Abstract
Water shortage is a key environmental factor that negatively effects plant growth. Lanzhou lily (Lilium davidii var. unicolor) is a perennial herbaceous drought-tolerant crop widely used as a food and medicine in China. The effects of drought stress on plant growth, osmotic pressure, and secondary metabolite content differ. Here, we investigate alterations in basic physiological processes and in the accumulation of osmolytes by Lanzhou lily under drought stress. Plants were grown at three drought intensities, being irrigated at 5, 15, and 25 day intervals (either throughout the study or during specific growth stages). Water stress markedly decreased plant height and leaf length. With increasing drought stress, the chlorophyll content, bulb weight, and contents of soluble sugars, polysaccharides, and fructose decreased. In contrast, the proline, glucose, and trehalose contents increased under severe drought stress. In addition, glucose and trehalose contents differed significantly under drought stress at different growth stages, whereas other indicators differed significantly only under drought stress throughout the growth period. Our results demonstrate that Lanzhou lily may adapt to drought stress in different growth stages were different. During the shoot stage, the adaptation strategy was to reduce the growth of aboveground part to sustain the underground parts, but during the bulbs expansion stage Lanzhou lily appears to adapt to drought stress by consuming nutrients from underground bulbs to sustain the growth of the aboveground parts and complete the plant's life cycle and by changing osmotic regulation and the levels of secondary metabolites to improve resistance to drought stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are many constraints on plant growth created by the natural environment and by agricultural conditions. Water stress is a global issue, particularly in regions with limited precipitation (Chaves et al. 2003; Madhava Rao et al. 2006; Farooq et al. 2009; Benesová et al. 2012). Water shortages impact many plants (Shao et al. 2008; Farooq et al. 2009; Bhargava and Sawant 2013). This stress reduces the quantity and quality of yield, biomass accumulation, and crop growth (Zlatev and Lidon 2012; Arash et al. 2013; Farooq et al. 2016).
As drought-induced stress continues, plants will respond and habituate to such conditions through the redistribution of photosynthetic products, metabolic changes, and the production of osmotic protectors (such as inorganic ions, soluble sugars, and proline), and other such compounds that protect the plant by increasing membrane stability. (Wang and Huang 2004; Langridge et al. 2006; Javadi et al. 2008). Usually, osmotic adjustments caused by the accumulation of various substances protect plants from the physiological effects of drought conditions (Zhang et al. 2005; Ye et al. 2012).
Sugar is an important carbon (C) and energy source for plant organisms. Moreover, soluble sugar is extremely sensitive to drought stress, which is primarily used to provide carbohydrates through source-sink cycles (Rosa et al. 2009). Drought stress induces soluble sugar accumulation, particularly sucrose, glucose, and fructose, which helps to improve osmoregulation (Praxedes et al. 2006; Wang et al. 2007). The accumulation of these solutes in cells improves the ability of cells to take up and retain water, so the content of these sugars directly affects the drought resistance of plants. These soluble sugars not only maintain water in the cells, but also maintain the stability of proteins, further improving drought resistance. Although sugar plays an indirect role in plant growth and development by regulating carbohydrate metabolism under drought stress, it is nevertheless extremely important (Gupta and Kaur 2005).
Lanzhou lily (Lilium davidii var. unicolor) is a common agricultural plant in China’s Gansu Province. Its cultivation is an important source of income for farmers in Lanzhou City (Fig. 1): it is grown in about 4000 ha in the Qilihe district, in about 2700 ha in the northern mountains and 4000 ha in the southern mountains of Yuzhong district, in more than 2700 ha in Yongjing district, and in more than 2000 ha in Lintao district. It is therefore essential for poverty alleviation. The lily is renowned for its large size, extreme sweet taste and its white jade-like color (Zhang et al. 2015).
Lanzhou is located in the northwestern part of China’s Loess Plateau, at an elevation ranging from 1800 to 2300 m. It has an arid climate, with an annual average temperature of 9.1 °C, and average precipitation ranges from 300 to 450 mm. The rainfall frequency and distribution are irregular, but are often insufficient to support an entire crop cycle. Since Lanzhou lily is mainly planted on hillsides, without irrigation, drought is the main factor that limits the crop’s yield and quality. Researchers in breeding programs have concentrated on plant cultivation techniques (Xu et al. 2009), the chemical composition of the crop (Li et al. 2012), the molecular biology of the species (Zhang et al. 2015), and technologies for processing plant parts (Gao et al. 2008). In contrast, its morphological characteristics and physiological adaption to drought stress have not yet been elucidated. Exploring the response of the Lanzhou lily to different degrees of drought stress will help identify and develop new genotypes that are better able to tolerant water deficits.
