Abstract
While the morphological changes in the leaves of low light adapted higher plants are well established, the architecture and lateral arrangement of the thylakoid membrane from plants grown under low light conditions are still not well explored. In the present work we compare the structural organization and thermal stability of thylakoid membranes isolated from pea plants adapted to moderate and low light conditions, and relate the observed structural changes to the functional capacity of the photosynthetic apparatus. In line with earlier reports we confirm that low light induces decrease in the chlorophyll a/b ratio and enlargement of grana membrane regions. Importantly, for the first time we demonstrate a significant thermal instability of low-light thylakoids that are reflected in lower heat needed to disassemble the lateral order of the photosynthetic complexes, as well as for the destabilization of the trimers and monomers of the major light-harvesting complex of photosystem II. Data suggest that this important regulatory complex might adopt different conformation at moderate and low light, which is caused by its specific lateral arrangement within the membrane and might be essential for its regulatory role. In functional terms low light decreases the photochemical activity of thylakoids due to partial photosystem II centers inactivation and impaired electron transport towards photosystem I without inhibiting the photosystems’ functionality, which suggests that the established structural changes play a part in the photosynthetic apparatus operation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Survival and acclimation of higher plants to the variable day light quantity and quality strongly depend on the ability of the photosynthetic apparatus to undergo timely adaptation processes. At a leaf level the adaptation mechanisms include changes of leaf area, thickness and position according to the incident light, as well as changes in the chloroplast number and rearrangement of already structured chloroplasts within leaf parenchyma cells. Generally, sun leaves are thicker and show higher photosynthetic capacity per unit area than shade leaves (Sims and Pearcy 1992; Murchie and Horton 1997; Oguchi et al. 2003). At a chloroplast level short- and long-term adaptation mechanisms involve reorganization of the pigment–protein complexes of the photosynthetic machinery accompanied by change in Rubisco activity and content (Brooks et al. 1988; Ballottari et al. 2007; Kirchhoff et al. 2007; Kouřil et al. 2013). The major light-harvesting complex of photosystem II (LHCII) is a crucial player in these processes: instant excessive sharp increase in light intensity is counteracted by dissipation of the excess excitation energy via non-photochemical quenching mechanism which requires alterations in LHCII conformation and aggregation (Johnson et al. 2011; Ruban 2016). There are also a number of reports revealing a strong link between the oligomerization state of LCHII in pea plants and PSII functionality (Apostolova et al. 2006; Ivanova et al. 2008; Dankov et al. 2009, 2011). For example it was shown that the enrichment of thylakoids with LHCII oligomers has a protective effect against high light (Dankov et al. 2009, 2011). In addition Janik et al. (2017) revealed that the LHCII trimers’ formation depends on the light intensity. The authors showed that the transition from low light to high light is associated with reduction of LHCII trimers and increase in LHCII monomers and dimmers, as well as with changes in LHCII configuration.
In conditions of short-term exposure to low light (LL) intensity and/or far-red-light enrichment part of LHCII complexes migrate and functionally associate with photosystem I (PSI) in a process known as “state transitions”, thus regulating the reduction state of the electron carriers in photosynthetic electron transport chain (Minagawa 2011; Horton 2012).
