Abstract
Organic farming and low-input production agroecosystems are the major components of sustainable agriculture; therefore, application of biofertilizers containing beneficial soil microorganisms to reduce environmental difficulties related to the use of chemical and manufactured fertilizers is the first step towards sustainability. Considering the importance of the production of medicinal and aromatic plant materials in sustainable ways, a 2-year field research was done as a split factorial experiment based on a randomized complete block design with three replicates. Three factors were investigated: (1) water-deficit stress in three levels comprising irrigation after 30%, 60%, and 90% depletion of available soil water (ASW) as main factor, (2) zeolite application (0 and 8 t ha− 1), and (3) seed inoculation with biofertilizers including nitroxin, phosphate barvar-2, and mixture of nitroxin × phosphate barvar-2, and the control (non-inoculation) as subplots. Results showed that the examined traits such as leaf relative water content, chlorophyll a and b, and total chlorophyll contents, leaf soluble sugars and proline contents, flower yield, essential oil percentage and yield, and irrigation water use efficiencies for flower yield (IWUEFY) and for essential oil yield (IWUEEOY) were significantly (p < 0.05) influenced by experimental treatments in dragonhead for both years. According to second-order interaction among treatment groups, the highest IWUEEOY (3.117 ml m− 3) was obtained in nitroxin × phosphate barvar-2 and zeolite treatments under 60% depletion of ASW. A positive and highly significant (r0.01 = 0.85 and 0.98) correlation was observed between essential oil content and essential oil yield and IWUEEOY, respectively. Regarding significant interaction between biofertilizers and zeolite, the treatment combinations of zeolite and nitroxin × phosphate barvar-2 could improve physiological functions and essential oil yield of dragonhead plants following irrigation after 60% depletion of ASW.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dracocephalum moldavica L. (known as Moldavian dragonhead) is an annual herb and aromatic medicinal plant belonging to the mint (Lamiaceae/Labiatae) family originated/naturalized from around southern Siberia and the Himalaya and typically cultivated in temperate areas of Eastern Europe and central Asia (Omidbaigi 2005). It includes approximately 45 species in the world, and has received much attention for use in several fields such as flavour and fragrance, food and pharmaceutical industries (Omidbeigi 1997).
Biosynthesis of secondary bioactive compounds/metabolites in the aromatic medicinal herbs is differently impacted by both genetics and environmental agents (Gholizade et al. 2010b). On the other hand, the phytochemical quality and quantity of plants are affected by both special biotic and abiotic environmental agents called elicitors (Clark and Menary 1980). However, the full control of environmental factors is not possible, but can be managed with specific procedures, in which plants under such adverse conditions seem to maximize their potential to provide optimum yield. To enhance plant productivity under environmental perturbation conditions, good agricultural practices to adequately address soil and water conservation objectives such as effective management of inputs in time, maintaining soil structure and organic content, scheduled irrigation based on plant requirements and soil characteristics, avoiding soil salinization through limiting water input to the plant requirements, preventing drainage and fertilizer run-off, understanding important physiological and biochemical pathways and various mechanisms of defense to reduce stress are mainly required (Hatami and Ghorbanpour 2014; Ibanez et al. 2010). The global demand for agricultural crops and products is increasing and is anticipated to be at least twofold as the world’s population reaches approximately 9.1 billion people by the year 2050. Both greater quantity and higher quality of food in response to increasing demand will need a greater emphasize on the agricultural section. Therefore, good agricultural operations along with effective inputs and technology use are one of the best tools to improve plant performance.
Organic production and low off-farm input farming systems are the major aims of sustainable agriculture; therefore, using biological fertilizers to diminish environmental concerns associated with the use of synthetic fertilizers is a main step towards sustainability. Moreover, to be environmentally friendly, the application of biofertilizers such as nitroxin (with free-living and or containing symbiotic and non-symbiotic nitrogen fixing bacteria) as well as phosphate-solubilizing soil microorganisms improves the quality of the soil physical, chemical and biological characteristics and enhances soil fertility and plant productivity without adverse impacts on the environment (Tahami et al. 2017).
Drought is one of the most significant environmental stressors/perturbations influencing growth, development and production of plants, especially in the arid and semi-arid areas of the world (Baiazidi-Aghdam et al. 2016). Water-deficit stress significantly impacts a broad spectra of plant developmental, physiological and biochemical processes (Geerts and Reas 2009) and productivity. Therefore, watering treatments must be applied efficiently to improve the sustainability of cultivation in agricultural ecosystems (Geerts and Reas 2009).
In countries where water shortage is the major restricting factor for cultivation and agriculture, researchers and farmers use some efficient ways to decrease adverse impacts of water deficit stress; one possible way to mitigating the influence of drought stress on plant performance is through the addition of natural zeolite to the soil environment (Manivannan et al. 2007). Zeolites may have great potential to be used as valuable materials in reclamation and restoration of soil and storing available soil water for plant growth and production (Zhang et al. 2007).
