Abstract
Regarding worldwide water shortage due to climate change impacts, reducing water consumption for plant irrigation without a significant reduction in plant yield is of great importance. Silicon is one of the beneficial elements for plants and has positive effects on plant physiology and biochemistry. A field experiment was conducted to evaluate the potassium silicate alleviating effect (K2SiO3; 0 (S0), 5 (S5), and 10 (S10) mM) on sweet corn yield at different irrigation cycles (70 as control (I70), 105 (I105), and 140 (I140) mm water evaporation from class A evaporation pan). Results showed that water-deficit reduced leaf photosynthetic pigments, relative water content, and yield components. However, soluble sugars, proteins, and proline content and the activity of superoxide dismutase, catalase, and ascorbic peroxidase of leaves were increased by water deficit. Foliar spraying of K2SiO3 effectively reduced the adverse effects of water deficit and improved the physiological traits and grain yield of plants. The S10I70 and S10I105 (786 and 788 g m−2, respectively) produced the greatest grain yield. The grain number per ear with the highest correlation with grain yield (r = 0.78) was found as the most determining yield component. It is concluded that the I105, due to reducing water consumption, along with S10, can be considered to obtain the desired performance in sweet corn.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sweet corn (Zea mays var. saccarata) is a variety of Zea mays that is ranked as the fourth most valuable crop among vegetables for fresh consumption and the second for the processing industries (Kalloo and Bergh 2012). Sweet corn is a mutant by a mutation in the locus SU, which is isolated from the dent corn (Levitt 1980). This gene leads to a double accumulation of sugars in the endosperm compared with regular corn. Fruits (ear) are the main purpose of sweet corn cultivation. Sweet corn fruits have a high nutritional value and are rich in minerals such as potassium, calcium, magnesium, and phosphorus, and protein, vitamins, and sugars.
Environmental stresses prevent plant genetic potential performance. Drought is a critical abiotic stress that limits crop productivity in areas subjected to water scarcity (Ashraf 2009). Plants show several mechanisms, such as the accumulation of soluble and active osmolytes, when facing drought stress and trying to maintain water absorption under such conditions. Amino acids, sugars, mineral ions, and soluble proteins are among the osmolytes that help plants absorb water and also regulate cell osmotic potential and protect cell membranes under drought stress conditions. One of the innate responses of plants to drought stress is the accumulation of proline, which participates in osmotic regulation (Cattivelli et al. 2008). Proline has also a protective role for macromolecules under stressful conditions (Esra et al. 2010). The proline accumulation is directly related to plant species and stress intensity (Johari-Pireivatlou 2010).
The plant water potential is closely related to the relative water content of leaves (O'Neill et al. 2006) and indicates the plant water content status and drought tolerance (Sairam and Srivastava 2001). Plants with relatively greater water content at the end of the stress period are more drought-tolerant (Basu 2004). In general, the lower relative water content of leaves decreases photosynthesis and, consequently, the overall yield (Lack et al. 2005). The production of oxygen radicals (ROS) is stimulated under environmental stresses such as drought. To scavenge the ROS, antioxidant enzyme activities are also significantly increased (Blokhina et al. 2003). Drought-tolerant species show higher antioxidant activity compared with drought-sensitive species. Therefore, antioxidant activities are vital in increasing plant tolerance to drought stress (Guo et al. 2010). Furthermore, these enzymes play a critical role in the production of lignin and plant resistance to living and non-living stresses (Milone et al. 2003).
Silicon is the second most abundant element on earth (28%). Although highly available on the soil surface, silicon can only be absorbed in the form of Si(OH)4 (Soylemezoglu et al. 2009). Cereals have the highest silicon levels (Epstein 1999). Although silicon is not essential for plant growth and its biological role is not well understood, several studies have reported its benefits in plants (Epstein 1999; Kaya et al. 2006). Through binding to calcium and pectin, silicon increases the strength of the cell wall and the resistance to cell damage caused by stresses. On the other hand, unlike calcium, silicon is highly mobile in plants. Potassium silicate (K2SiO3), with high solubility in water, is a useful chemical fertilizer for cash crops (Balakhnina and Borkowska 2013). Silicon is considered a supplementary element in various plants and increases photosynthesis, leaf stability, leaf chlorophyll concentration, and yield quality, and reduces evapotranspiration (Liang et al. 1996). The application of silicon improved plant growth, dry and fresh weight, and yield of corn (Zea mays) plants (Kaya et al. 2006).
