Abstract
Iron deficiency that is induced by high soil pH is a major factor affecting plant growth in calcareous soils and in some areas that have been reclaimed following industrial activities. Since the effects of high soil pH commonly involve Fe deficiency, in this study, we examined whether Fe provided to part of the root system exposed to low pH would alleviate high pH stress in paper birch (Betula papyrifera), trembling aspen (Populus tremuloides) and red-osier dogwood (Cornus stolonifera) seedlings. The plants were grown in a controlled environment growth room in mineral nutrient solution at pH 5 and 9 and provided with either 0 or 40 µM Fe in a split-root system for 8 weeks. At the end of the treatments, plant dry weights, net photosynthesis, transpiration rates, root ferric-chelate reductase activity, and leaf chlorophyll concentrations were measured, and elemental analyses were carried out in young leaves. The results demonstrated high root zone pH affected Fe, P and Zn concentrations in young leaves. In the three considered species, plants with part of their root system exposed to pH 5 had higher dry weights, net photosynthesis, and transpiration rates compared with the plants with the whole root system immersed in pH 9 solution. High root zone pH reduced photosynthesis, transpiration rates, leaf chlorophyll concentrations and the uptake of Fe, P, and Zn in plants. Partial exposure of the root system to low pH and Fe supply reduced leaf chlorosis and partly alleviated the high pH stress in the studied plants by improving Fe uptake, but did not alleviate root growth reductions.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Approximately, 30% of the world’s cultivated land are covered with calcareous soils (Kobayashi and Nishizawa 2012). The associated high soil pH profoundly inhibits plant growth and productivity. High pH soils can limit root water uptake and reduce transpiration, photosynthesis, and shoot growth of plants (Zhang et al. 2013). In high pH, sensitive species such as lupin (Lupinus angustifolius L.), growth, root surface area, root elongation, and root hair formation were negatively affected by high root zone pH (Tang et al. 1993). In high pH soil, the availability of some essential nutrients to plants, including Fe, Mn, Cu, and Zn, is reduced due to low solubility and mobility (Comerford 2005; George et al. 2012). In addition to natural high pH soils, in the oil sands reclamation areas in Northeastern Alberta, Canada, soil pH values commonly exceed 8.0 (Howat 2000), due to the usage of large amount of NaOH during the oil extraction processes; in fact, the tailing sands have been used as a reconstruction substrate in reclamation (Chalaturnyk et al. 2002; Fung and Macyk 2000).
Iron deficiency is common in plants growing in high pH soils (George et al. 2012). Fe is an essential micronutrient in various plant physiological processes including photosynthesis, respiration, and chlorophyll biosynthesis (Kobayashi and Nishizawa 2012). Iron deficiency can alter chloroplast structure and reduce photosynthetic rate (Eberhard et al. 2008). As soil pH increases from 4 to 8, the concentration of soluble Fe3+ decreases from 10−8 to 10−20 M (Läuchli and Grattan 2017). Plants have developed two principal strategies for Fe uptake when Fe is deprived. Strategy I is used mainly by non-graminaceous plants while strategy II is adopted mainly by graminaceous monocots. In strategy I plants, upon Fe deprivation, a set of genes are expressed on encoding the proton ATPase, ferric chelate reductase FRO2 and metal transporter IRT1 in root surface cells. Other factors including Fe-deficiency induced transcription factor 1 (FIT1) and IRT2 are also involved in regulation of Fe uptake and homeostasis (Colangelo and Guerinot 2004; Vert et al. 2009). Strategy I plants modulate the biochemical processes of extruding inorganic ions, amino acids, organic acids, phenolics, flavins, etc. in response to changing environment and internal characteristics (Młodzińska 2012; Zocchi et al. 2007). The strategy II responses include secretion of high affinity ferric iron chelators termed phytosiderophores (PS) in response to iron deficiency. Fe(III)–PS complexes are then assimilated into root cells via transporters (Jeong and Connolly 2009).
The rhizosphere pH may vary in different microsites depending on root respiration, organic acid extrusion, redox reactions, and microorganism activities (Hinsinger et al. 2003). In fact, rhizosphere pH has been reported to be up to 1–2 pH units either higher or lower than bulk soil pH (Colombo et al. 2014). The split-root system was commonly used to study plant responses to heterogenous nutrition regimes. Coppa et al. (2018) recently used a split-root approach to study iron and sulfur interplay in tomato. Plants need a relatively small amount of micronutrients including Fe. Exposure of part of the root system to low pH and to high pH, was expected to provide sufficient amount of Fe for the whole plant. It was also of interest to determine whether the differences in sensitivity to high pH of the different plant species can be explained by the nutritional factors (Zhang and Zwiazek 2016a).
