds.
The metabolic activity decreased due to a reduction in seed water content during desiccation. Therefore, a decrease in water content increases the time seeds take to germinate, which is due to reduced metabolism and, consequently, more time is needed for water reabsorption, metabolic reactivation, and germination (Calderón-Hernández and Pérez-Martínez 2018). We found this behavior for the two types of desiccation. The T50 increased with decreasing seed water content.
The characterization of some species as to the ability of seeds to tolerate desiccation evidence that this tolerance is generally lost at the end of the germination process by root protrusion (Castro et al. 2017). The seeds of Bowdichia virgilioides Kunth (Fabaceae), Cedrela fissilis Vell. (Meliaceae), Enterolobium contortisiliquum (Vell.) Morong (Fabaceae), Handroanthus impetiginosus (Mart. Ex DC.) Mattos (Bignoniaceae), and Libidibia ferrea (Mart. ex Tul.) L.P. Queiroz (Fabaceae) decreased their capacity to complete germination or recommence growth, respectively, from an average of 60% before root protrusion to 0%. Upon completion of germination, we also observed a decrease in the ability to restore tolerance to desiccation of these species (Maia et al. 2011).
Some studies have shown that, during the germination process, the mechanisms that provide seeds with tolerance to desiccation are deactivated (Guimaraes et al. 2016). Thus, root protrusion signifies the loss of this tolerance for many species (Dekkers et al 2015). With the intensification of seed metabolism and the consumption of reserves, there is a progressive deactivation of mechanisms involved in desiccation tolerance until it is completely lost (Nascimento et al. 2007). However, the period of loss of tolerance to desiccation varies for seeds of tree species. In studies on Anadenanthera colubrina (Vell.) Brenan (Fabaceae), Castro, Guimarães, and Faria observed that seeds of this species totally lost their tolerance to desiccation after root protrusion. The seeds of Sesbania virgata (Cav.) Pers. (Fabaceae) lose tolerance to desiccation when roots reach 2 mm in length. In our study, the seeds of T. aurea with roots of up to 10 mm tolerated extreme desiccation, indicating a much greater tolerance than that of all species mentioned previously at the critical moment of plant ontogeny.
The desiccation tolerance pattern is related to the environment where seeds are formed. The high relative air humidity at the moment of dispersal favors the synthesis of macromolecules, which are important to provide a greater or a lesser tolerance to desiccation (Guimarães et al. 2016; Ribeiro et al. 2016). The ability of seeds to tolerate desiccation, even after germination, can characterize a great survival capacity even in environments with greater water restrictions (Vieira et al. 2010). Although A. colubrina seeds submitted to desiccation have reduced the formation of normal seedlings after 12 h of imbibition (Guimaraes et al. 2016), the seedlings of T. aurea managed to establish themselves even after total desiccation with roots of up to 10 mm, showing a great tolerance of seedlings to the loss of water to the medium during their establishment.
The different strategies for resuming of development presented by T. aurea seedlings demonstrated the high degree of tolerance to desiccation of this species. The growth of secondary roots, formation of a new root, and expansion of cotyledons before root development showed the resilience of the species to resume seedling development after desiccation. The maintenance of development, even after the death of the primary root, evidenced the high degree of tolerance to the desiccation of this species, making it an interesting model for future studies. Only one of these strategies was demonstrated in the study by Angelovici, Galili, Fernie, and Fait after Handroanthus impetiginosus (Mart. Ex DC.). Mattos seedlings suffered desiccation that led to the death of the roots, and they resumed development with the production of adventitious roots.
At the end of the LEA proteins and non-reducing sugars accumulated; including sucrose, stachyose, and raffinose (Hundertmark and Hincha 2010). The expression of LEA proteins is related to the acquisition of desiccation tolerance in orthodox seeds (Buitink and Leprince 2008). Non-reducing sugars, on the other hand, fill the free spaces between macromolecules. Such spaces are created by the loss of water during desiccation (Xie et al. 2021). The desiccation tolerance of Handeliondendron bodinieri seeds is closely related to the soluble sugar content and protein content, and the storage of sugars and the stability of proteins provide this tolerance (Xie et al. 2021). However, although seeds did not show changes in protein content during desiccation in experiment I, we observed a 50% increase in the contents of reducing sugars when we subjected T. aurea seeds to the highest degree of desiccation in both types of desiccation evaluated.
The ability of some species to maintain tolerance to desiccation after germination is related to adaptation to stress (Daws et al. 2007). The decrease in oligosaccharide content is related to the advancement of the soaking process, and the loss of ability to tolerate desiccation in some species coincides with the increase in reducing sugars (Koster and Leopold 1988). The decrease in contents of reducing sugars in seedlings that have undergone desiccation and preservation of protein content may have enabled the maintenance of meristematic cells during desiccation and allowed the resumption of twinning and seedling development. This can be justified because, at the advanced stages of orthodox seed maturity, there is a reduction in the level of monosaccharides, which allows the formation of oligosaccharides (Kigel and Galili 1995).