According to the changes in the drought period patterns, it is vital to adopt appropriate strategies to prevent a reduction in potential performance and actual yield of crops (Sasani et al. 2004) and reduce water usage for growing plants without significant reduction in their productivity. Due to the limitation of the crop year and irrigation water in the drought regions of the world, finding strategies to improve crop tolerance to stressful conditions, e.g., drought and high temperature, helps to increase plant production. Such C4 plants as corn with high nutritional value and fertility strength might be an efficient alternative for hot and dry regions due to their relative drought tolerance (Khazaei et al. 2016); hence, improving the crop drought tolerance enables cultivating the crops in hot and dry summers as the second cultivation. Besides, corn is a relatively high water-consuming crop, and reducing water consumption without any significant reduction in its productivity seems necessary.
The effects of various abiotic stresses, including drought, salinity, cold, and heavy metals toxicity were effectively reduced by the silicon element (Liang et al. 2007). Although previous studies elucidated the beneficial effects of silicon compounds on plant growth and yield, limited information has still been released on the silicon effects on different plant species, especially sweet corn, and their physiochemical traits under different climatic conditions. Therefore, a comprehensive study using different physiobiochemical assays was carried out aimed to evaluate whether: (1) sweet corn productivity can be alleviated by silicon under water deficit conditions, (2) silicon can impact the physiochemical traits to improve the crop yield, and (3) sweet corn water requirement can be reduced by silicon without any significant reduction in the crop yield.
Materials and methods
Experimental procedure
The experiment was conducted at the Faculty of Agriculture, the Vali-e-Asr University of Rafsanjan (55º 55´ N, 30º 22´ W, 1526 m asl) in 2018. Irrigation regimes in three levels [70 as control (I70), 105 (I105), and 140 (I140) mm day−1 evaporation from the class A evaporation pan, and the foliar spraying of potassium silicate (K2SiO3) in three levels (zero; as control, 5, and 10 mM; S0, S5, and S10, respectively) were considered as the experimental factors (Khalili et al. 2013; Bayazidi Aqdam 2014; Karvar et al. 2022). The irrigation was applied when the determined value of water for each treatment was evaporated from the pan. For example, for the control treatment, when 70 mm of water was evaporated from the pan, irrigation was applied. If daily evaporation is 10 mm, for instance, after 7 days 70 mm of water is evaporated from the pan and it is time for irrigation, and the same procedure was carried out for other treatments.
Seeds of sweet corn (cv. Chase) were sown in four rows 40 cm apart and three meters long in plots 1 × 3 m (12.5 plants m−2) and in soil depth of 4–5 cm in early July. Surface irrigation was performed right after sowing and continued weekly for 4 weeks. At the 4–6 leaf stage (30 days after planting, DAP), irrigation treatments were applied. Foliar spraying of K2SiO3 at the rate of 416 l ha−1 was simultaneously applied by a handheld spray twice at 2 weeks intervals (at the 4–6 leaf stage and 14 days after the first spray). Weeds were removed by hand every 15 days. Nitrogen (urea, 46% N) at the rate of 66 N kg ha−1 was applied twice (at the V5 and V12; 5-leaf and 12-leaf stages, respectively). Table 1 shows the physicochemical characteristics of the experimental soil. Based on the Domarten climatic classification, Rafsanjan has an arid climate (mean annual precipitation of 75.5 mm). Temperature and precipitation during the growing season are shown in Fig. 1. (Karvar et al. 2022).
Sampling and measurements
The ear leaves were sampled and immediately frozen in liquid nitrogen and transferred to − 75 °C for further measurement of the physiochemical traits.
Leaf physiological traits
Chlorophyll fluorescence
The leaf chlorophyll fluorescence was measured using a handheld PEA Chlorophyll Fluorimeter shortly before the beginning of pollination between 11:00 and 13:00 h (Hansatech, UK). Three leaves of different plants from each treatment were covered by the clips, the chlorophyll fluorimeter was placed on the clip after 15 min of darkness and the maximum quantum yield of PSII photochemistry (Fv/Fm) was recorded (Ahmadi-Lahijani et al. 2018).
Relative water content (RWC)
The relative water content of leaves was measured using Eq. (1): (Ritchie et al. 1990)
Here, FW, TW, and DW are fresh weight, turgid weight, and dry weight, respectively.
Leaf biochemical traits
Photosynthetic pigments
Leaf chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid content (chla, chlb, and chlt, respectively) were assayed using the following equations (Lichtenthaler and Wellburn 1983).
Here, A663, A646, and A470 are the absorption rate at 663, 646, and 470 nm, respectively.
Soluble sugars and sucrose content
Van Handel (1968) and Irigoyen et al. (1992) methods were used to assaying leaf sucrose and soluble sugars content, respectively. The sucrose and glucose standard curves were used to assay the sucrose content.
Proline content
Leaf proline content was measured using the method of Bates et al. (1973). The supernatant absorption rate was read at a wavelength of 515 nm spectrophotometrically. l-Proline with concentrations of 0, 2, 4, 16, and 32 mg l−1 was used to prepare proline standards. The concentration of the samples was calculated in ppm using the standard curve.