In the present study, we examined the growth and physiological responses of paper birch (Betula papyrifera), trembling aspen (Populus tremuloides), and red-osier dogwood (Cornus stolonifera) to different root zone pH and Fe nutrition levels. These species are commonly planted in oil sands reclamation areas and belong to the strategy I plant species. In previous studies, paper birch (Betula papyrifera) and red-osier dogwood (Cornus stolonifera) were found to be relatively sensitive to high pH, while trembling aspen (Populus tremuloides) was relatively tolerant (Calvo-Polanco et al. 2017). The objectives of this study were to investigate the responses of these species to heterogeneous root zone pH differing in Fe availability to evaluate the role of Fe in plant tolerance of high pH. We hypothesized that a partial exposure of the root system to low pH conditions would alleviate the effects of high pH applied to the other part of the root system.
Materials and methods
Plant material and growth conditions
One-year-old dormant seedlings of paper birch (Betula papyrifera), trembling aspen (Populus tremuloides), and dogwood (Cornus stolonifera) were obtained from the Boreal Horticultural Services Ltd., Bonnyville, Alberta, Canada. The roots of the seedlings were gently washed to remove potting medium and the seedlings were transplanted into aerated 50% modified Hoagland’s solution (pH 5.8) (Epstein 1972) in the controlled environment growth chamber. The conditions were: 22/18 °C (day/night) temperature, 65 ± 10% relative humidity, and 16-h photoperiod with 300 ± 50 μmol m−2 s−1 photosynthetic photon flux density (PPFD) obtained from full-spectrum fluorescent bulbs (Philips high output, F96T8/TL835/HO, Markham, ON, Canada).
A semi-automated hydroponic split-root system was used in this study (Zhang and Zwiazek 2016b). Two 11 L plastic tubs (31 cm length × 18 cm width × 40 cm height) were glued together to construct as a split-root growth container. For each treatment, five split-root growth containers were connected to two separate 40 L plastic barrels (40.5 cm length × 31 cm width × 62 cm height) by polyvinyl chloride (PVC) tubing. A water pump (Model 9.5 950GPH, Danner MFG Inc., New York, NY, USA) was placed in each barrel to circulate nutrient solution between the split-root growth containers and the barrels. All split-root growth containers had spouts installed into their sides with 1-m-long tubing to facilitate drainage. In this way, the two parts of each split-root growth container were supplied with a different solution, and the solution could constantly circulate and provide sufficient oxygen to plants (~ 8 mg L−1 O2). The split-root growth container was covered by a Styrofoam lid with four 3.8 cm holes in its center for holding of the seedlings. The roots of the seedlings were inserted into one side of the split-root growth container secured with foam plugs for 2 weeks. The expanded roots were evenly divided and placed into the two parts of the split-root container. There were four seedlings per species in each split-root container.
Experimental treatments
The plants were treated with two pH levels (5 and 9) and two Fe levels (0 and 40 µM Fe-EDTA). The 40 µM Fe treatment was applied in the Milli-Q water to eliminate the effects of other nutrients that are present in Hoaglandʼs solution and isolate Fe as a single factor in plant responses to pH. The combination of treatments is illustrated in Fig. 1. The pH was adjusted to 5 and 9 with KOH or H2SO4 as described below. The solution pH was controlled within ± 0.1 range using the split-root setup. In this system, the pH controller (PHCN-70, Omega Engineering Inc., Laval, QC, Canada) connected with a pH electrode (Orion 9106 BNWP gel-filled combination pH electrode, Thermo Scientific, Rochester, NY), which was immersed in the solution to read the solution pH values and connected to an electronic valve (Model 8260G071 120/60 ASCO Valve, Inc., Florham Park, NJ, USA) to add automatically either 5% (w/w) KOH or 1% (v/v) H2SO4 solution. The treatments lasted for 8 weeks and the solution was replaced every 2 weeks.
Net photosynthetic (Pn) and transpiration (E) rates
After 8 weeks of treatment, Pn and E were measured using the infrared gas analyzer (LI-6400, LI-COR, Lincoln, Nebraska USA) at 400 μmol m−2 s−1 PPFD with five seedlings (n = 5) per treatment per species. Fully developed leaves with minimal or no necrosis on the uppermost branches were selected for the measurements. The reference CO2 concentration was 400 μmol mol−1 and the flow rate was 200 μmol s−1 in the leaf chamber. The leaf chamber temperature was kept at 20 °C. The measurements were taken from 8:00 to 12:00 h.
Dry weights
At the end of the treatments, shoot and root dry weights were determined in five seedlings (n = 5) per treatment for each tree species. The seedlings were separated into roots (taken separately from each of the two split containers), stems, and leaves and dried in an oven at 70 °C for 72 h. The leaves for chlorophyll measurement were detached from the stems and freeze dried at − 80 °C for 72 h. The total dry weights were the sum of the dry weights of stems, roots, and leaves from each plant, and the combined weights for leaves and stems were referred to as the shoot dry weights.