Materials and methods
Plant material
In this study, we focused on Lanzhou lily. The lilies were planted in black polyethylene pots that were 24 cm deep and 28 cm in diameter. We covered the bottom of each pot with crushed stone, and covered the stone with a mixture of organic matter and soil (3:1 v/v). Each pot contained approximately 7.0 kg of this mixture. We planted five lily bulbs in each pot, and the total bulb weight was at least 153 g. When 99.2% of the lilies had emerged, we began the drought stress treatment, but the water content (w/w) in all treatments was the same before the drought stress began. The pot experiment was conducted in a greenhouse from March to November 2017 at the Gaolan Research Station of the Chinese Academy of Sciences (Gaolan County, Lanzhou, Gansu Province, China; 36°13″N 103°47″E). We planted 48 pots in a randomized split-plot block design with six drought intensity treatments (i.e., n = 8 pots per treatment). For each measurement, we analyzed the plant materials in one of the eight pots per treatment; the value of the measurement represents the average of the values of the five bulbs in that pot. All of the bulbs were homogeneous at the start of the experiment.
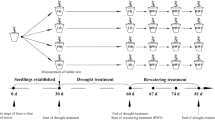
Experimental design and water treatments
The pots in each treatment were watered at different time intervals either throughout the study period or during different growth stages (Table 1). We used this approach to create six levels of drought stress. In the first three treatments, a consistent level of moisture stress was maintained throughout the growth period: the control group was watered every 5 days, the moderate drought stress group was watered every 15 days, and the severe drought stress group was watered every 25 days. In the final three treatments, which began 25 days after the start of the experiment, the soil in the pots was allowed to dry to between 6 and 7% w/w moisture content only during certain growth stages (and followed the control watering regime, with watering every 5 days, during all other stages). Severe water stress was imposed by withholding water for 25 days only during certain growth stages: during the shoot growth, flowering, or bulb expansion stages. Every 5 days, the pot’s weight was recorded to allow calculation of the amount of water that should be added. When the soils in the different stress treatments reached the specified water content, we sampled the middle leaves and analyzed the parameters described in the rest of the “Materials and methods” section.
Growth measurements
Plant height (CM) was measured accurately with a ruler. The lengths and widths of the fully expanded young leaves in the middle of the plant were measured with a ruler during the different stages of drought stress.
Chlorophyll content (SPAD units)
Chlorophyll content was determined using a portable chlorophyll meter (Minolta SPAD-502, Japan). Three different positions of the middle leaves lily plants were selected for measurement, and the average value was used to represent the chlorophyll content of the whole leaf.
Analysis of lipid peroxidation
We measured the level of lipid peroxidation of the leaf tissue using the malondialdehyde content, which we measured using the 5% thiobarbituric acid method described by Hudges et al. (1999).
Proline content of leaves
The method described by Pesci and Beffagna (1984) was used to determine fresh leaf proline accumulation.
Soluble sugars content of leaves
We used the phenol–sulfuric acid to quantify the total soluble sugars (Dubois et al. 1956). In summary, we homogenized 0.1 g of fresh leaves in deionized water, and centrifuged the mixture at 6000 rpm for 15 min. We then removed the supernatant and increased it to a total volume of 10 mL by adding deionized distilled water. We then measured the absorbance at 485 nm using a UV-1200 spectrophotometer (Jinan Like Medical Instrument Co., Ltd., Jinan, China).We determined the soluble sugar contents using glucose as the standard.
Leaf polysaccharide content
We used the method of Zhang et al. (2016) for the extraction of polysaccharides, based on ultrasonication at a temperature of 60 °C. We then precipitated the filtrates by increasing the solution to 3 × its original volume by adding absolute ethanol for 24 h at 4 °C, and then washed the precipitates with acetone and evaporated the solution to obtain the crude polysaccharides.
Soluble and reducing sugar contents of the bulbs
We used the method of Johnson et al. (1964) to measure the glucose and fructose contents of the bulbs, and the anthrone method (Lin et al. 2013) to determine the trehalose and soluble sugar contents. We used van Handel’s (1968) method to measure the sucrose content and the 3,5-dinitrosalicylic acid method to measure the reducing sugars content (Yang et al. 2017).
Statistical methods
We performed our statistical analysis using version 19.0 of the SPSS statistics software (https://www.ibm.com/analytics/). We used one-way ANOVA to detect differences among treatments, and when the ANOVA results were significant, we used Duncan’s multiple-range tests to detect differences between pairs of values. We defined significance at P < 0.05. We used version 9.0 of the OriginPro software (https://www.originlab.com/) to prepare the graphs.