Prolonged growth (including early stages of development) at high light intensity provokes increase in chlorophyll (chl) a/b ratio due to higher photosystem II (PSII) content and lowering of the overall synthesis of LHCII (Lhcb1, Lhcb2, Lhcb5 and Lhcb6 content being influenced the most), thus leading to increased CO2 assimilation rates and electron transport rates (Melis and Harvey 1981; Leong and Anderson 1984; Anderson et al. 1988; Bailey et al. 2001; Ballottari et al. 2007). On the contrary acclimation to low irradiation typically leads to higher LHCII content (thus, lower chl a/b ratio) and more extended grana formation (Leong and Anderson 1984; Anderson et al. 1988; Bailey et al. 2001; Ballottari et al. 2007; Kouřil et al. 2013). It was shown that Arabidopsis plants respond with increased Lhcb3 and Lhcb6 and reduced Lhcb4.3 expression under LL conditions, leading to enrichment of LHCII trimers that are moderately associated to PSII (Kouřil et al. 2013; Albanese et al. 2016). While LL induces the formation of neatly ordered 2D arrays of PSII-LHCII supercomplexes and hexagonal lipid phase separation in spinach thylakoids (Kirchhoff et al. 2007), in Arabidopsis the relative abundance of the ordered membrane domains was identical at low and optimal light conditions but they did differ in the extent of packing of the supercomplexes (Kouřil et al. 2013). These results show that the protein–protein and protein–lipid interactions play active role in the light adaptation process, probably in a plant- and light-specific manner.
To gain more insight into the mechanism of LL adaptation of higher plants, in this work we study the structural organization and thermal stability of thylakoid membranes isolated from pea plants adapted to moderate (ML) and LL conditions, and relate those structural features to the functional capacity of the photosynthetic apparatus.
Materials and methods
Plant material
Pea (Pisum sativum) seedlings cv. RAN1 were cultivated hydroponically in tap water for 14 days at controlled conditions: temperature 21 °C, 8 h light/16 h darkness photoperiod and either 200 μmol photons s−1 m−2 (ML) or 20 μmol photons s−1 m−2 (LL) growth light intensity provided by 6500 K LED lamps.
Thylakoid membranes isolation
Thylakoids were isolated from 14-day old pea plants according to the protocol described in Petrova et al. (2018). Isolated thylakoids were resuspended in 20 mM Tricine (pH 7.6), 250 mM sorbitol and 5 mM MgCl2 buffer media supplemented with 30% glycerol and stored at − 20 °C until further use. Prior to performing the experiments, the membranes were washed twice in the same buffer. The chl a and b concentrations of thylakoid acetone extracts were determined spectrophotometrically according to Arnon (1949). Grana fractionation of thylakoids was performed according to Chow et al. (1980). Thylakoid membranes were adjusted to 0.1 mg chl ml−1 with the resuspension media and digitonin detergent was added gradually to 0.5% final concentration. After 15 min incubation on ice in the dark the thylakoid suspensions were diluted 6.7 times and centrifuged for 30 min at 10,000×g. The total chlorophyll content of grana containing pellets was compared to that of thylakoids before fractionation allowing for calculation of the percentage of grana abundance in ML and LL thylakoids.
For each experimental method five individual repetitions were performed.
Circular dichroism
Circular dichroism (CD) spectra of thylakoid suspensions were recorded by Jobin–Yvon CD6 dichrograph in the spectral range 400–750 nm with increment 1 nm, integration time 0.5 s and 2 nm bandpass. The thylakoids concentration was 15 μg chl ml−1. For the temperature series the CD spectra were recorded at 20 °C after the samples were incubated for 5 min at temperatures ranging from 20 to 80 °C, increasing at an increment of 5 °C. The spectra were smoothed and corrected for buffer contribution with the aid of Origin 8.0 software. For the CD bands at (−)679 nm and (+)694 nm, a reference intensity value at 750 nm was subtracted, while the value at 620 nm was used as reference for the (+)506 nm CD band. The intensity of the doublet CD band (+)484/(−)473 nm was obtained by subtraction of the intensities at 484 and 473 nm. The temperature at which the intensity of specific CD bands reaches half of its intensity at 25 °C was regarded as transition temperature.
Differential scanning calorimetry
The calorimetric profiles of isolated ML and LL thylakoids were obtained by means of DASM-4 microcalorimeter (Privalov, Pushchino). Thylakoids with chl concentration of 1–1.3 mg chl ml−1 were scanned in the range 30–100 °C with scanning rate 0.5 °C/min. Thermograms were processed with Origin 8.0 software. Buffer–buffer scan and sigmoidal baseline were subtracted from the original scans, and they were further normalized to the total chl content of the samples. Temperature of denaturation (Tm, the temperature at the peak) and excess heat capacity (c exP , the amplitude) of the resolved thermal transitions were determined.