Leaf chlorophyll concentrations
Chlorophyll a and b concentrations in old and young leaves were determined in five seedlings per treatment (n = 5) for each species. The old leaves were those that expanded fully before the treatments and the young leaves were those that were formed after the start of treatments and were close to the shoot apex. After freeze drying, the leaves were ground in the Thomas Wiley Mini-Mill (Thomas Scientific, NJ, USA). Each pulverized leaf sample (10 mg) was extracted with 8 ml of the extraction solvent. Chlorophyll from paper birch and trembling aspen leaves was extracted with dimethylsulfoxide (DMSO) at 65 °C for 22 h, and from dogwood with methanol at 55 °C for 22 h. Different solvents were used since the DMSO extracts of dogwood leaves were black, which interfered with chlorophyll analysis. Chlorophyll concentrations were measured in the DMSO extracts at 648 nm and 665 nm, and in the methanol extracts at 652 nm and 665 nm with the spectrophotometer (Ultrospec, Pharmacia LKB, Uppsala, Sweden). Total chlorophyll concentration was calculated using the Arnon’s equation for the DMSO extracts (Barnes et al. 1992) and the MacKinney’s equation for the methanol extracts (Sestak et al. 1971).
Ferric-chelate reductase (FCR) activity
Root tips (1-cm long) of five seedlings per species (n = 5) were excised from each treatment, as this part of root was supposed to have the highest FCR activity (İpek et al. 2017). Each root sample was rinsed in 0.2 mM CaSO4 and placed in a test tube filled with 0.2 mM CaSO4. The test tube with roots was placed in an ice box and taken to the laboratory. For about 0.2 g root (fresh weight), 10 ml of assay solution was added. The assay solution containing 0.2 mM CaSO4, 0.1 mM Fe-EDTA, and 0.3 mM BPDS (bathophenanthrolinedisulfonic acid) (Cohen et al. 1997) was adjusted to pH 5 or 9 with either 5% (w/w) KOH or 1% (v/v) H2SO4 as required. One test tube containing 10 ml assay solution with no roots was used as a control. The tubes were shaken in the dark at room temperature for 1 h. After that, the absorbance of each solution was measured with the spectrophotometer (Ultrospec, Pharmacia LKB, Uppsala, Sweden) at 535 nm. The concentration of Fe(II)-BPDS was calculated using the molar extinction coefficient of 22.14 mM−1cm−1 (Cohen et al. 1997).
Elemental analysis of young leaves
The concentrations of Ca, Cu, Fe, Mg, Mn, P, and Zn were determined in young leaves in five seedlings per treatment per species (n = 5). Young leaves were freeze dried and ground with a Thomas Wiley Mini-Mill. Each sample (0.3–0.4 g dry weight) was digested with 10 ml 70% HNO3 and heated for 10 min at 185 °C in a microwave oven (Mars 5 Microwave Accelerated Reaction System, CEM, Matthews, NC, USA). After complete digestion, the solution was diluted with Milli-Q water to 40 ml. The extracts were then filtered and analyzed by ICP-MS (inductively coupled plasma mass spectrometry) (Zarcinas et al. 1987) in the Radiogenic Isotope Facility at the University of Alberta, Edmonton, AB, Canada.
Experimental design and statistical analysis
All data were analyzed with the GLM model of SAS (Version 9.3, SAS Institute Inc., Cary, NC, USA) to determine significant differences (p < 0.05). The data for the root dry weights of the split roots and chlorophyll concentration of old and young leaves were analyzed using the paired t test. One-way ANOVA was used to detect significant differences between the means. Residuals were checked for normality and homogeneity of variances. The Log10 function was used to transform the data that violated the ANOVA assumptions. Comparisons between different treatment means were conducted by the Tukey’s test.
Pearson correlation analysis was conducted on the dataset of mean values for each response variable at each treatment and for each species. Principal component analysis (PCA) of leaf element (B, Ca, Cu, Fe, K, Mg, Mn, P, Zn) concentrations was performed to separate effects of treatments, using select() in the ‘dplyr’ and rda() in the ‘vegan’ packages in R v. 3.5.2 (http://www.r-project.org/).
Results
Total dry weights and shoot to root dry weight (s/r) ratios
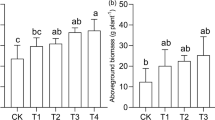
The total dry weights of the considered species were lower in plants grown in Hoagland’s solution of pH 9 compared with those grown in Hoagland’s solution of pH 5 (Fig. 2a, b, c). Total dry weights of paper birch and trembling aspen in pH 5:5 and pH 5:9 (5H–9Fe and 9H–5Fe) treatments were similar (Fig. 2a, b), while for dogwood, the total dry weights were higher when subjected to pH 5:5 treatments compared with the other treatments (Fig. 2c). The trends for s/r ratios were similar in the three species and the s/r ratios in 9H–5Fe treatment were lower compared with the other three treatments (Fig. 2d, e, f).
Effects of pH and Fe supply in a split-root system on total dry weights and shoot to root dry weight ratios in paper birch, trembling aspen, and dogwood. Different letters above the bars indicate significant differences (p < 0.05) between treatments within each plant species. Means (n = 5) ± SE are shown. The symbol “H” refers to Hoagland’s solution without Fe and “Fe” refers to Fe-EDTA (40 µM) in Milli-Q water. The subscripts are pH levels
Gas exchange
The Pn and E of the studied species with half roots exposed to the low pH (5H–9Fe and 9H–5Fe treatments) had similar values compared with those in the pH 5:5 treatment (Fig. 3). Both Pn and E followed the same pattern in the three studied species, while that their lowest values were observed in pH 9:9 treatment (Fig. 3).