Leaf protein and antioxidant
Five hundred mg of fresh ear leaves stored at − 75 ℃ were ground. The samples were then extracted in 5 ml potassium phosphate buffer (50 mM, pH of 7.5) containing Polyvinylpyrrolidone (PVP) and EDTA (1 mM), and centrifuged for 20 min at 4000 rpm at 4 °C. The enzymatic activity and protein content of leaves was measured using the extract.
Soluble protein content
Twenty μl of the extract was diluted in 80 μl of the extraction buffer. The fresh dye Coomassie blue (Bradford reagent) (5 ml) was then added to the solution and stirred for 2 min. After 5 min, the absorption rate was read at 595 nm spectrophotometrically (Spekol-1500). Leaf protein concentration was calculated according to the standard curve obtained from bovine albumin serum (BSA) (Bradford 1976).
Antioxidants content
Fifty μl of the extract was used to assay enzymatic antioxidants, including superoxide dismutase (SOD) (EC 1.15.1.1), Catalase (CAT) (EC 1.11.1.6), and Peroxidase (APX) (EC 1.11.1.7) by methods described by Beauchamp and Fridovich (1971), Dhindsa et al. (1981), and Nakano and Asada (1981), respectively.
Yield and yield components
Grain yield (GY)
Plants were harvested at the physiological maturation (R5–R6) to measure the grain yield and yield component from an area of 1 m2.
Yield components
Three ears per replication (n = 9) were harvested at the grain dough stage (R4–R5) and immediately weighed to measure ear fresh weight (EFW). The samples were then dried at 70 ℃ to constant weight for ear dry weight (EDW). The grain number per ear (GN) was counted after drying in the oven (n = 9). The grain thousand weight (GTW) was measured using Eq. 6:
Here, GW is the grain weight.
Harvest index (HI)
The harvest index was obtained by dividing the economic yield (ear) (EY) by the biological yield (aerial parts) (BY) according to Eq. (7):
Statistical analysis
A randomized complete block design in a split-plot arrangement with three replications was used. The irrigation treatment in three levels was assigned to main plots and foliar application of K2SiO3 at three levels (3 × 3) to subplots. Data were subjected to ANOVA using SAS v.9.1 software. Statistical significance, where indicated, is at least at the 5% level of probability as determined by the analysis of variance and Duncan’s test. Correlation among parameters was computed when applicable.
Results
Physiological traits
Leaf chlorophyll fluorescence
Foliar application of K2SiO3 and deficit irrigation interacted to affect the maximum quantum yield of PSII photochemistry (Fv/Fm). Increasing the water deficit intensity reduced Fv/Fm, but K2SiO3 significantly improved Fv/Fm at each deficit irrigation treatment. The highest Fv/Fm was observed in I70S10, which was 22% higher than I70S0 (control), although there was no significant difference with I105S10 (Fig. 2A).
The maximum quantum yield of PSII photochemistry (A) and relative water content (B) of sweet corn leaves at different irrigation regimes (mm water evaporation from class A evaporation pan) and foliar sprayed with various concentrations of K2SiO3. The control plants (70) were sprayed with water. 70, 105, and 140: 70-, 105-, and 140-mm water evaporation from the class A evaporation pan, respectively. Data are the means of three replicates. Means with the same letter are not significantly different (p ≤ 0.05) based on the Duncan test
Leaf relative water content (RWC)
The interactive effect of deficit irrigation and foliar application of K2SiO3 was significant on the leaf RWC. The lowest leaf RWC was obtained from 140 mm irrigation and non-foliar application of K2SiO3 (Fig. 2B). The lower soil moisture diminished the leaf RWC. Nevertheless, at each level of irrigation, foliar application of 5 and 10 mM K2SiO3 increased leaf RWC and reduced the effects of deficit irrigation. The highest RWC was observed in I70S10 which was 4 and 18% higher compared with I70S0 and I140S0, respectively.
Biochemical traits
Leaf pigments content
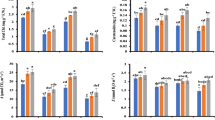
Chlorophyll b content was affected by the irrigation treatments; chlb showed a decreasing trend with a delay in the irrigation. The lowest Chlb was observed in I140; leaf chlb content decreased by 12% at I140 compared with I70 (Fig. 3). The highest leaf chla, chlb, chlt content, and chlorophyll a/b were observed in S10 when K2SiO3 was applied (Table 2 and Fig. 3). For instance, chlorophyll a, b, total chlorophyll content, and chlorophyll a/b were increased 32, 13, 32, and 22%, respectively, at S10 compared with S0. Deficit irrigation, K2SiO3, and their interaction significantly affected leaf carotenoid content. I105S10 showed the greatest leaf carotenoid content with 42% higher values compared with the control (I70S0) (Fig. 4A).