JIP test
Multifunctional Plant Efficiency Analyzer (M-PEA, Hansatech Instruments Ltd. King’s Lynn, UK) was used to record prompt fluorescence induction curves of intact fully developed pea leaves. The protocol applied involved recording of fluorescence intensity for 1 s after application of high-intensity (4000 μmol photons m−2 s−1, 620 nm) excitation light. The JIP test results reported by us are based on four independent experiments with about 30 repetitions for each variant.
PSII photochemistry
To probe the photochemical activity of PSII we measured the rate of oxygen evolution via Clark type electrode (DW1, Hansatech) as in Ivanova et al. (2008) with slight modifications. Isolated thylakoid membranes were resuspended in 20 mM MES buffer (pH 6.5), 10 mM NaCl, 5 mM MgCl2 and 330 mM sucrose to a final concentration of 25 μg chl ml−1 and supplemented with 0.4 mM 1,4-parabezoquinone. The measurements were performed at 22 °C applying saturating white light.
Results
As a first step in our study we examined to which extent LL adaptation of pea plants changed the chl a/b ratio and the grana stacking under our experimental conditions. We established that the growth of plants at LL intensity led to 5.5% lower chl a/b ratio for isolated thylakoid membranes than the growth at ML intensity (Table 1); most probably this was a result of increased LHCII content at low light, in accordance with a number of studies (Lichtenthaler et al. 1982a; Kirchhoff et al. 2007; Kouřil et al. 2013; Albanese et al. 2016).
The amount of chl in isolated grana membranes, used as a measure of grana abundance, was found to be higher by about 37% in LL than in ML thylakoids (Table 1), confirming that larger grana stacks are formed at low light as reported earlier (Lichtenthaler et al. 1982a, b).
To compare the lateral (macro)organization of the photosynthetic complexes within the ML and LL thylakoid membranes we employed CD. The CD spectra of ML and LL isolated thylakoids (Fig. 1a) recorded at 20 °C were comprised of the characteristic bands already reported for intact thylakoids. Based on a number of publications the origin of some of the CD bands can be attributed to specific components of the photosynthetic membranes: long-range pigment–pigment interactions originating from chirally ordered PSII supercomplexes domains within the membrane plane [(−)679 nm and (+)694 nm bands]; β-carotene bound to PSII cores assembled in chirally ordered domains [(+)506 nm band]; LHCII trimers [(+)484/(−)473 nm band pair]; LHCII monomers [(−)657 nm band] (Garab et al. 1988; Dobrikova et al. 2003; Garab and van Amerongen, 2009; Tóth et al. 2016; Petrova et al. 2018).
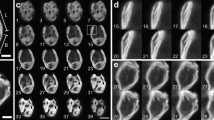
CD spectra of ML (a) and LL (b) thylakoid membranes recorded at different temperatures. For clarity arrows indicate the direction in which the intensity of the main CD bands [(+)506 nm, (−)657 nm, (−)679 nm and (+)694 nm] is changing with the increase in temperature. Temperature dependence of the intensity of (−)657 nm (c) and (−)679 nm (d) CD bands recorded for ML (full squares) and LL (open circles) thylakoids. For clarity the data are normalized to the value at 25 °C
While the thermal stability of (+)679 nm band was not affected by the light regimen, all other above-mentioned CD bands were less stable upon LL than upon ML condition (Table 2; Fig. 1). The extent of destabilization was the highest for the CD bands reflecting the LHCII monomer and trimer stability, i.e., the transition temperatures for the (+)484 nm/(−)473 nm and (−)657 nm bands determined for LL samples were found to be lower by 4–6 °C compared to ML ones. On the other hand the CD bands related to the macro-organization of the thylakoid membranes [i.e., (+)506 nm and (+)694 nm bands] had transition temperatures ca. 3 °C lower for LL than for ML samples (Table 2; Fig. 1).