Effects of pH and Fe supply in a split-root system on net photosynthetic rates (Pn) and transpiration rates (E) in paper birch, trembling aspen, and dogwood seedlings. Different letters above the bars indicate significant differences (p < 0.05) between treatments within each plant species. Means (n = 5) ± SE are shown. The symbol “H” refers to Hoagland’s solution without Fe and “Fe” refers to Fe-EDTA (40 µM) in Milli-Q water. The subscripts are pH levels
Root dry weights and ferric-chelate reductase (FCR) activity
Paper birch and dogwood showed similar responses to the pH 5:5 treatment; in fact, their root dry weights were higher in the Hoagland’s-supplied side (Fig. 4a, c). When subjected to pH 5H–9Fe treatment, the root dry weights of paper birch, trembling aspen, and dogwood were four-, six-, and six-fold higher in the pH 5 side than those in the pH 9 side, respectively (Fig. 4a, b, c). However, for 9H–5Fe treatment, trembling aspen responded differently compared with the other two species in that the root dry weights were significantly higher in the Hoagland’s-supplied side with the pH 9 (Fig. 4b). Concerning the pH 9:9 treatments, the root dry weights of paper birch were higher in the side supplied with Fe, while the root dry weights of dogwood was higher in the side supplied with Hoagland’s solution (Fig. 4a, c).
Effects of pH and Fe supply in a split-root system on root dry weights and FCR (ferric-chelate reductase) activity in the Hoagland’s and Fe sides of paper birch, trembling aspen, and dogwood. The asterisk above the bars indicates significant differences (p < 0.05) between two sides of root dry weight and FCR activity determined by the paired t-test. Means (n = 5) ± SE are shown. The symbol “H” refers to Hoagland’s solution without Fe and “Fe” refers to Fe-EDTA (40 µM) in Milli-Q water. The subscripts are pH levels
The FCR activities in paper birch and trembling aspen were little affected by the treatments (Fig. 4d, e). Concerning dogwood, the FCR activity was significantly higher in the Fe-supplied side in the pH 5:5 treatment and in the Hoagland’s-supplied side in the 9H–5Fe treatment (Fig. 4f).
Chlorophyll concentrations
In the three examined species, the highest chlorophyll concentration in old (Chl_O) and young (Chl_Y) leaves was observed in the pH 5:5 treatment and the concentrations were similar in young and old leaves (Fig. 5a, b, c). In paper birch and trembling aspen, Chl_Y dramatically decreased compared with Chl_O in the 5H–9Fe treatment (Fig. 5a, b), while in dogwood, Chl_Y increased compared with Chl_O in the 5H–9Fe treatment (Fig. 5c).
Effects of pH and Fe supply in a split-root system on total chlorophyll (concentrations (chlorophyll a + chlorophyll b) in old and young leaves of paper birch, trembling aspen, and dogwood. The old leaves were those expanded fully before the treatments and the young leaves were those sprouted after the start of treatments and were close to the shoot apex. Different letters above the bars (lowercase letters for old leaves, uppercase letters for young leaves) indicate significant differences (p < 0.05) between treatments within each plant species. Means (n = 5) ± SE are shown. The symbol “H” refers to Hoagland’s solution without Fe and “Fe” refers to Fe-EDTA (40 µM) in Milli-Q water. The subscripts are pH levels
Elemental concentrations in young leaves
In paper birch and trembling aspen, leaf Fe concentrations varied with the soluble Fe concentration in the treatment solutions. For the 5H–5Fe and 9H–5Fe treatments with Fe supplied in the low pH side, the leaf Fe concentrations were about one- to twofold higher compared with the remaining treatments (Fig. 6a, b). In dogwood, the highest leaf Fe concentration was found in the pH 5:5 treatments (Fig. 6c).
Effects of pH and Fe supply in a split-root system on Ca, Cu, Fe, Mg, Mn, P and Zn concentrations in young leaves of paper birch, trembling aspen, and dogwood seedlings, presented as the percentages of values measured at 5H–5Fe treatment in young leaves. Different letters above the bars indicate significant differences (p < 0.05) between treatments within each plant species. Means (n = 5) ± SE are shown. The symbol “H” refers to Hoagland’s solution without Fe and “Fe” refers to Fe-EDTA (40 µM) in Milli-Q water. The subscripts are pH levels
In paper birch, the leaf Ca and Mg concentrations were about 100% higher in 5H–9Fe treatment compared with the other treatments (Fig. 6a). The leaf Cu and Mn concentrations were little affected by the applied treatments. The leaf P and Zn concentrations were dramatically higher in 5H–5Fe and 5H–9Fe treatments compared with the remaining treatments (Fig. 6a). The responses of leaf P and Zn concentrations in trembling aspen and dogwood followed a similar trend (Fig. 6b, c).
In trembling aspen, significant differences in leaf Ca, Mg, and Mn concentrations were not detected among the treatments. Leaf Cu concentration was slightly higher in the 5H–9Fe treatment (Fig. 6b) than other treatments.