Chlorophyll b and protein content of sweet corn leaves at different irrigation regimes (mm water evaporation from class A evaporation pan). 70, 105, and 140: 70-, 105-, and 140-mm water evaporation from the class A evaporation pan, respectively. Data are the means of three replicates. Means with the same letter are not significantly different (p ≤ 0.05) based on the Duncan test
Carotenoids (A), sucrose (B), soluble sugars (C), and proline (D) content of sweet corn leaves foliar spayed with various concentrations of K2SiO3 (mM) under different irrigation regimes (mm water evaporation from class A evaporation pan). The control plants (70) were sprayed with water. 70, 105, and 140: 70-, 105-, and 140-mm water evaporation from the class A evaporation pan, respectively. Data presented are the means of three replicates. Means with the same letter are not significantly different (p ≤ 0.05) based on the Duncan test
Leaf sucrose and soluble carbohydrates content
Deficit irrigation and foliar application of K2SiO3 interacted to affect leaf sucrose and soluble carbohydrate content. Leaf soluble carbohydrates and sucrose content were the highest at I140S10; 81% and 3.8 times, respectively, greater than the control (I70S0) (Fig. 4B, C).
Leaf proline content
The interaction of deficit irrigation and foliar application of K2SiO3 affected leaf proline content. Leaf proline content tended to increase by increasing deficit irrigation. The greatest leaf proline content was also obtained from I140S10; 2.5 times higher compared with the control (Fig. 4D).
Leaf protein content
Deficit irrigation and K2SiO3 application significantly affected leaf protein content. Increasing water deficit intensity decreased leaf protein content; the lowest leaf protein content was recorded in I140 by a 24% decrease compared with the control (Fig. 3B). However, K2SiO3 increased leaf protein content; application of 10 mM K2SiO3 enhanced leaf protein content by 28% compared with S0 (Table 2).
Leaf enzymatic antioxidants
Leaf SOD, CAT, and APX content were increased by deficit irrigation and foliar supplication of K2SiO3. Deficit irrigation (I140) increased SOD, CAT, and APX content by 100, 140, and 30%, respectively, compared with the control. However, foliar K2SiO3 spraying also increased the content of the enzymes. Leaf SOD, CAT, and APX content were increased by 44, 33, and 12%, respectively, in S10 compared with S0 (Table 3).
Yield and yield component
The effect of deficit irrigation and K2SiO3 foliar application was significant on the ear fresh yield, BY, and GTW. Deficit irrigation (I140) decreased ear fresh yield by 29% compared with the control. Similarly, reducing the soil moisture (I140) diminished GTW and BY by 18 and 43%, respectively, compared with I70 (Table 3). Deficit irrigation and K2SiO3 foliar application interacted to affect GN, HI, GY, and the grain weight percentage per ear (GWPE) (Fig. 5A–D). The greatest GY was obtained from I70S10 and I105S10, by a 28% increase compared with I70S0. Decreasing the available soil moisture led to a decrease in GN per ear, but the foliar application of K2SiO3 significantly reduced the adverse effect of deficit irrigation. The greatest GN was observed in I105S10 by a 34% increase compared with the control (I70S0). Reducing soil water moisture, I105 and I140, decreased GN by 15 and 35%, respectively, compared with I70, while applying 10 mM K2SiO3 in I105 and I140 increased GN by 55 and 37%, respectively, compared with I105S0 and I140S0.
Grain yield (A), grain number (B), grain weight percentage per ear (C), and harvest index (D) of sweet corn leaves foliar spayed by various concentrations of K2SiO3 (mM) under different irrigation regimes (mm water evaporation from class A evaporation pan). The control plants (70) were sprayed with water. 70, 105, and 140: 70-, 105-, and 140-mm water evaporation from the class A evaporation pan, respectively. Data are the means of three replicates. Means with the same letter are not significantly different (p ≤ 0.05) based on the Duncan test
The overall trend of grain weight percentage per ear showed that GWPE increased with the delay in irrigation. The foliar application of K2SiO3 also increased GWPE; the highest GWPE was obtained from I140S10, which was 18% higher than the control (I70S0) (Fig. 5C). The harvest index was increased by deficit irrigation and the foliar application of K2SiO3. The highest HI was obtained from I140S10 by a ~ 27% increase compared with I70S0 and I140S0 (Fig. 5D).
Discussion
The increase in the antioxidant enzyme content can be due to the induction of oxidative stress and the production of reactive oxygen radicals (ROS). One of the solutions to scavenge ROSs is increasing antioxidant enzyme content (Hayat et al. 2007). Plants defend against environmental stress by both enzymatic and non-enzymatic mechanisms. Besides acting as an osmolyte, proline has an antioxidative role in protecting plants. The positive correlation coefficient between the leaf enzymatic; i.e., SOD, CAT, and SOD, and non-enzymatic; i.e., proline, antioxidants showed that those mechanisms were coupled (Fig. 6). The high content of sucrose, proline, and antioxidant enzymes in response to deficit irrigation could indicate the importance of these organic compounds in response to drought stress.