To further characterize the heat-induced denaturation of the protein components in ML and LL thylakoids we applied DSC. Thermograms of ML and LL thylakoids featured 8 endothermic transitions denoted as T1–T8, four of which were well-pronounced peaks centered at 60 °C, 65 °C, 71–73 °C, and 88 °C, respectively, and the other four (denoted by *) were not clearly discernible and appeared as shoulders at 57 °C, 63 °C, 68 °C, and 81 °C. The resolved denaturation temperatures and their excess heat capacities are summarized in Table 3. The first endothermic event occurring at 57 °C, ascribed to the loss of order in horizontal (lateral macroorganization) and/or vertical (stacking) direction (Dobrikova et al. 2003; Petrova et al. 2018) was downshifted on the average by 1.5 °C in LL compared to ML thylakoids. The transition occurring at 73 °C in ML thylakoids, that is due to LHCII denaturation (see Petrova et al. 2018), was also destabilized by 2 °C in LL samples (Fig. 2; Table 3). Its shift towards lower temperatures most probably led to the slight increase of the amplitude of the transitions below 70 °C (Fig. 2; Table 3).
We assessed the functional consequences of low light adaptation by recording prompt fluorescence induction curves of intact ML and LL leaves applying JIP test (as described and interpreted in Strasser et al. 2004 and Goltsev et al. 2016). Selected JIP parameters for intact leaves are presented in Fig. 3 and Table 4. LL adaptation led to decrease in the total amount of active PSII reaction centers (reflected by the parameter RC/CS0) by about 13% which resulted in proportional (ca. 14%) enlargement of their effective light-harvesting antenna (ABS/RC) as compared to moderate light-adapted plants. The latter result is in accordance with the report of Albanese et al. (2016) on the dependence of functional antenna size of PSII on light intensity. These effects were associated with higher level of excitation energy dissipation for processes other than photochemistry (DI0/RC) in LL than in ML plants. The flux of electrons passing through PSII and reaching the acceptor side of PSI was also reduced by about 17% (RE0/RC) under LL conditions. While the efficiency of electron transport from Q −A to PQ (ψEo) did not exhibit significant decline in LL, the electron transport from PQ to PSI end acceptors (δRo) was significantly decreased. The overall performance of the photosynthetic machinery of LL-adapted plants as judged by the PItotal parameter strongly decreased compared to ML ones. The initial slope of the fluorescence induction curves reflecting the QA reduction rate at the beginning of illumination (M0 parameter) and the maximum quantum yield of PSII (φPo) did not change upon acclimation of plants to LL intensity. The functional connectivity of PSII complexes (P2G) was not significantly different for the two studied light conditions.
Oxygen evolution measurements have also revealed different PSII photochemical activity for the two light conditions. The oxygen yield for NL thylakoids was found to be 60.9 ± 5.3 μmolO2.chl−1 h−1, while it was about 20% lower for LL samples, i.e., 48.6 ± 3.6 μmolO2.chl−1 h−1.
Discussion
In this work we demonstrate that pea plants’ acclimation to LL intensity affects the structural organization and thermal stability of the photosynthetic complexes at multiple levels, i.e., membrane 3D architecture, lateral protein arrangement and LHCII stability.