In dogwood, the lowest leaf Ca, Mg, and Mn concentrations were observed in the 9H–5Fe treatments; significant differences were not found in the leaf Cu concentrations across all treatments (Fig. 6c).
Correlation and PCA analyses
In paper birch, aspen and dogwood, the net photosynthesis and transpiration rates were significantly correlated (Table 1). In paper birch, leaf Fe and K concentrations were negatively correlated. In aspen, young leaf chlorophyll concentration was positively correlated with Fe concentration while negatively correlated with Mn concentration. In dogwood, leaf Ca concentration was positively correlated with Mn and P concentration.
The PCA obviously separated the effects of different treatments on leaf element concentration (Fig. 7). For all three species, on the PCA plot the component loadings of Fe were different from other elements. In paper birch, Fe majorly represented PC2, while in dogwood Fe majorly represented PC1. In paper birch, the PC1 was mainly explained by Mn and Zn concentration; neither PC1 nor PC2 explained the leaf Ca, P, Mg and Cu concentrations.
Discussion and conclusion
In the considered plant species, the highest total dry weights, Pn, and E, were detected in the 5H–5Fe treatment and the lowest in the 9H–9Fe treatment. In the pH 5:9 treatments (5H–9Fe and 9H–5Fe), with the root partially exposed to the low pH medium, most of the plant growth and physiological parameters improved compared with the 9H–9Fe treatment. The different side arrangement of the Hoagland’s solution and Fe supply in the pH 5:9 treatment mainly affected biomass allocation and elemental concentrations in leaves, but had minor effects on total dry weights, total chlorophyll concentrations, Pn, and E. These results confirm the hypothesis that partial exposure of root system to low pH could alleviate the stress of high pH.
The total dry weights of paper birch and aspen dramatically decreased in the 9H–9Fe treatment, while in dogwood, the total dry weights decreased when high pH was applied. This appears to be consistent with the previous study that showed strong growth reductions in dogwood when subjected to high pH (Calvo-Polanco et al. 2017). The mean dry weight of dogwood seedlings was around 120 g, while the total dry weights of paper birch and trembling aspen seedlings were about 14 g and 10 g, respectively, after 8 weeks of the 5H–5Fe treatment. Among the studied species, dogwood showed relatively highest growth rates. The decreased total dry weights of dogwood under high pH conditions may be due to inadequate nutrients to support the high rate of growth.
Plants have different strategies to allocate biomass in order to optimize growth and physiological performance. The shoot:root dry weight ratios of paper birch, aspen and dogwood were lowest at 9H–5Fe treatment (Fig. 2). Since the total root dry weights were similar at 5H–9Fe and 9H–5Fe (Fig. 4), the lower shoot:root dry weight ratios indicated that the shoot growth was inhibited at 9H–5Fe, when all other essential nutrients excluding Fe were supplied only at pH 9. The same findings were reported in our previous studies (Zhang et al. 2013, 2015). In the 5H–5Fe and 5H–9Fe treatments, paper birch, trembling aspen, and dogwood grew a larger root system in the Hoaglandʼs-supplied side. In the 9H–5Fe treatment, although Hoagland’s solution was supplied in the high pH side, it was expected that more roots of the studied species would be present in the low pH side since high pH inhibits root growth. Paper birch and dogwood followed this pattern, but aspen had higher root dry weights in the side containing Hoagland’s solution regardless of high pH. These results suggest that the root growth of aspen was relatively tolerant to high pH, but sensitive to the nutrition; as a high nutrient demanding species (Alban 1982), this root growth pattern in aspen could facilitate nutrient uptake and support growth. Since Hoagland’s solution used in this study was Fe-free, the Fe-deficiency response of proton extrusion to lower the apoplastic pH may also play a role in this process when the roots were in the high pH side (Jeong and Connolly 2009).
In the present study, due to the inconvenience of handling the relatively large size of the whole root system, for the FCR activity assay we used root tips instead of intact roots. Our previous studies showed that when the root tips were stored on ice and the assay was completed in a short time, the excision stress did not significantly affect FCR activity of root tips compared with intact roots (Zhang and Zwiazek 2016a). In the strategy I plants, the reduction of Fe3+ to Fe2+ by FCR is thought to be an obligatory and rate-limiting step in Fe uptake (Grotz and Guerinot 2002; Curie and Briat 2003; Schmidt 2003). In dogwood, the FCR activity was much higher in the Fe-supplied side in the 5H–5Fe treatment than other treatments. These results are in agreement with earlier results, which showed that the reduction activity increased in the Fe-supplied side in a split-root experiment while Fe-free grown roots had lower FCR activity (Schikora and Schmidt 2001). This response contributed to more Fe uptake under uneven Fe concentrations. For 9H–5Fe treatment, the responses of root FCR activity were complex since the FCR activity was affected by both high pH and Fe concentration. On the one hand, the Fe3+-reduction rate is pH dependent and the optimal pH for FCR activity in vivo is around 5.5 (Moog and Brüggemann 1994). FCR activity may be severely depressed by high root apoplastic pH (Toulon et al. 1992; Mengel 1994). On the other hand, as discussed above, the root Fe3+-reduction capacity may increase in the side with sufficient Fe supplied under uneven Fe conditions. Therefore, it was expected that the root FCR activity in the low pH side with sufficient Fe supplied (5Fe) would be higher compared with the high pH side with Fe deprivation (9H). However, concerning dogwood, the FCR activity was higher in the Hoagland’s-supplied side at pH 9 (9H) in 9H–5Fe treatment. Our previous study also demonstrated that root FCR activity of paper birch and dogwood was higher at pH 7 and 9 compared with pH 5 in Hoagland’s solution (Zhang and Zwiazek 2016a). This may reflect a general response of plant roots to nutrient deficiency and when part of the root system was exposed to the 9H treatment, plants tended to increase the enzyme activity in order to absorb more nutrients from the Hoagland’s-supplied side. However, more research is required to clarify the relationship between FCR activity and high pH under Fe-deficiency conditions.