Corrplot analysis of the leaf physiology, biochemistry, and yield components of sweet corn plants exposed to deficit irrigation and foliar application of K2SiO3 parameters. APX ascorbate peroxidase, BY biological yield, Car carotenoids content, CAT catalase, Chlt total chlorophyll content, Fv/Fm maximum quantum yield of PSII photochemistry, GN grain number per ear, GW thousand grain weight, HI harvest index, Prol proline content, Prot protein content, RWC relative water content, SOD superoxide dismutase, SS soluble sugars content. The darker colors represent a higher correlation (blue and red, positive and negative correlations, respectively)
Silicon treatment has been reported to increase enzymatic activity in plants (Chakraborty and Tongden 2005). Silicon protects the plant against environmental stress by stimulating growth and increasing the activity of antioxidant enzymes (Balakhnina and Borkowska 2013). In a study on wheat (Triticum aestivum) plants, silicon increased the levels of antioxidant enzymes, including CAT and SOD (Behtash et al. 2010). Silicon deposition on the cuticle layer increases its thickness and reduces the transpiration from the leaf surface under stressful conditions (Ma and Yamaji 2006), which could help the plant maintain leaf RWC. On the other hand, silicate can improve leaf RWC through osmoregulation strategies to improve the plant water absorption ability and maintain cell membrane stability. Leaf RWC was positively correlated with the leaf chlt and Fv/Fm (Fig. 6), indicating the direct role of leaf water content on photosynthesis and photosynthetic products, leading to better plant growth.
The maximum quantum yield of PSII was improved by the foliar application of K2SiO3. The cellular turgor of leaves decreases under water deficit and reduces in the stomatal aperture, and interrupts leaf gas exchanges. The electron transport chain is also disrupted by the stomatal closure and reduces the quantum yield of photosystem II (PSII). However, K2SiO3 probably by strengthening the plant tolerance mechanisms increased water absorption and stomatal opening. Leaf Fv/Fm was positively correlated with leaf chlt content (Fig. 6). The Fv/Fm assays the PSII efficiency and is an efficient indicator of species resistance to environmental stresses (Kiani et al. 2008; Ahmadi-Lahijani et al. 2018). Fv/Fm showed a decreasing trend in stressful conditions, and plants with higher Fv/Fm were more stress-tolerant (Zafari et al. 2012).
Potassium silicate had a more increasing effect on leaf chla compared with chlb. The chlb is considered an auxiliary pigment and increasing the damage of free radicals resulting from delayed irrigation destroys chlb molecules. In a study to evaluate the silicon effect on corn (Zea mays) plants, the total chlorophyll content was increased by 20% under drought stress conditions (Moghaddam et al. 2011). Silicon increases α-Amino levulinate, a precursor to chlorophyll production in plants; therefore, silicon is likely to increase the chlorophyll content by increasing this substance (de Siqueira et al. 1999). Research shows that silicon increased the chloroplast size and the number of granules (Xie et al. 2014). Positive correlations between the leaf chlt and Fv/Fm and yield components indicate the important role of higher leaf chl content to improve photosynthetic apparatus and crop productivity (Fig. 6).
An overall increase in leaf carotenoid content was observed by a delay in irrigation due to lower photosynthetic efficiency and increased free radicals resulting from reduced plant access to soil moisture. Under stressful conditions, carotenoids as non-enzymatic antioxidants prevent the photooxidation of chlorophyll molecules (Lawlor and Cornic 2002). Potassium silicate plays a crucial role in increasing leaf carotenoid content. A positive correlation was observed between leaf carotenoids and chlt content (Fig. 6). This correlation coefficient indicated that carotenoid activity and their presence in the cell are closely related to chla due to the spatial position of the two pigments (chloroplast) and the function of the carotenoid (photoprotection of chla, neutralization of free radicals, and the absorption and transfer of incident light energy to reaction centers).
In general, soluble sugars act as osmotic regulators and stabilize the cell membranes and regeneration (Slama et al. 2007). Soluble sugars are increased to maintain cell turgor by the starch breakdown and the accumulation of simple sugars. Various studies have reported an increase in soluble sugar content by silicon application, which can be attributed to a reduction of oxidative stress and the protection of macromolecules such as proteins (Epstein 1999; Kaya et al. 2006; Detmann et al. 2013). Since silicon increases the photosynthetic pigments and, therefore, the plant photosynthetic rate, it can increase the soluble sugars by protecting macromolecules and reducing oxidative stress (Savvas and Ntatsi 2015). The correlation results show that soluble sugar content was negatively correlated with leaf chlt and carotenoids (Fig. 6). These negative correlations were likely due to lower photosynthetic pigments content imposed by the deficit irrigation, which was inversely related to leaf soluble sugars and sucrose content changes.