T. aurea seeds showed high tolerance to desiccation even under low free water content after germination. Therefore, the ability to tolerate desiccation of the studied seeds is not lost after germination. Seeds with up to 10 mm of radicle can tolerate water loss and resume growth when rehydrated. Furthermore, the different desiccation methods did not influence the ability of the seed to tolerate water loss.
The biochemical analyses of the treatments evaluated in the present study demonstrated that the promoted desiccation caused alteration in the levels of reducing sugars and related substances. The seeds that were subjected to greater water loss in the evaluated treatments had an increase in this type of sugar; despite this, they managed to withstand desiccation and germinate.
The high tolerance to desiccation in species of our study makes them an interesting model for studies on the mechanisms involved with the resumption of growth and germination after the submission of tissues to very low water contents. This tolerance may be related to the ability to maintain, in the case of seedlings, the meristematic region intact to damages caused by the water leaving the tissues, which, after rehydration, promotes the formation of new roots, a topic that deserves investigation in future works. Thus, the understanding of the anatomical, physiological, and molecular aspects involved with the tolerance of this species can guide in expanding the limits of tolerance to desiccation in other species.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alpert P (2005) The limits and frontiers of desiccation-tolerant life. Integr Comp Biol 45:685–695. https://doi.org/10.1093/icb/45.5.685
Alves JJA (2009) Degradação da Caatinga: Uma investigação ecogeográfica. Caatinga 22:126–135
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Brito WADL, Pereira KTO, Nogueira NW, Torres SB, Paiva EPD (2020) Evaluation of viability of Tabebuia aurea seeds through tetrazolium test. Revista Caatinga 33:993–999. https://doi.org/10.1590/1983-21252020v33n414rc
Buitink J, Leprince O (2008) Intracellular glasses and seed survival in the dry state. CR Biol 331:788–795. https://doi.org/10.1016/j.crvi.2008.08.002
Cabral EL, Barbosa DCDA, Simabukuro EA (2003) Armazenamento e germinação de sementes de Tabebuia aurea (Manso) Benth. & Hook. F. ex. S. Moore. Acta Botanica Brasilica 17:609–617. https://doi.org/10.1590/S0102-33062003000400013
Calderón-Hernández M, Pérez-Martínez LV (2018) Seed desiccation tolerance and germination of four Puya (Bromeliaceae) high-andean tropical species from Colombia. Caldasia 40:177–187. https://doi.org/10.15446/caldasia.v40n1.67740
Castro LE, Guimarães CC, Faria JMR (2017) Physiological, cellular and molecular aspects of the desiccation tolerance in Anadenanthera colubrina seeds during germination. Braz J Biol 77:774–780. https://doi.org/10.1590/1519-6984.00616
Clegg JS (2005) Desiccation tolerance in encysted embryos of theanimal extremophile. Artemia Integr Comp Biol 45(715–724):2005. https://doi.org/10.1093/icb/45.5.715
Climate Data (2018) Clima Canindé de São Francisco. https://pt.climate-data.org/america-do-sul/brasil/sergipe/caninde-de-sao-francisco-42944/ Accessed 26 June 2018
Daws MI, Bolton S, Burslem DFR et al (2007) Loss of desiccation tolerance during germination in neo-tropical pioneer seeds: implications for seed mortality and germination characteristics. Seed Sci Res 17:273–281. https://doi.org/10.1017/S0960258507837755
Dekkers BJ, Costa MCD, Maia J et al (2015) Acquisition and loss of desiccation tolerance in seeds: from experimental model to biological relevance. Planta 241:563–577
Farooq M, Basra SMA, Ahmad N et al (2005) Thermal hardening: a new seed vigor enhancement tool in rice. J Integr Plant Biol 47:187–193. https://doi.org/10.1111/j.1744-7909.2005.00031.x
Gois DV, Melo FP, Melo R (2022) Risco à desertificação nos municípios de Canindé de São Francisco e Poço Verde (SE). Revista Ciência Geográfica 26:03–126. https://doi.org/10.18817/26755122.26.01.2022.2875
Gomes-Junior RA, Moldes CA, Delite FS et al (2006) Antioxidant metabolism of coffee cell suspension cultures in response to cadmium. Chemosphere 65:1330–1337. https://doi.org/10.1016/j.chemosphere.2006.04.056
Guimaraes CC, Maia J, Faria JMR et al (2016) Changes in gene expression and soluble carbohydrate contents during the imbibition and re-induction of desiccation tolerance in Peltophorum dubium seeds. Seed Sci Technol 44:125–137. https://doi.org/10.15258/sst.2016.44.1.20
Hundertmark M, Hincha DK (2010) LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics 9:118–122. https://doi.org/10.1186/1471-2164-9-118
ISTA - International Rules for Seed Testing (1993) Seed Science and Technology 21: 363 (ssupplement)