In the three studied species, both Chl_Y and Chl_O in the treatments involving high pH dramatically decreased compared with the 5H–5Fe treatment. Exposing part of the root system to lower pH had little beneficial impact on chlorophyll concentrations for the three species. This could be due to decreased concentrations of some of the leaf elements including Fe. Iron deficiency can diminish chlorophyll biosynthesis by affecting Fe-containing enzymes which catalyze the formation of chlorophyll precursors (Ouchane et al. 2004). The Fe concentration in young leaves was lower in the three studied species in the 5H–9Fe treatment compared with that in the 5H–5Fe treatment. In the 5H–9Fe treatment, the Chl_Y in paper birch and aspen was significantly lower compared with Chl_O, while the Chl_Y in dogwood was much higher compared with Chl_O. In the 9H–5Fe treatment, Chl_Y was similar to Chl_O in paper birch and aspen, this might be due to the increased concentration of Fe in young leaves. Iron is generally considered to be an immobile element in plants; however, under Fe deficiency, Fe of mature leaves was found to be remobilized to the young leaves and shoot apex in bean (Phaseolus vulgaris L.) (Zhang et al. 1995). The mobility of Fe may vary between plant species. Dogwood was found to better maintain lower root xylem sap pH compared with paper birch when exposed to high pH (Zhang and Zwiazek 2016a). Therefore, it can be speculated that dogwood may be able to retranslocate more Fe from the old to the young leaves. In the PCA plot, the leaf Fe concentration mainly represented PC1 in dogwood, which was different from paper birch and aspen. This may explain that the chlorophyll concentrations of young leaves of dogwood were less affected by high pH conditions in 5H–9Fe treatment compared with paper birch and aspen.
Although exposure of parts of the roots to low pH did not increase chlorophyll concentrations in the 5H–9Fe and 9H–5Fe treatments, particularly in aspen and dogwood, it had a beneficial effect on Pn in all three species and Pn decreased in the 9H–9Fe treatment compared with 5H–5Fe treatment. Photosynthesis can be influenced by stomatal factors and the efficiency of the photosynthetic system. The similar patterns of Pn and E reductions indicated that stomatal conductance was likely to be the main contributor to the decrease of Pn. High pH can reduce root water flow and, consequently, stomatal conductance (Kamaluddin and Zwiazek 2004). Although the efficiency of the photosynthetic system in the 5H–9Fe and 9H–5Fe treatments may have been reduced due to either Fe or other nutrient deficiencies, treatment duration might have been too short to significantly impact Pn.
Due to the effects of high pH on the uptake of some of the essential nutrients, we expected that the plants would have the best nutrient status in the 5H–5Fe treatment. However, the elemental analysis of young leaves demonstrated that some of the nutrient concentrations were either higher or similar in the 5H–9Fe treatments compared with those in the 5H–5Fe treatments; these included Ca, Mg, P, and Zn in paper birch, Cu, P, and Zn in trembling aspen, and Ca, Mg, Mn, P, and Zn in dogwood. In the 5H–9Fe treatment, half of the root system was exposed to high pH in the Fe-supplied side. Therefore, Fe deficiency and high pH stress may both have the possibility to induce stress responses in the 5H–9Fe treatment. The Fe-deficiency response could improve not only Fe uptake but also that of other nutrients. The uptake of several micronutrients including Mn and Zn in sunflower (Helianthus annuus L.) was enhanced by Fe deficiency and the uptake rate was slightly lower than that of Fe (Römheld et al. 1982). Additionally, more chelating substances such as phenolics were likely extruded under Fe deficiency, which could facilitate the transport of cations across the plasma membrane (Römheld and Marschner 1981). Another possibility may be that elevated pH triggered a signal from the 9Fe-side roots to the 5H-side roots and lead to increased activities of enzymes involved in nutrient uptake. Previous split-root experiments conducted on Arabidopsis indicated that the long distance molecular signals generated in shoots regulated root responses, particularly those of FCR activities under Fe starvation stress (Schikora and Schmidt 2001; Vert et al. 2003). Evidence from studies on cucumber (Cucumis sativus) suggests that ethylene plays a major role in this modulating process (Waters et al. 2007). Fe and Mn deficiencies are common in plants growing in high pH soils (George et al. 2012). However, in the present study, as Fe supply under high pH condition was a treatment factor, in three studied plant species leaf Fe and Mn concentrations showed negative correlation, particularly in aspen. In the PCA plot, leaf Fe and Mn concentrations also represented different principal components in paper birch, aspen and dogwood.