The greatest leaf proline content was obtained from I140S10; 2.5 times higher than the control. Accordingly, Amin et al. (2009) observed an increase in proline and sugar levels of okra (Hibiscus esculentus L.), which were directly related to plant tolerance to drought stress. Drought stress also increased the accumulation of proline in corn (Zea mays) leaves (Li et al. 2010). Besides reducing the osmotic potential and scavenging free radicals, proline reduces the risks of protein breakdown in stressful conditions and prevents ammonia accumulation in cells (Jyoti and Yadav 2012). Leaf proline content was positively correlated with soluble sugar (Fig. 6), indicating their essential role in osmotic regulation under drought stress. Since growth is the first process that slows down or ceases due to reduced water uptake by the plant while photosynthesis continues, the accumulation of carbohydrates might also enhance the osmolytes content.
With the highest correlation with GY, grain number per ear was the most determining grain yield component (Fig. 6). Deficit irrigation reduces the grain number by a delay in tasseling initiation or causes tassel abortion. As the growth rate slows down, tassels appear with a delay, and the interval between pollination and tassel emergence increases, leading to a decrease in the grain number, or no grain formation (Bänzinger 2000). However, the greatest GN per ear obtained from I70S10 and I105S10 indicated that the intensity of water stress in I105 was mild and the foliar application of potassium silicate could compensate for the water stress damage to plants.
Under drought stress conditions, plant growth and development and cell division diminish due to reduced cell turgor pressure. Simultaneously, they will reduce the grain storage capacity, and finally, the grain size and weight (Reezi et al. 2009; Ahmadi-Lahijani and Emam 2016). Mean grain weight is directly determined by the photosynthetic rate and can be improved by any factor that alleviates the environmental stress effects on plants. On the other hand, any factor that restricts water availability reduces nutrient absorption by the plant, resulting in reduced yield components (Wang et al. 2017). It was found that silicate significantly increased plant growth and led to an increase in grain yield in maize (Zea mays) plants (Kaya et al. 2006). The grain weight percentage per ear was increased with deficit irrigation and the foliar application of K2SiO3. Due to the higher growth of ears under normal irrigation, the number of late-formed spikelets (which often do not produce seeds or produce small grains) likely reduced the grain weight percentage per ear.
The harvest index determines the efficiency of the photoassimilates translocation toward the grains. However, in the present study, changes in the HI were interesting; the highest HI was obtained from I140S10. The plant likely experienced a decreased reproductive growth and reduced economic yield and HI due to high vegetative growth at the control irrigation. The HI was positively correlated with leaf chlt content and grain yield (Fig. 6). Silicon was reported to increase plant grain yield more than biological yield, leading to an improved HI (Zuccarini 2008). The application of K2SiO3 enhanced the crop yield and improved HI by improving the photosynthetic procedure.
In summary, deficit irrigation reduced the soil available water and induced drought stress to sweet corn plants; however, the intensity of the stress was mild in I105 and some traits (especially the yield components) were not significantly diminished compared with the control. Since deficit irrigation at the rate of 105 mm evaporation reduces the consumption of irrigation water without a significant adverse effect on plants, this level of irrigation can be considered for the production of sweet corn in the second cultivation (summer). The foliar spraying of K2SiO3 at both 5 and 10 mM reduced the effects of drought stress on sweet corn plants and improved the physiological traits and crop yield. The foliar application of 10 mM potassium silicate could compensate for the water stress damages imposed on plants and can be expected to improve optimal yield under water deficit conditions. Potassium silicate application at levels above 10 mM seems to be still feasible on sweet corn.
Author contribution statement
MK: data collecting, software, writing—original draft. AA: resources, supervision, validation. AR: validation, resources. SM-H: validation, investigation. MJA-L: software, formal analysis, writing—original draft, writing—review and editing.