In line with previous works our data show that the grana formed under LL conditions are larger than those formed in ML plants. In addition, for the first time, we demonstrate that the structural stability of LL thylakoids is lower than the one of ML membranes upon heat treatment as evidenced by both DSC (downshift of T1 transition upon LL conditions by 1.5 °C on the average, Table 3) and CD (2 °C lower transition temperatures of (+)694 nm and (+)506 nm CD bands for LL than ML samples, Table 2) measurements. This implies that the formation of larger grana at LL does not result in increase in the extent of stacking, i.e., the amount of heat needed to disassemble the 3D structure of thylakoids. By comparing the CD spectra of thylakoids from different plant and algae species, Tóth et al. (2016) have convincingly shown that there is no strict correlation between the grana number and dimensions and the intensities of the major CD bands; the authors have presented evidences implying that the (+)694 nm and (+)506 nm CD bands are more sensitive to the lateral arrangement of the PSII supercomplexes within the stacked membranes, while the (−)679 nm band is more tightly related to the stacking itself. The fact that in LL thylakoids the thermal stability of the (−)679 nm CD band is not affected by the growth light while those of (+)694 nm and (+)506 nm CD bands are decreased suggests that the thermal destabilization under our experimental conditions is prompted by protein rearrangement in the plane of grana membranes. This would mean that heating of thylakoids isolated from LL pea plants results first in loss of lateral order followed by membrane unstacking, while in ML pea samples these processes occur in parallel under our experimental conditions.
The presented DSC and CD data also show that LHCII monomers and trimers in LL plants are thermodynamically less stable than in ML thylakoids. The destabilization of the LHCII-associated DSC transition under LL conditions by 1.4 °C on the average is lower than the reduction by 4–6 °C of the transition temperatures of the CD bands assigned to the denaturation of LHCII trimers [(+)484/(−)473 nm CD band] and monomers [(−)657 nm CD band], but this is most probably an effect resulting from the different heating protocols applied in the two types of experiments. Nevertheless, both methods show that LHCII structure is affected by the growth regimen. The altered thermal stability and thermodynamic properties of LHCII in LL-adapted plants might be due to differential enrichment or depletion of some of the Lhcb proteins’ (iso)forms (as reported by Kouřil et al. 2013 and Albanese et al. 2016) or as a result of the ability of LHCII to adopt different conformation depending on the light conditions, in line with the findings of Janik et al. (2017). This change in the LHCII conformation might be associated with different oligomerization state of LHCII and/or different lateral arrangement of the complexes. In either case altered protein–protein and protein–lipid interactions in the two types of samples should be involved, that might be essential for the regulatory function of LHCII complex.
Although in this work we have not studied thoroughly the exact lateral protein order under the two light conditions, on the basis of previous findings it can be suggested that LL affects the ordering and extent of association of the PSII supercomplexes as a mechanism of coping with limited resources (Kirchhoff et al. 2007; Kouřil et al. 2013). The effect observed for LL plants is opposite to that previously reported by us for unstacked thylakoids, where the LHCII thermal stability was strongly increased (Petrova et al. 2018). Therefore, it will be of a great interest to further study the relation between LHCII stability and the thylakoid membrane macroorganization in more detail.
In our experimental conditions, we found that the chl a/b ratio of LL plants was increased by 5.5% (Table 1). Although small, this change is significant and within the expected values established in previous chl a/b vs. light intensity curves (Leong and Anderson 1984; Bailey et al. 2001), and suggests synthesis of additional LHCII molecules in LL-adapted pea plants. According to Kouřil et al. (2013) these additionally synthesized complexes belong to the population of non-PSII-attached LHCII molecules. Therefore, an interesting question to pursue further is whether they contribute to the observed changes in LHCII thermal stability or the LHCII molecules bound to PSII supercomplexes have prominent effect on the registered LHCII destabilization.