In conclusion, high pH conditions reduced net photosynthesis, transpiration rates, leaf chlorophyll concentrations and uptake of Fe, P, and Zn in plants. Partial exposure of the root system to low root zone pH and Fe supply mitigated leaf chlorosis and alleviated the high pH stress in the studied plants by enhanced Fe uptake. The improvement may not only be attributed to acidified spots affecting solubility and uptake of micronutrients under high pH condition, but also to the fact that the general stress responses in the high pH side modified the responses in the low pH side. Under Fe deficiency, dogwood had higher Fe utilization efficiency compared with paper birch and trembling aspen under high pH stress. The roots of trembling aspen could tolerate high pH in order to obtain more nutrients. Findings from this study could potentially be applied in revegetation practices at reconstructed landforms with high soil pH problems.
Author contribution statement:
JJZ designed the experiments. FX conducted the experiments, and collected the data. FX, XT and WQZ analyzed the data. All authors contributed to the writing of the paper.
References
Alban DH (1982) Effects of nutrient accumulation by aspen, spruce, and pine on soil properties. Soil Sci Soc Am J 46:853–861
Barnes J, Balaguer L, Manrique E, Elvira S, Davison A (1992) A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ Exp Bot 32:85–100
Calvo-Polanco M, Zhang W, Macdonald E, Señorans J, Zwiazek JJ (2017) Boreal forest plant species responses to pH: ecological interpretation and application to reclamation. Plant Soil 420:195–208
Chalaturnyk RJ, Don Scott J, Özüm B (2002) Management of oil sands tailings. Pet Sci Technol 20:1025–1046
Cohen CK, Norvell WA, Kochian LV (1997) Induction of the root cell plasma membrane ferric reductase (an exclusive role for Fe and Cu). Plant Physiol 114:1061–1069
Colangelo EP, Guerinot ML (2004) The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 16:3400–3412. https://doi.org/10.1105/tpc.104.024315
Colombo C, Palumbo G, He JZ, Pinton R, Cesco S (2014) Review on iron availability in soil: interaction of Fe minerals, plants, and microbes. J Soil Sedim 14:538–548. https://doi.org/10.1007/s11368-013-0814-z
Comerford NB (2005) Soil factors affecting nutrient bioavailability. In: BassiriRad H (ed) Nutrient acquisition by plants. Ecological Studies (Analysis and Synthesis), vol 181. Springer, Berlin, pp 1–14. https://doi.org/10.1007/3-540-27675-0_1
Coppa E, Celletti S, Pii Y, Mimmo T, Cesco S, Astolfi S (2018) Revisiting Fe/S interplay in tomato: a split-root approach to study the systemic and local responses. Plant Sci 276:134–142
Curie C, Briat JF (2003) Iron transport and signaling in plants. Annu Rev Plant Biol 54:183–206
Eberhard S, Finazzi G, Wollman FA (2008) The dynamics of photosynthesis. Annu Rev Genet 42:463–515
Epstein E (1972) Mineral nutrition of plants: principles and perspectives. Wiley, New York
Fung MYP, Macyk TM (2000) Reclamation of oil sands mining areas. In: Barnhisel RI, Darmody RG, Daniels WL (eds) Reclamation of drastically disturbed lands. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison
George E, Horst WJ, Neumann E (2012) Adaptation of plants to adverse chemical soil conditions. In: Marschner P (ed) Marschner’s Mineral Nutrition of Higher Plants, 3rd edn. Academic press, Cambridge, Massachusetts, United States, pp 409–472
Grotz N, Guerinot ML (2002) Limiting nutrients: an old problem with new solutions? Curr Opin Plant Biol 5:158–163
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59
Howat D (2000) Acceptable salinity, sodicity and pH values for boreal forest reclamation. Environmental Sciences Division. Edmonton Alberta. Report # ESD/LM/00-2. ISBN 0-7785-1173-1 (printed edition) or ISBN 0-7785-1174-X (on-line edition)
İpek M, Aras S, Arıkan Ş, Eşitken A, Pırlak L, Dönmez MF, Turan M (2017) Root plant growth promoting rhizobacteria inoculations increase ferric chelate reductase (FC-R) activity and Fe nutrition in pear under calcareous soil conditions. Sci Hortic 219:144–151
Jeong J, Connolly EL (2009) Iron uptake mechanisms in plants: functions of the FRO family of ferric reductases. Plant Sci 176:709–714. https://doi.org/10.1016/j.plantsci.2009.02.011
Kamaluddin M, Zwiazek JJ (2004) Effects of root medium pH on water transport in paper birch (Betula papyrifera) seedlings in relation to root temperature and abscisic acid treatments. Tree Physiol 24:1173–1180
Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 63:131–152
Läuchli A, Grattan SR (2017) Plant stress under non-optimal soil pH. In: Shabala S (ed) Plant stress physiology, 2nd edn. CAB International, Wallingford, p 210