Results
Growth analysis
Table 2 shows the growth analysis results. The plant height and leaf length parameters decreased significantly with increasing drought stress. When drought stress was applied during the shoot growth period, the plant height, length of the leaf blade, and leaf length to width ratio were significantly reduced compared to the control. Compared to the control, drought stress treatments did not have a significant effect on plant height and leaf width during the flowering stage; however, the leaf length as well as the leaf length to width ratios were considerably lower than the control. When the drought was applied only during the bulb expansion stage, none of the growth parameters differed significantly from those in the control.
Chlorophyll content (SPAD units)
Figure 2a shows the impact of drought stress on chlorophyll content (Fig. 2a). When drought was applied throughout the study period, the chlorophyll SPAD value decreased significantly compared to the control, by 22.9% and 41.3% under moderate and severe stress, respectively. With the drought stress applied only during certain periods, there was no significant difference in the chlorophyll SPAD value compared to the control.
Changes in the physiological parameters of Lanzhou lily under drought stress: a chlorophyll content; b bulb weight; c soluble sugars content; d polysaccharide content; e proline content; f malondialdehyde (MDA) content. Values are means ± SD (n = 8). FW fresh weight. Values of a parameter followed by different letters differ significantly (ANOVA followed by Duncan’s multiple-range tests (P < 0.05). Drought stress: C control, MS moderately severe, S severe, SS severe only during the shoot growth stage, SF severe only during the flowering stage, SBE severe only during the bulb expansion stage
Bulb weight
Figure 2b shows the effects of drought stress on bulb weight. Drought stress during the growth period significantly the decreased bulb weight compared to the control. The bulb weight decreased by 73.5% compared to the control under severe drought stress. Under moderate drought stress, the bulb weight (60 g) decreased by 50.3% compared to the control. With the drought stress applied only during the shoot growth period, there was no significant difference in bulb weight compared to the control, but when drought was applied during flowering or bulb expansion, bulb weight decreased significantly (by 22.7 and 39.2%, respectively).
Soluble sugar concentrations
Figure 2c shows the effects of drought stress on the leaf soluble sugar content. With drought stress applied throughout the growth period, the soluble sugar content decreased significantly compared to the control; under moderate and severe drought stress, the concentration decreased by 30.2 and 34.5%, respectively. With drought stress applied only during specific periods, the decrease compared to the control (35.4%) was only significant during the flowering period.
Leaf polysaccharide concentrations
Figure 2d shows the impact of the different drought stress treatments on polysaccharide content in leaves. With drought stress applied throughout the growth period, the leaf polysaccharide content decreased compared to the control, but the decrease (20.5%) was only significant under severe drought. With drought stress applied only during specific periods, the decrease compared to the control (17.16%) was only significant during the flowering period.
Proline concentration
Figure 2e shows that the proline content was affected by drought. With drought stress applied throughout the growth period, the leaf proline content increased with increasing drought stress, and the difference was significant under severe stress. The magnitude of the increase compared to the control was 13.4%. With drought stress applied only during specific periods, there was no significant difference from the control.
Malondialdehyde concentrations
Malondialdehyde is produced by peroxidation of the cell’s lipid membrane, and therefore represents the intensity of the damage to the cell membrane. With drought stress applied throughout the growth period, there was no significant difference compared to the control (Fig. 2f). With drought stress applied only during the flowering and bulb expansion periods, malondialdehyde increased significantly compared to the control; the content was 32% and 37% greater than in the control due to drought stress during the flowering and bulb expansion stages, respectively.
Changes in sucrose concentrations
Soluble sugars are the main substance of osmotic regulation, and plants exhibit effective osmotic regulation that help them to adapt to drought stress. For example, sucrose, fructose and trehalose, being important osmotic protectors, important roles in osmotic regulation, protecting cell membranes from damage, and removing reactive oxygen species produced under abiotic and biological stress (Silva et al. 2010; Keunen et al. 2013; Singh et al. 2015).
Sucrose is the main product of photosynthesis in higher plants. The effect of drought stress on plants will impact the availability of photosynthetic C in the form of sucrose (Farrar et al. 2000; O’Hara et al. 2013). When drought stress was applied throughout the growth period, the bulb sucrose concentration decreased significantly with increasing drought stress compared to the control treatment (Fig. 3a). When the drought stress was applied only during certain stages, only drought during the bulb expansion stage significantly decreased the bulb sucrose content.
Changes in sugar contents (per unit fresh weight [FW]) in the bulbs of Lanzhou lily. a sucrose content; b glucose content; c fructose content; d trehalose content; e soluble sugar content; f reducing sugar content. Values are mean ± standard deviation (n = 8). Values of a parameter followed by different letters differ significantly (ANOVA followed by Duncan’s multiple-range tests (P < 0.05)
Changes in glucose concentrations
Under drought stress treatments respective of the entire growth period, the glucose content of bulbs increased significantly (by 22.5% compared to the control) under severe drought stress (Fig. 3b). When drought stress was applied only during certain stages, bulb glucose content was significantly higher compared to the control during the shoot growth and bulb expansion stages (by 43.9% and 14.4%, respectively).