JIP test of intact pea leaves showed that the established alterations in the thermal stability of LL thylakoids are associated with changes in the photosynthetic processes in a way that leads to lowering of the probability of reduction of PSI end acceptors, thus reducing the overall productivity of the whole electron transport chain. The data revealed lower number of active (QA reducing) PSII reaction centers under LL than ML conditions, which is most probably the reason for the lower oxygen evolution associated with LL thylakoids as already reported (Chow et al. 1991; Park et al. 1997; Bailey et al. 2001). It should also be taken into account that the oxygen evolution rate depends strongly on the oligomerization state of LHCII (as demonstrated by Ivanova et al. 2008), however, this factor is not explicitly studied in the current work. On the other hand the maximum photochemical quantum yield of active PSII remains unaffected at LL conditions which proves that these complexes per se are well adapted to low light conditions and do not experience any inhibition of their function. PSI appears less active since the electron transport from PQ towards PSI is less efficient under LL conditions. Thus, the overall decrease in the photosynthetic performance of LL plants (PItotal) can be traced out to a partial deactivation of PSII reaction centers and less-efficient electron transport to PSI acceptor side. Our data also revealed that the functional cooperativity of the PSII complexes did not change with the light conditions, at least within the sensitivity limits of the applied JIP test.
Conclusions
In this work we have compared the structural arrangement and stability of isolated ML and LL thylakoids, as well as the functional performance of ML and LL pea plants. Our data revealed that LL adaptation leads to increase in LHCII content, enlargement of granas and formation of alternative, thermally unstable lateral arrangement of PSII and LHCII complexes. In functional terms LL-adapted thylakoids performed less efficiently due to partial PSII centers inactivation and impaired electron transport towards PSI, but do not experience severe inhibition of their functions. In conclusion our data demonstrate that the structural remodeling of the photosynthetic complexes is an important part of the low light adaptation of higher plants.
Author contribution statement
NP cultivated plants, designed the experimental part, performed significant part of the experimental work, analyzed data and wrote part of the manuscript; SS conducted spectroscopic experiments; MP performed JIP measurements; ST performed calorimetric measurements; SGT consulted and revised the manuscript; SK analyzed data, wrote part of the manuscript, revised and edited it. All authors read the manuscript and approved the submission.
References
Albanese P, Manfredi M, Meneghesso A, Marengo E, Saracco G, Barber J, Morosinotto T, Pagliano C (2016) Dynamic reorganization of photosystem II supercomplexes in response to variations in light intensities. Biochim Biophys Acta Bioenerg 1857:1651–1660. https://doi.org/10.1016/j.bbabio.2016.06.011
Anderson JM, Chow WS, Goodchild DJ (1988) Thylakoid membrane organisation in sun/shade acclimation. Funct Plant Biol 15:11–26. https://doi.org/10.1071/PP9880011
Apostolova EA, Dobrikova AG, Ivanova PI, Petkanchin IB, Taneva SG (2006) Relationship between the organization of the PSII supercomplex and the functions of the photosynthetic apparatus. J Photochem Photobiol B 83:114–122. https://doi.org/10.1016/j.jphotobiol.2005.12.012
Arnon D (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Bailey S, Walters RG, Jansson S, Horton P (2001) Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta 213:794–801. https://doi.org/10.1007/s004250100556
Ballottari M, Dall’Osto L, Morosinotto T, Bassi R (2007) Contrasting behavior of higher plant photosystem I and II antenna systems during acclimation. J Biol Chem 282:8947–8958. https://doi.org/10.1074/jbc.M606417200