Mengel K (1994) Iron availability in plant tissues-iron chlorosis on calcareous soils. Plant Soil 165:275–283
Młodzińska E (2012) Alteration of plasma membrane H+-ATPase in cucumber roots under different iron nutrition. Acta Physiol Plant 34:2125–2133
Moog R, Brüggemann W (1994) Iron reductase systems on the plant plasma membrane—a review. Plant Soil 165:241–260
Ouchane S, Steunou AS, Picaud M, Astier C (2004) Aerobic and anaerobic mg-protoporphyrin monomethyl ester cyclases in purple bacteria a strategy adopted to bypass the repressive oxygen control system. J Biol Chem 279:6385–6394
Römheld V, Marschner H (1981) Effect of Fe stress on utilization of Fe chelates by efficient and inefficient plant species. J Plant Nutr 3:551–560
Römheld V, Marschner H, Kramer D (1982) Responses to Fe deficiency in roots of “Fe-efficient” plant species. J Plant Nutr 5:489–498
Schikora A, Schmidt W (2001) Iron stress-induced changes in root epidermal cell fate are regulated independently from physiological responses to low iron availability. Plant Physiol 125:1679–1687
Schmidt W (2003) Iron solutions: acquisition strategies and signaling pathways in plants. Trends Plant Sci 8:188–193
Sestak Z, Catský J, Jarvis PG et al (1971) Plant photosynthetic production: Manual of Methods. Dr. W. Junk Publishers, The Hague
Tang C, Robson A, Longnecker N, Greenway H (1993) Physiological responses of lupin roots to high pH. In: Barrow J (ed) Plant nutrition—from genetic engineering to field practice. Springer, Dordrecht, pp 755–758
Toulon V, Sentenac H, Thibaud JB, Davidian JC, Moulineau C, Grignon C (1992) Role of apoplast acidification by the H+ pump. Planta 186:212–218
Vert GA, Briat JF, Curie C (2003) Dual regulation of the Arabidopsis high-affinity root iron uptake system by local and long-distance signals. Plant Physiol 132:796–804. https://doi.org/10.1104/pp.102.016089
Vert G, Barberon M, Zelazny E, Séguéla M, Briat J-F, Curie C (2009) Arabidopsis IRT2 cooperates with the high-affinity iron uptake system to maintain iron homeostasis in root epidermal cells. Planta 229:1171–1179
Waters BM, Lucena C, Romera FJ, Jester GG, Wynn AN, Rojas CL, Alcántara E, Pérez-Vicente R (2007) Ethylene involvement in the regulation of the H+-ATPase CsHA1 gene and of the new isolated ferric reductase CsFRO1 and iron transporter CsIRT1 genes in cucumber plants. Plant Physiol Biochem 45:293–301
Zarcinas B, Cartwright B, Spouncer L (1987) Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Commun Soil Sci Plant Anal 18:131–146
Zhang W, Zwiazek JJ (2016a) Effects of root medium pH on root water transport and apoplastic ph in red-osier dogwood (Cornus sericea) and paper birch (Betula papyrifera) seedlings. Plant Biol 18:1001–1007
Zhang W, Zwiazek JJ (2016b) Responses of reclamation plants to high root zone pH: effects of phosphorus and calcium availability. J Environ Qual 45:1652–1662
Zhang C, Römhel R, Marschner H (1995) Retranslocation of iron from primary leaves of bean plants grown under iron deficiency. J Plant Physiol 146:268–272
Zhang W, Calvo-Polanco M, Chen ZC, Zwiazek JJ (2013) Growth and physiological responses of trembling aspen (Populus tremuloides), white spruce (Picea glauca) and tamarack (Larix laricina) seedlings to root zone pH. Plant Soil 373:775–786
Zhang W, Xu F, Zwiazek JJ (2015) Responses of jack pine (Pinus banksiana) seedlings to root zone pH and calcium. Environ Exp Bot 111:32–41. https://doi.org/10.1016/j.envexpbot.2014.11.001
Zocchi G, De Nisi P, Dell’Orto M, Espen L, Gallina PM (2007) Iron deficiency differently affects metabolic responses in soybean roots. J Exp Bot 58:993–1000. https://doi.org/10.1093/jxb/erl259
Acknowledgements
This project was funded through research grants to JJZ by Natural Sciences and Engineering Research Council of Canada (NSERC) and Canada’s Oil Sands Innovation Alliance (COSIA). We thank Seonghee Lee for her advices in writing the manuscript, and Mónica Calvo-Polanco and Jorge Señorans for their kind research assistance. We are also grateful to the three anonymous reviewers for their comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Feller.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, F., Tan, X., Zhang, WQ. et al. Effects of iron and root zone pH on growth and physiological responses of paper birch (Betula papyrifera), trembling aspen (Populus tremuloides) and red-osier dogwood (Cornus stolonifera) seedlings in a split-root hydroponic system. Acta Physiol Plant 41, 142 (2019). https://doi.org/10.1007/s11738-019-2933-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-2933-7