Data Availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- APX:
-
Ascorbate peroxidase
- BY:
-
Biological yield
- CAT:
-
Catalase
- chlt:
-
Total chlorophyll content
- chlb :
-
Chlorophyll b content
- chla :
-
Chlorophyll a content
- EFW:
-
Ear fresh weight
- EDW:
-
Ear dry weight
- F v /F m :
-
The maximum quantum yield of PSII photochemistry
- GN:
-
Grain number per ear
- GY:
-
Grain yield
- GWPE:
-
The grain weight percentage per ear
- GTW:
-
Grain thousand weight
- HI:
-
Harvest index
- K2SiO3 :
-
Potassium silicate
- RWC:
-
Relative water content
- SOD:
-
Superoxide dismutase
References
Ahmadi-Lahijani MJ, Emam Y (2016) Post-anthesis drought stress effects on photosynthesis rate and chlorophyll content of wheat genotypes. J Plant Physiol Breed 6:35–52
Ahmadi-Lahijani MJ, Kafi M, Nezami A, Nabati J, Erwin J (2018) Effect of 6-benzylaminopurine and abscisic acid on gas exchange, biochemical traits, and minituber production of two potato cultivars (Solanum tuberosum L.). J Agric Sci Technol 20:129–139
Amin B, Mahleghah G, Mahmood HMR, Hossein M (2009) Evaluation of interaction effect of drought stress with ascorbate and salicylic acid on some of physiological and biochemical parameters in okra (Hibiscus esculentus L.). Res J Biol Sci 4:380–387
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv 27:84–93
Balakhnina T, Borkowska A (2013) Effects of silicon on plant resistance to environmental stresses. Int Agrophys 27:225–232. https://doi.org/10.2478/v10247-012-0089-4
Bänzinger M (2000) Breeding for drought and nitrogen stress tolerance in maize: From theory to practice. Cimmyt
Basu MS, Nautiyal PC (2004) Improving water use efficiency and drought tolerance in groundnut by trait-based breeding programmes in India, vol 2. Brisbane, Australia. pp 98
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bayazidi Aqdam MT (2014) The effect of titanium dioxide nanoparticles on morphological and physiological properties of oil flax under drought stress conditions. Shahid Madani University of Azerbaijan
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Behtash F, Tabatabaii S, Malakouty M, Sorour-Aldin M, Ustan S (2010) Effect of cadmium and silicon on growth and some physiological aspects of red beet. J Agr Sci Sustain Prod 2:53–67
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annal Bot 91:179–194
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cattivelli L et al (2008) Drought tolerance improvement in crop plants: an integrated view from breeding to genomics. Field Crop Res 105:1–14
Chakraborty U, Tongden C (2005) Evaluation of heat acclimation and salicylic acid treatments as potent inducers of thermotolerance in Cicer arietinum L. Curr Sci 89(2):384–389
de Siqueira S, Moreira M, Mosquim P, José I, Ferreira F, Sediyama C (1999) Simulation of the transgenic soybean tolerant to glyphosate through explant cultivation. Rev Bras Fisiol Veg 17:(1)95–107
Detmann K, Araújo W, Martins S, Fernie AR, DaMatta F (2013) Metabolic alterations triggered by silicon nutrition: is there a signaling role for silicon? Plant Signal Behav 8:e22523
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Epstein E (1999) Silicon. Annu Rev Plant Biol 50:641–664
Esra K, İşlek C, Üstün AS (2010) Effect of cold on protein, proline, phenolic compounds and chlorophyll content of two pepper (Capsicum annuum L.) varieties. Gazi Univ J Sci 23:1–6
Guo X-Y, Zhang X-S, Huang Z-Y (2010) Drought tolerance in three hybrid poplar clones submitted to different watering regimes. J Plant Ecol 3:79–87
Hayat S, Ali B, Ahmad A (2007) Salicylic acid: biosynthesis, metabolism and physiological role in plants. In: Hayat S, Ahmad A (eds) Salicylic acid: a plant hormone. Springer, Dordrecht. https://doi.org/10.1007/1-4020-5184-0_1
Irigoyen J, Einerich D, Sánchez-Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:55–60
Johari-Pireivatlou M (2010) Effect of soil water stress on yield and proline content of four wheat lines. Afr J Biotech 9(1):36–40
Jyoti B, Yadav SK (2012) Comparative study on biochemical parameters and antioxidant enzymes in a drought tolerant and a sensitive variety of horsegram (Macrotyloma uniflorum) under drought stress. Am J Plant Physiol 7:17–29
Kalloo G, Bergh B (2012) Genetic improvement of vegetable crops. Newnes
Karvar M, Azari A, Rahimi A, Maddah-Hosseini S, Ahmadi-Lahijani MJ (2022) Titanium dioxide nanoparticles (TiO2-NPs) enhance drought tolerance and grain yield of sweet corn (Zea mays L.) under deficit irrigation regimes. Acta Physiol Plant 44:1–14
Kaya C, Tuna L, Higgs D (2006) Effect of silicon on plant growth and mineral nutrition of maize grown under water-stress conditions. J Plant Nutr 29:1469–1480
Khalili M, Naghavi MR, Aboughadareh AP, Rad HN (2013) Effects of drought stress on yield and yield components in maize cultivars (Zea mays L.). Int J Agron Plant Prod 4:809–812