Changes in fructose concentrations
Under drought stress treatments respective of the entire growth period, the fructose content of bulbs decreased significantly with increasing stress (Fig. 3c), by 30.7% and 38.6%, respectively, compared to the control. When the drought stress was applied only during certain stages, the bulb fructose concentration decreased significantly compared with the control.
Changes in trehalose concentrations
Under drought stress treatments respective of the entire growth period, the trehalose content of bulbs decreased significantly under moderate drought stress (by 13.8%), but increased significantly (by 8.9%) under severe stress (Fig. 3d). When the drought stress was applied only during certain stages, drought stress during the shoot growth phase significantly increased the bulb trehalose concentration compared to control, whereas drought stress at the flowering stage significantly decreased the trehalose concentration. There was no effect with drought during the bulb expansion stage.
Changes in soluble sugar concentrations
Under drought stress treatments respective of the entire growth period, the soluble sugar content of bulbs decreased significantly under moderate drought stress (by 8.9%) and sever stress (by 9.6%) (Fig. 3e). When the drought stress was applied only during certain stages, the bulb total soluble sugar contents decreased significantly compared to the control for all three stages.
Changes in reducing sugar concentrations
Under drought stress treatments respective of the entire growth period, the decreasing sugar content of bulbs did not differ significantly from the control (Fig. 3f). When the drought stress was applied only during certain stages, the bulb reducing sugar concentrations only decreased significantly with stress during the flowering period.
Discussion
Morphological and physiological adaptations of Lanzhou lily to drought stress throughout the growth period
For plants, drought is a main environmental stress, obstructing growth, reducing flower production, and decreasing grain filling (Farooq et al. 2009; Singh et al. 2015). Cell division, elongation and differentiation are the main factors of plant growth and development; subsequently, these stages are impacted by drought (Bhargava and Sawant 2013; Ding et al. 2013; Osakabe et al. 2014). In the present study, the plant height, leaf width, and leaf length were all significantly decreased by drought stress (Table 2). Drought limits plant growth and development by decreasing photosynthesis, constraining metabolic processes, and decreasing nutrient availability (Hu and Schmidhalter 2005; Bohnert et al. 2006). The effects of drought stress on plant height and leaf length of potato were consistent with the present results (Deblonde and Ledent 2001). Based on the above results, the Lanzhou lily limits its leaf width, length and growth to maintaining source activity as one of the tolerance mechanism (Albacete et al. 2014).
Drought stress has a serious negative effect on chlorophyll and yield. With increasing drought stress, the adverse impacts on the bulbs became increasingly serious, especially when severe drought stress was applied during the bulb expansion stage, leading to significantly decreased bulb weight compared to the control, as was the case for the size of the aboveground parts of the plant. The leaf chlorophyll content has been found (Saravia et al. 2016) to be a good early predictor of stress caused by drought. Under moderate and severe drought stress, the SPAD value decreased by 22.9% and 41.3%, respectively, compared to the control (Fig. 2a), and the bulb weight decreased by 50.3 and 73.5%, respectively (Fig. 2b). However, with severe drought applied only during specific stages, the chlorophyll value and bulb weight decreased later in the growth period, with the largest decreases occurring with drought during the shoot growth and bulb expansion periods. This suggests that chlorophyll synthesis occurs mainly during the shoot growth and flowering stages, and that the pigment may not be produced or may be lost during the bulb expansion stage (Fig. 2a). A deficiency in water will directly affect the rate of photosynthesis, which is associated with the stomatal closure induced by drought stress (Flexas et al. 2006; Chaves et al. 2009), and in the present study (Liu et al. 2013), this decrease may also have resulted from the lower SPAD value (Fig. 2a).
We also found that the malondialdehyde content of Lanzhou lily leaves under drought stress increased significantly with drought during the flowering and bulb expansion stages, to 1.32 and 1.37 times, respectively, the control value. Even under severe drought stress applied throughout the growing season, the leaf malondialdehyde content increased very little, suggesting that physiological adaptation prevented serious damage to the cell membranes. Wei et al. (2010a) found similar results in Lilium longiflorum, in which the malondialdehyde content increased slowly under severe stress. In contrast, the proline content increased significantly under severe drought stress, which consistent with the present results (Pei et al. 2010; Liu et al. 2016), suggesting that proline may be the main osmotic-regulating substance. The polysaccharides are important secondary metabolites, and we found that the leaf polysaccharide content decreased significantly under drought stress (Fig. 2d), which contradicts previous results for Dendrobium moniliforme (Wu et al. 2016) and Arabidopsis (Balsamo et al. 2015). The main reason for this contradiction is likely to be differences in how the species respond to drought as well as differences in the watering regime.