Brooks A, Portis AR, Sharkey TD (1988) Effects of irradiance and methyl viologen treatment on ATP, ADP, and activation of ribulose bisphosphate carboxylase in spinach leaves. Plant Physiol 88:850–853
Chow WS, Thorne SW, Duniec JT, Sculley MJ, Boardman NK (1980) The stacking of chloroplast thylakoids: effects of cation screening and binding, studied by the digitonin method. Arch Biochem Biophys 201:347–355. https://doi.org/10.1016/0003-9861(80)90520-2
Chow WS, Hope AB, Anderson JM (1991) Further studies on quantifying photosystem II in vivo by flash-induced oxygen yield from leaf discs. Funct Plant Biol 18:397–410
Dankov K, Dobrikova A, Bogos B, Gombos Z, Apostolova E (2009) The role of anionic lipids in LHCII organization and in photoinhibition of photosynthetic apparatus. Compt Rend Acad Bulg Sci 62(8):941–948
Dankov KG, Dobrikova AG, Ughy B, Bogos B, Gombos Z, Apostolova EL (2011) LHCII organization and thylakoid lipids affect the sensitivity of the photosynthetic apparatus to high-light treatment. Plant Physiol Biochem 49:629–635. https://doi.org/10.1016/j.plaphy.2011.02.019
Dobrikova AG, Várkonyi Z, Krumova SB, Kovács L, Kostov GK, Todinova SJ, Busheva MC, Taneva SG, Garab G (2003) Structural rearrangements in chloroplast thylakoid membranes revealed by differential scanning calorimetry and circular dichroism spectroscopy. Thermo-optic effect. Biochemistry 42:11272–11280. https://doi.org/10.1021/bi034899j
Garab G, van Amerongen H (2009) Linear dichroism and circular dichroism in photosynthesis research. Photosynth Res 101:135–146. https://doi.org/10.1007/s11120-009-9424-4
Garab G, Wells S, Finzi L, Bustamante C (1988) Helically organized macroaggregates of pigment-protein complexes in chloroplasts: evidence from circular intensity differential scattering. Biochemistry 27:5839–5843
Goltsev VN, Kalaji HM, Paunov M, Bąba W, Horaczek T, Mojski J, Kociel H, Allakhverdiev SI (2016) Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russ J Plant Physiol 63:869–893. https://doi.org/10.1134/S1021443716050058
Horton P (2012) Optimization of light harvesting and photoprotection: molecular mechanisms and physiological consequences. Philos Trans R Soc B 367:3455–3465. https://doi.org/10.1098/rstb.2012.0069
Ivanova PI, Dobrikova AG, Taneva SG, Apostolova EL (2008) Sensitivity of the photosynthetic apparatus to UV-A radiation: role of light-harvesting complex II–photosystem II supercomplex organization. Radiat Environ Biophys 47:169–177. https://doi.org/10.1007/s00411-007-0139-7
Janik E, Bednarska J, Sowinski K, Luchowski R, Zubik M, Grudzinski W, Gruszecki WI (2017) Light-induced formation of dimeric LHCII. Photosynth Res 132:265–276. https://doi.org/10.1007/s11120-017-0387-6
Johnson MP, Goral TK, Ruban AV (2011) Photoprotective energy dissipation involves the reorganization of photosystem II light-harvesting complexes in the grana membranes of spinach chloroplasts. Plant Cell 23:1468–1479. https://doi.org/10.1105/tpc.110.081646
Kirchhoff H, Haase W, Wegner S, Danielsson R, Ackermann R, Albertsson PA (2007) Low-light-induced formation of semicrystalline photosystem II arrays in higher plant chloroplasts. Biochemistry 46:11169–11176. https://doi.org/10.1021/bi700748y
Kouřil R, Wientjes E, Bultema JB, Croce R, Boekema EJ (2013) High-light vs. low-light: effect of light acclimation on photosystem II composition and organization in Arabidopsis thaliana. Biochim Biophys Acta Bioenerg 1827:411–419. https://doi.org/10.1016/j.bbabio.2012.12.003
Leong TY, Anderson JM (1984) Adaptation of the thylakoid membranes of pea chloroplasts to light intensities. I. Study on the distribution of chlorophyll-protein complexes. Photosynth Res 5:105–115. https://doi.org/10.1007/BF00028524