Khazaei M, Galavi M, Dahmarde M, Moosavi-Nik SM, Zamani GR, Mahdi-Nejad N (2016) Effect of drought stress on osmolyte accumulation, photosynthetic pigment and growth of three foxtail millet (Setaria italica L.) species. Environ Stress Crop Sci 9(2):149–162
Kiani SP, Maury P, Sarrafi A, Grieu P (2008) QTL analysis of chlorophyll fluorescence parameters in sunflower (Helianthus annuus L.) under well-watered and water-stressed conditions. Plant Sci 175:565–573
Lack S, Naderi A, Siadat S, Ayenehband A, Nour-Mohammadi N (2005) Effects of water deficiency stress on yield and nitrogen efficiency of grain corn hybrid SC. 704 at different nitrogen rates and plant population. J Water Soil Sci 14:1–14
Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25:275–294
Levitt J (1980) Responses of plants to environmental stresses. Water, radiation, salt, and other stresses (No. Ed. 2). Academic Press. pp 607
Li F, Wei C, Zhang F, Zhang J, Nong M, Kang S (2010) Water-use efficiency and physiological responses of maize under partial root-zone irrigation. Agr Water Manag 97:1156–1164
Liang Y, Shen Q, Shen Z, Ma T (1996) Effects of silicon on salinity tolerance of two barley cultivars. J Plant Nutr 19:173–183
Liang Y, Sun W, Zhu Y-G, Christie P (2007) Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut 147:422–428
Lichtenthaler H, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Portland Press Ltd
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trend Plant Sci 11:392–397
Milone MT, Sgherri C, Clijsters H, Navari-Izzo F (2003) Antioxidative responses of wheat treated with realistic concentration of cadmium. Environ Exp Bot 50:265–276
Moghaddam N, Arvin M, Nezhad GK, Maghsoudi K (2011) Effect of salicylic acid on growth and forage and grain yield of maize under drought stress in field conditions. Seed Plant Prod J 27(1):41–55
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
O’Neill PM, Shanahan JF, Schepers JS (2006) Use of chlorophyll fluorescence assessments to differentiate corn hybrid response to variable water conditions. Crop Sci 46:681–687
Reezi S, Kalantari MBS, Okhovvat SM, Jeong BR (2009) Silicon alleviates salt stress, decreases malondialdehyde content and affects petal color of salt-stressed cut rose (Rosa xhybrida L.) Hot Lady. Afr J Biotech 8:1502–1508
Ritchie SW, Nguyen HT, Holaday AS (1990) Leaf water content and gas-exchange parameters of two wheat genotypes differing in drought resistance. Crop Sci 30:105–111
Sairam R, Srivastava G (2001) Water stress tolerance of wheat (Triticum aestivum L.): variations in hydrogen peroxide accumulation and antioxidant activity in tolerant and susceptible genotypes. J Agron Crop Sci 186:63–70
Sasani S, Jahansooz M, Ahmadi A (2001) The effects of deficit irrigation on water-use efficiency, yield and quality of forage pearl millet. In: Proceedings 4th International crop science congress, Brisbane, Australia
Savvas D, Ntatsi G (2015) Biostimulant activity of silicon in horticulture. Sci Hortic 196:66–81
Slama I, Ghnaya T, Hessini K, Messedi D, Savouré A, Abdelly C (2007) Comparative study of the effects of mannitol and PEG osmotic stress on growth and solute accumulation in Sesuvium portulacastrum. Environ Exp Bot 61:10–17
Soylemezoglu G, Demir K, Inal A, Gunes A (2009) Effect of silicon on antioxidant and stomatal response of two grapevine (Vitis vinifera L.) rootstocks grown in boron toxic, saline and boron toxic-saline soil. Sci Hortic 123:240–246
van Handel E (1968) Direct microdetermination of sucrose. Anal Biochem 22:280–283
Wang JY, Turner NC, Liu YX, Siddique KH, Xiong YC (2017) Effects of drought stress on morphological, physiological and biochemical characteristics of wheat species differing in ploidy level. Func Plant Biol 44:219–234
Xie Z, Song F, Xu H, Shao H, Song R (2014) Effects of silicon on photosynthetic characteristics of maize (Zea mays L.) on alluvial soil. Sci World J 2014:6. https://doi.org/10.1155/2014/718716
Zafari M, Ebadi A, Jahanbakhsh S (2012) Effect of mycorrhiza on water deficit resistance in alfalfa. University of Mohaghegh Ardabili, p 99 (in Persian)
Zuccarini P (2008) Effects of silicon on photosynthesis, water relations and nutrient uptake of Phaseolus vulgaris under NaCl stress. Biol Plant 52:157–160
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by O. Ferrarese-Filho.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karvar, M., Azari, A., Rahimi, A. et al. Potassium silicate reduces water consumption, improves drought tolerance, and enhances the productivity of sweet corn (Zea mays) under deficit irrigation. Acta Physiol Plant 45, 38 (2023). https://doi.org/10.1007/s11738-022-03510-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-022-03510-7