Osmotic regulation by changes in concentrations of several sugars
A plant’s ability to undergo osmotic adjustment by changing concentrations of soluble sugars is closely related to the duration and intensity of drought stress, the plant’s resistance to drought, and the plant tissue that is affected (Zlatev and Lidon 2012). Soluble sugars primarily exist in the form of sucrose, glucose, and fructose in Lilium (Rees 1994). Our results showed that drought stress significantly increased the content of glucose in Lanzhou lily bulbs (Fig. 3b), except at moderate stress, but significantly decreased the contents of fructose and sucrose (Fig. 3a, c). The increased glucose was previously shown to enhance the drought resistance of Lanzhou lily because the glucose induced stomatal closure to reduce water loss and enhanced the plant's adaptability to the drought stress (Osakabe et al. 2013). Nevertheless, the sucrose concentration decreased (Fig. 3a). This may be because sucrose can be decomposed into glucose and fructose, but the much lower amounts of fructose recorded most likely reflect the synthesis of fructans by Lanzhou lily bulbs (Kameli 1990) under drought stress. However, the decreased sucrose content may simply be associated with inhibition of photosynthesis, which is supported by the decreased leaf SPAD content (Fig. 2a), or by increased respiratory consumption under drought stress.
Trehalose affects the biosynthesis of stored carbohydrates, and its concentration is known to be connected with plant tolerance drought stress (Cortina and Culiáñez-Macià 2005; Iordachescu and Imai, 2008; Smeekens et al. 2010). Cortina and Culiáñez-Macià (2005) found that trehalose can improve the drought and salt stress resistance of tomatoes. Our results showed that severe drought stress significantly increased the bulb’s trehalose content, suggesting that the trehalose had an osmotic regulation effect. Hence, the Lanzhou lily responded to drought stress by altering it osmotic regulation to maintain its survival under stress.
Plants typically adjust soluble sugar content to cope with various stresses through osmotic regulation (Wu et al. 2014). In this study, the decrease in water potential also reduced the total contents of leaves soluble sugars, which agrees with previous results for cassava (Alfredo et al. 2004). In addition, the soluble sugar content in the bulbs of Lanzhou lily was much higher than that in the leaves. When Lanzhou lily suffers from drought, soluble sugars in its leaves can be transported into the bulb to increase its drought resistance. However, because we did not measure this transport, it will be necessary to confirm this hypothesis in future research.
The reducing sugars content in bulbs showed no significant response to drought stress, except for a slight but significant decrease with severe drought stress applied during the flowering period. This confirms previous results in L. longiflorum bulbs (Wei et al. 2010b). This indicates that the role of reducing sugars in osmotic adjustment is limited in Lanzhou lily.
Morphological and physiological adaptations of Lanzhou lily to drought stress during different growth stages
At the shoot growth stage, plants require the most water to support growth, so a lack of water will severely limit the plant’s height growth. Similarly, leaf length increases mainly during the shoot growth and flowering stages, so a water shortage during these stages can greatly reduce leaf growth. This result is similar to previous results for potato under drought (Van loon 1981). In our study, the weight of the lily bulb decreased by 22.7 and 39.2%, respectively, during the flowering and bulb expansion stages. Rykaczewska (2017) studied the response of potatoes to drought stress during the seedling and flower bud stage, and she found a considerable reduction in yield. The timing of flowering strongly determines the reproductive success of plants, and carbohydrate metabolism is thought to play a crucial role in the regulation of flowering (Liu et al. 2017). In addition, decreases in the amount of pollen can explain the decrease of seed yield (Yadav et al. 2004).
During the shoot growth period, plant height and leaf length significantly decreased by 21.5% and 16.9%, respectively, but the bulbs weight increased by 0.9%, which indicated that the strategy of the Lanzhou lily to adapt to drought is to reduce the growth of its aboveground components to promote the development of its belowground components. However, under drought conditions during the bulb expansion stage, plant height and leaf length did not change significantly, but the bulbs weight significantly decreased by 39.2%. Moreover, sucrose, fructose and soluble sugars in the bulbs decreased by 39.4%, 14.2% and 15%, respectively, while soluble carbohydrate concentrations directly affected the development of lily bulbs (Miller and Langhans 1990), which indicated the ability of the Lanzhou lily to adapt to drought stress by consuming nutrients from its belowground components (bulbs) to sustain the growth of its aboveground components, thus completing its life cycle.