Lichtenthaler HK, Kuhn G, Prenzel U, Buschmann C, Meier D (1982a) Adaptation of chloroplast-ultrastructure and of chlorophyll-protein levels to high-light and low-light growth conditions. Z Naturforsch C 37:464–475. https://doi.org/10.1515/znc-1982-5-619
Lichtenthaler HK, Kuhn G, Prenzel U, Meier D (1982b) Chlorophyll-protein levels and degree of thylakoid stacking in radish chloroplasts from high-light, low-light and bentazon-treated plants. Physiol Plant 56:183–188. https://doi.org/10.1111/j.1399-3054.1982.tb00322.x
Melis A, Harvey GW (1981) Regulation of photosystem stoichiometry, chlorophyll a and chlorophyll b content and relation to chloroplast ultrastructure. Biochim Biophys Acta Bioenerg 637:138–145. https://doi.org/10.1016/0005-2728(81)90219-X
Minagawa J (2011) State transitions—the molecular remodeling of photosynthetic supercomplexes that controls energy flow in the chloroplast. Biochim Biophys Acta Bioenerg 1807:897–905. https://doi.org/10.1016/j.bbabio.2010.11.005
Murchie EH, Horton P (1997) Acclimation of photosynthesis to irradiance and spectral quality in British plant species: chlorophyll content, photosynthetic capacity and habitat preference. Plant Cell Environ 20:438–448. https://doi.org/10.1046/j.1365-3040.1997.d01-95.x
Oguchi R, Hikosaka K, Hirose T (2003) Does the photosynthetic light-acclimation need change in leaf anatomy? Plant Cell Environ 26:505–512. https://doi.org/10.1046/j.1365-3040.2003.00981.x
Park IIY, Chow WS, Anderson JM (1997) Antenna size dependency of photoinactivation of photosystem II in light-acclimated pea leaves. Plant Physiol 115:151–157. https://doi.org/10.1104/pp.115.1.151
Petrova N, Todinova S, Paunov M, Kovács L, Taneva S, Krumova S (2018) Thylakoid membrane unstacking increases LHCII thermal stability and lipid phase fluidity. J Bioenerg Biomembr 50:425–435. https://doi.org/10.1007/s10863-018-9783-7
Ruban AV (2016) Nonphotochemical chlorophyll fluorescence quenching: mechanism and effectiveness in protecting plants from photodamage. Plant Physiol 170:1903–1916. https://doi.org/10.1104/pp.15.01935
Sims DA, Pearcy RW (1992) Response of leaf anatomy and photosynthetic capacity in Alocasia macrorrhiza (Araceae) to a transfer from low to high light. Am J Bot 79:449–455. https://doi.org/10.2307/2445158
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence, vol 19. Advances in photosynthesis and respiration. Springer, Dordrecht, pp 321–362
Tóth TN, Rai N, Solymosi K, Zsiros O, Schröder WP, Garab G, van Amerongen H, Horton P, Kovács L (2016) Fingerprinting the macro-organisation of pigment–protein complexes in plant thylakoid membranes in vivo by circular-dichroism spectroscopy. Biochim Biophys Acta Bioenerg 1857:1479–1489. https://doi.org/10.1016/j.bbabio.2016.04.287
Acknowledgements
This work is supported by Grant Number DFNP 17-138 (N.P.), Program for career development of young scientists and PhD students in Bulgarian Academy of Sciences 2017 and partially by Bulgarian Ministry of Education and Science under the National Research Programme “Young scientists and postdoctoral students” approved by DCM # 577/17.08.2018 (NP). The authors are grateful to Assoc. Prof. Anelia Dobrikova for her help with oxygen evolution experiments.
Funding
This study was funded by Program for career development of young scientists and PhD students in Bulgarian Academy of Sciences 2017 (Grant Number DFNP 17-138, NP) and partially supported by Bulgarian Ministry of Education and Science under the National Research Programme “Young scientists and postdoctoral students” approved by DCM # 577/17.08.2018 (NP).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Kovacik.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Petrova, N., Stoichev, S., Paunov, M. et al. Structural organization, thermal stability, and excitation energy utilization of pea thylakoid membranes adapted to low light conditions. Acta Physiol Plant 41, 188 (2019). https://doi.org/10.1007/s11738-019-2979-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-2979-6