Kigel J, Galili G (1995) Seed development and germination Marcel Dekker, New York
Koster KL, Leopold AC (1988) Sugars and desiccation tolerance in seeds. Plant Physiol 88:829–832. https://doi.org/10.1104/pp.88.3.829
Maia J, Dekkers BJW, Provart NJ et al (2011) The re-establishment ofdesiccation tolerance in germinated Arabidopsis thaliana seeds and its associated transcriptome. PLoS One 6:1–11. https://doi.org/10.1371/journal.pone.0029123
Matilla AJ (2022) The orthodox dry seeds are alive: a clear example of desiccation tolerance. Plants 11:10–20
Miller GL (1959) Use of dinitrosalicilic acid reagent for determination of reducing sugar. Anal Chem 31(426–428):1959. https://doi.org/10.1021/ac60147a030
Nascimento WMO, Novembre ADLC, Cicero AM (2007) Consequências fisiológicas da dessecação em sementes de açaí (Euterpe oleracea Mart). Revista Brasileira De Sementes 29:38–43. https://doi.org/10.1590/S0101-31222007000200006
Nassari PJ, Keshavulu K, Rao M, Reddy KCS, Raheem A (2014) Post-harvest drying of tomato (Lycopersicon esculentum Mill) seeds to ultra-low moisture safe for storage using desiccant (zeolite) beads and their effects on seed quality. Am J Res Com 2:74–83
Oliver MJ, Farrant JM, Hilhorst HW et al (2020) Desiccation tolerance: avoiding cellular damage during drying and rehydration. Ann Rev Plant Biol 71:435–460. https://doi.org/10.1146/annurev-arplant-071219-105542
Pereira WVS, Faria JMR, Tonetti OAO et al (2018) Loss of desiccation tolerance in seeds of tree species during germination: theoretical and practical implications. Revista Árvore 42:1–9. https://doi.org/10.1590/1806-90882018000500003
Pereira Lima JJ, Buitink J, Lalanne D, Rossi RF et al (2017) Molecular characterization of the acquisition of longevity during seed maturation in soybean. PLoS One 12:1–25. https://doi.org/10.1371/journal.pone.0180282
Ranal MA, Santana DG (2006) How and why to measure the germination process? Revista Brasileira De Botânica 29:1–11. https://doi.org/10.1590/S0100-84042006000100002
Ribeiro DE, Alvarenga AA, Martins JR et al (2016) Germination and re-induction of desiccation tolerance in seeds of Senna multijuga (Rich.) Irwin Et Barn (Rich.) Irwin et Barn. Ciência Florestal 26:1133–1140. https://doi.org/10.5902/1980509825031(2016)
Smolikova G, Leonova T, Vashurina N et al (2021) Desiccation tolerance as the basis of long-term seed viability. Int J Mol Sci 22:101. https://doi.org/10.3390/ijms22010101
StatSof (2016) STATISTICA 13. StatSoft South America. http://www.statsoft.com.br. Accessed 04 June 2018
Thompson R (2017) Letters to the twenty-first century botanist. Second series: “What is a seed? Bot Lett 165:3–5. https://doi.org/10.1080/23818107.2018.1476177
Tweddle JC, Dickie JB, Baskin CC et al (2003) Ecological aspects of seed desiccation sensitivity. J Ecol 91:294–304. https://doi.org/10.1046/j.1365-2745.2003.00760.x
Van Buren R, Man Wai C, Pardo J et al (2018) Desiccation tolerance evolved through gene duplication and network rewiring in Lindernia. Plant Cell 30:2943–2958. https://doi.org/10.1105/tpc.18.00517
Vieira CV, Silva EAA, Alvarenga AA et al (2010) Stress-associated factors increase after desiccation of germinated seeds of Tabebuia impetiginosa Mart. Plant Growth Regul 62:257–263. https://doi.org/10.1007/s10725-010-9496-3
Walters C, Hill LM, Wheeler LJ (2005) Dying while dry: kinetics and mechanisms of deterioration in desiccated organisms. Integr Comp Biol 45:751–758. https://doi.org/10.1093/icb/45.5.751
Wyse SV, Dickie JB (2017) Taxonomic affinity, habitat and seed mass strongly predict seed desiccation response: a boosted regression trees analysis based on 17 539 species. Ann Bot 121:71–83. https://doi.org/10.1093/aob/mcx128
Xie C, Liu T, Guo S et al (2021) Effects of ultra-dry storage on seed germination and seedling growth of Handeliondendron bodinieri. Silva Fennica 55:1–21. https://doi.org/10.14214/sf.10509
Acknowledgements
This study was funded by the Sergipe State Research and Innovation Support Foundation (FAPITEC).
Author information
Authors and Affiliations
Contributions
CS and MV conceived and designed research. CS conducted experiments. CG contributed new reagents or analytical tools. CS and MV analyzed data. CS wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Additional information
Communicated by S. Santos.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santos, C.S., de Siqueira, C.G. & Meiado, M.V. Desiccation sensitivity of fresh and germinating seeds of Tabebuia aurea: physiological and biochemical implications. Acta Physiol Plant 46, 58 (2024). https://doi.org/10.1007/s11738-024-03676-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-024-03676-2