Sugars perform diverse functions in living organisms (Paul and van Dijck 2011). In the present study, drought stress during the flowering period significantly reduced the bulb’s fructose, trehalose, reducing sugars, and total soluble sugars contents compared to the control. The flowering period may be the main period of sugar transformation. A severe deficiency of water and nutrients can reduce the synthesis of sugar, thus reducing the overall sugar content. During the bulb expansion stage, the bulb sucrose, fructose, and total soluble sugar contents decreased compared to control values, but the glucose content increased significantly compared to the control. In comparison with levels with drought during flowering, drought during bulb expansion significantly decreased the sucrose content, but significantly increased the glucose and trehalose contents. This is because autotrophic organisms synthesize sugars, and principally sucrose. Sucrose cleavage in plants is catalyzed by invertases and produces glucose and fructose, but the reaction requires water. In contrast, fructose synthesis from sucrose is catalyzed by synthases (Koch 2004). The balance between these competing processes is complex, as is how sugar affects plant growth. Thus, sucrose cleavage may be slowed by water stress, thereby affecting the balance between these and other sugar metabolic reactions. However, additional research on enzymatic changes will be necessary to support this hypothesis.
Conclusions
Our results showed that in Lanzhou lily, drought decreases plant height, leaf length, leaf width, the leaf length to width ratio, leaf chlorophyll content (SPAD value), yield (bulb weight), and leaf polysaccharide content, but increases the contents of some osmoregulators (proline, glucose, and trehalose). Based on our research, the adaptation strategies of Lanzhou lily to drought in different growth stages were different. During the shoot stage, the adaptation strategy of Lanzhou lily was to reduce the growth of aboveground part to sustain the underground parts, but during the bulbs expansion stage Lanzhou lily appears to adapt to drought stress by consuming nutrients from underground bulbs to sustain the growth of the aboveground parts and complete the plant's life cycle. Lanzhou lily is a drought-tolerant crop, but excessive drought can still reduce its quality and yield. In addition, proline, sucrose, glucose, and trehalose appear to play important roles in osmotic regulation under drought stress. The starch content, the changes in activity of enzymes related to sugar metabolism, and the expression of sugar-related genes under drought stress will be key points to elucidate in future research.
Author contributions statement
ZKX conceived the project; YBZ performed the experiments with the help of WML; YJW, RYW and ZHG analyzed the data; WML wrote the paper. All authors read and approved the manuscript.
References
Albacete AA, Cristina M-A, Francisco P-A (2014) Hormonal and metabolic regulation of source–sink relations under salinity and drought: from plant survival to crop yield stability. Biotechnol Adv 32:12–30
Alves AA, Setter TL (2004) Abscisic acid accumulation and osmotic adjustment in cassava under water deficit. Environ Exp Bot 51:259–271
Arash N, Hossain PZ, Golam F (2013) Drought tolerance in wheat. Sci World J 2013:610721
Balsamo R, Boak M, Nagle K, Peethambaran B, Layton B (2015) Leaf biomechanical properties in Arabidopsis thaliana polysaccharide mutants affected drought survival. J Bionmech 15(48):4124–4129
Benesová M, Holá D, Fischer L, Jedelsky PL, Hnilicka F, Wilhelmová N, Rothová O, Kocová M, Procházková D, Honnerová J, Fridrichóva L, Hnilicková H (2012) The physiology and proteomics of drought tolerance in maize: early stomatal closure as a cause of lower tolerance to short-term dehydration? PLoS ONE 7(6):e38017
Bhargava S, Sawant K (2013) Drought stress adaptation: metabolic adjustment and regulation of gene expression. Plant Breeding 132:21–32
Bohnert HJ, Gong Q, Li P, Ma S (2006) Unraveling abiotic stress tolerance mechanisms—getting genomics going. Curr Opin Plant Biol 9:180–188
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought from genes to the whole plant. Funct Plant Biol 30(3):239–264
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot Lond 103:551–560
Cortina C, Culiáñez-Macià FA (2005) Tomato abiotic stress enhanced tolerance by trehalose biosynthesis. Plant Sci 169(1):75–82
Ding Y, Tao Y, Zhu C (2013) Emerging roles of microRNAs in the mediation of drought stress response in plants. J Exp Bot 64(11):3077–3086
Deblonde PMK, Ledent JF (2001) Effects of moderate drought conditions on green leaf number, stem height, leaf length and tuber yield of potato cultivars. Eur J Agron 14:31–41
Dubois MKA, Gilles JK, Hamilton PA, RebersFred S (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 38:350–356
Farooq FK, Mohammad HS, Ahmad E (2016) Changes in some physiological and osmotic parameters of several pistachio genotypes under drought stress. HortScience 198:44–51
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. J Sustain Agr 29:153–188
Farrar J, Pollock C, Gallagher J (2000) Sucrose and the integration of metabolism in vascular plants. Plant Sci 154:1–11
Flexas J, Bota J, Galmés J, Medrano H, Ribas-Carbó M (2006) Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Plant Physiol 127(3):343352
Gupta AK, Kaur N (2005) Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J Biosci 30(5):761–776
Gao GQ, Yang H, Zhao YS, Wu GF (2008) Study on technology of extracting pectin from Lily. China Food Addit 6:86–90
Hu YC, Schmidhalter U (2005) Drought and salinity: a comparison of their effects on mineral nutrition of plants. J Plant Nutr Soil SC 168:541–549
Hudges DM, Delong JM, Forney FC, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Iordachescu M, Imai R (2008) Trehalose biosynthesis in response to abiotic stresses. J Integr Plant Biol 50:1223–1229
Javadi T, Arzani K, Ebrahimzadeh H (2008) Study of proline, soluble sugar, and chlorophyll a and b changes in nine Asian and one European pear cultivar under drought stress. Acta Hortic 769:241–246
Johnson G, Lambert C, Johnson DK (1964) Plant tissue analysis, colorimetric determination of glucose, fructose, and sucrose in plant materials using a combination of enzymatic and chemical methods. J Agric Food Chem 12(3):216–219
Keunen E, Peshev D, Vangronsveld J, Ende W, Cuypers A (2013) Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept. Plant Cell Environ 36:1242–1255
Kameli A (1990) Metabolic responses of wheat plants to water stress and their role in drought resistance. Ph.D. thesis, University of Sheffield, Sheffield
Khan MB, Hussain N, Iqbal M (2001) Effect of water stress on growth and yield components of maize variety YHS 202. J Res Teach 12:15–18
Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7:235–246
Langridge P, Paltridge N, Fincher G (2006) Functional genomics of abiotic stress tolerance in cereals. Comp Funct Genom 4:343–354
Lin KH, Huang MY, Huang WD, Hsu MH, Yang ZW, Yang CM (2013) The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci Hortic Amst 150:86–91
Liu JG, Wang Y, Song DX, Wang DX, Cui LJ, Sun EY, Yi B (2017) Effects of drought stress on the accumulation of dry matter and yield of sunflower. Liaoning Agric Sci 3:1–8
Li X, Zhang F, Li YC, Wu QH (2012) Comparative study on the polysaccharide contents and antioxidant activities of different parts of Lanzhou Lily. Sci Technol Food Ind 24:018
Liu D, Wu LT, Muhammad SN, Liu HB, Deng XQ, Xu L, Zhang F, Zhou WJ (2013) 5-Aminolevulinic acid enhances photosynthetic gas exchange, chlorophyll fluorescence and antioxidant system in oilseed rape under drought stress. Acta Physiol Plant 35:2747–2759
Liu D, Hu LY, Ali B, Yang AG, Wan GL, Xu L, Zhou WJ (2016) Influence of 5-aminolevulinic acid on photosynthetically related parameters and gene expression in Brassica napus L. under drought stress. Soil Sci Plant Nutr 62:254–262
Madhava Rao KV, Raghavendra AS, Janardhan Reddy K (eds) (2006) Physiology and molecular biology of stress tolerance in plants. Springer, Dordrecht. https://so.hiqq.com.cn/
Miller WB, Langhans RW (1990) Low temperature alters carbohydrate metabolism in Easter lily bulbs. HortScience 25:463–465
O’Hara LE, Paul MJ, Wingler A (2013) How do sugars regulate plant growth and development? New insight into the role of trehalose-6-phosphate. Mol Plant 6(2):261–274
Osakabe Y, Osakabe K, Shinozaki K, Tran LSP (2014) Response of plants to water stress. Front Plant Sci 5:1–7
Osakabe Y, Shinozaki Y, Shinozaki K, Tran LSP (2013) ABA control of plant macro element membrane transport systems in response to water deficit and high salinity. New Physiol 202:35–49
Praxedes SC, Da Matta FM, Loureiro ME (2006) Effects of long-term soil drought on photosynthesis and carbonhydrate in mature robusta coffee (Coffea canephora Pierre var. kouillou) leaves. Environ Exp Bot 56:263–273
Paul M, Van Dijck P (2011) How do sugars regulate plant growth? Plant Sci 2:70
Pesci P, Beffagna N (1984) Inhibiting effect of fusicoccin on abscisic acid induced proline accumulation in barley leaf segments. Plant Sci 36:7–12
Pei ZF, Ming DF, Liu D, Wan GL, Geng XX, Gong HJ, Zhou WJ (2010) Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J Plant Growth Regul 29:106–115
Rees TA (1994) Plant physiology: virtue on both sides. Curr Biol 4:557–559
Rosa M, Prado C, Podazza G, Interdonato R, González JA, Hilal M, Prado FE (2009) Soluble sugars—metabolism, sensing and abiotic stress. Plant Signal Behav 4(5):388–393
Rykaczewska K (2017) Impact of heat and drought stresses on size and quality of the potato yield. Plant Soil Environ 63:40–46
Saravia D, Roxana Farfán Vignolo E, Gutiérrez R, Mendiburu FD, Schafleitner R, Bonierbale M, Khan MA (2016) Yield and physiological response of potatoes indicate different strategies to cope with drought stress and nitrogen fertilization. Am J Potato Res 93(3):288–295
Silva EN, Ferreira-Silva SL, Viégas RA, Silveira JAG (2010) The role of organic and inorganic solutes in the osmotic adjustment of drought-stressed Jatropha curcas plants. Environ Exp Bot 69(3):279–285
Singh M, Kumar J, Singh S, Singh V, Prasad S (2015) Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Rev Environ Sci Biol 14:407–426
Shao HB, Chu LY, Jaleel CA, Zhao CX (2008) Water-deficit stress-induced anatomical changes in higher plants. Cr Biol 331(3):215–225
Smeekens S, Ma J, Hanson J, Rolland F (2010) Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13:274–279
Van Handel E (1968) Direct microdetermination of sucrose. Anal Biochem 22:280–283
Van Loon CD (1981) The effect of water stress on potato growth, development, and yield. Am Potato J 58(1):51–69
Wang YF, Wang QX, Li WAS (2007) The effects of NaCl and Na2SO4 stress on the infiltration regulation of maize seedlings. Corn Sci 15(5):69–75
Wang ZL, Huang BR (2004) Physiological recovery of Kentucky bluegrass from simultaneous drought and heat stress. Crop Sci 44:1729–1736
Wei CB, Tan Y, Zhang P, Zhang FY (2010a) Study on the physiological response of the leaves and bulbs to the drought stress. J Chang Jiang Veg 6:26–29
Wei CB, Zhang P, Zhang FY, Tan Y (2010b) Study on physiological changes and drought resistance of longa lily under drought stress. J Anhui Agric Sci 38(4):1814–1816
Wu SW, Hu CX, Tan QL, Nie ZJ, Sun XC (2014) Effects of molybdenum on water utilization, antioxidative defense system and osmotic-adjustment ability in winter wheat (Triticum aestivum) under drought stress. Plant Physiol Biochem 83:365–374
Wu XL, Yuan J, Luo AX, Chen Y, Fan YJ (2016) Drought stress and re-watering increase secondary metabolites and enzyme activity in dendrobium moniliforme. Ind Crops Prod 94:385–393
Xu LF, Ma FW, Liang D (2009) Plant regeneration from in vitro cultured leaves of Lanzhou lily (Lilium davidii var. unicolor). Sci Hortic Amst 919:458–461
Yadav RS, Hash CT, Bidinger FR, Devos KM, Howarth CJ (2004) Genomic regions associated with grain yield and aspects of post flowering drought tolerance in pearl millet across environments and tester background. Euphytica 136:265–277
Yang QN, Zhou QJ, Wu SJ, Wang YB, Zhang M, Hong Y, Nong HZ, Huang CH (2017) Comparison of 3,5-dinitrosalicylic acid method and enzymatic method in the determination of sugar and sucrose content in sweet corn. J Agric Sci Technol Iran 19(11):125–131
Ye JB, Tu SM, Shi XH, Yang XZ, Huang J (2012) Ecological response at the cytological level of the root primary structure in mycorrhiza-inoculated mulberry saplings to gradient water stress. J Southwest China Norm Univ 34(8):67–72
Zhang DY, Wan Y, Xu JY, Wu GH, Li L, Yao XH (2016) Ultrasound extraction of polysaccharides from mulberry leaves and their effect on enhancing antioxidant activity. Carbohyd Polym 137:473–479
Zhang XH, Cai HY, Wang YF, Liao DZ (2005) Effects of drought stress on seeding growth and resistance physiology of early-maturing flue-cured tobacco. Chin Sci Bull 21(11):189–192
Zhang YB, Wang YJ, Meng J, Xie ZK, Wang RY (2015) Development of an immunochromatographic strip test for rapid detection of lily symptomless virus. J Virol Methods 220:13–17
Zlatev Z, Lidon FC (2012) An overview on drought induced changes in plant growth, water relations and photosynthesis. Emir J Food Agric 24:57–72
Acknowledgements
This work was financially supported by the Gansu Science and Technology Major Project (Grant number 182D2NA010), the Science and Technology Service Network Initiative of the Chinese Academy of Sciences (Grant number KFJ-STS-QYZD-120).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Zhou.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, W., Wang, Y., Zhang, Y. et al. Impacts of drought stress on the morphology, physiology, and sugar content of Lanzhou lily (Lilium davidii var. unicolor). Acta Physiol Plant 42, 127 (2020). https://doi.org/10.1007/s11738-020-03115-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03115-y