With respect to the importance of water in agricultural sector and the spread of water shortage by population growth especially in arid and semi-arid areas of the world, cultivation of water-efficient medicinal plants such as dragonhead in a sustainable manner seems advantageous for these regions. Little is known regarding the use of biofertilizers and zeolite under different watering conditions and their double and triple interactions on physiological functions and efficiency of irrigation for flowering parts and essential oil yield in field-grown D. moldavica L. plants. Thus, the present work was aimed to investigate the impacts of zeolite and bacterial biofertilizer application on physiological characteristics, essential oil content and yield of D. moldavica L.under deficit water stress conditions in two continuous years.
Materials and methods
Plant materials, experimental setup and treatments
A 2-year, 2014 and 2015, field research was performed at the experimental area of the Seed and Plant Improvement Institute in Karaj, Iran (latitude 35°48′N, longitude 51°26′E, altitude 1321 m above mean sea level) with relatively temperate and arid climate, and mean annual precipitation and temperature of 250 mm and 14.2 °C, respectively. The daily precipitation and air temperature during the experiment in both 2014 and 2015 years are given in Fig. 1. The soil samples were taken from 0 to 30 cm depth approximately 2 months before sowing, and the related physical and chemical properties of the tested soil are presented in Table 1. According to the soil chemical analysis, macronutrients except nitrogen were at sufficient levels; therefore, only a nitrogen-containing fertilizer; urea (46% N), was applied at a rate of 140 kg ha− 1 basis to the soil before commencing irrigation treatment.
This study was performed as a split factorial experiment based on a randomized complete block design (RCBD) with three replicates. Three factors were investigated: (1) drought stress in three irrigation regimes [(consisting irrigation after 30% (I1), 60% (I2), and 90% (I3) depletion of available soil water)] as main factor, (2) zeolite application (0 and 8 t ha− 1), and (3) seed inoculation with biofertilizers including nitroxin, phosphate barvar-2, and a mixture of nitroxin × phosphate barvar-2, and the control (non-inoculation with biofertilizers) as subplots. Nitroxin (containing 107 CFU/ml free-living nitrogen-fixing bacteria including Azotobacter sp., Azospirillum sp., and phosphate-solubilizing bacteria from Pseudomonas genus) is the traditional name of biological nitrogen. Also, phosphate barvar-2 (phosphate-solubilizing bacteria) containing Pantoea agglomerans (p5) and Pseudomonas putida (p13) (each mL contains 108 microorganisms). The field was deeply (35–40 cm) ploughed in the late autumn after being fallowed, and then was ploughed superficially (10–12 cm) in the early spring and finally levelled by hand trowels. Experimental plots consisted of six rows that were 3 m long and 0.75 m apart (each plot size: 2.25 m2), which were prepared after soil arrangement. There were 4 m gaps between the blocks, and a 1.5 m alley was considered between the plots to avoid lateral water movement and other interferences.
The seeds of D. moldavica L. were provided from Pakan Bazr Co. Esfahan, Iran. The seed viability was further examined before starting research and was determined to be 98% on average.
The seeds were planted in the end of April in soil at a uniform depth of 1 cm, and seedling emergence subsequently occurred about 3 weeks after planting (mid/late May).Thinning operations were done at the end of the second week of emergence, so that the distance between plants on the lines and plant spacing among lines was 12.5 and 40 cm, respectively. Weeding operations were carried out manually without using any chemical herbicides. No pesticides and fungicides were used as well during both years of the experiment. Flowering process initiated approximately 60 days after emergence (i.e. mid/late July). The plants were finally harvested at 60–70% flowering stage (i.e. about 95 days after emergence or late August) in the both experiment years.
The zeolite physicochemical characteristics are also given in Table 1. A random sample was taken from ten plants per each plot area after leaving out 1 m from the edge of the field (i.e. beginning and end of each experimental plot), where plants grow differently due to the margin/and or border effect.
Irrigation treatment
In all experimental plots, dragonhead plants were watered in a uniform manner when 30% of soil moisture, namely available soil water (ASW), was drained or depleted for 25 days (i.e. 4–6 leaf growth stage). Then when the plants were approximately 15 cm tall (i.e. 45 days after sowing or mid June), watering regimes (irrigation treatments) were applied and continued until plants reached 60–70% flowering stage (i.e. 115 days after sowing). All experimental operations such as soil ploughing, seed preparation, cultivation, treatments, etc. were repeated in the following year (2015). Similarly, for 2015 the plots containing plants were irrigated two times in May–June (4–6 leaf growth stage) and the irrigation treatments were initiated in mid-June and continued until early August 2015.
The volume of water accumulated in rhizosphere (the root zone of the soil) between field capacity (FC) and the permanent wilting point (PWP) is described as ASW, which can be easily used by plants. ASW was calculated based on the following equation:
where WFC and WPWP are the gravimetric soil–water content (%) at FC and PWP, respectively, Bd refers to the value of soil bulk density (g cm− 3) and V indicates the soil layer volume (m3) at the depth of the root zone in D. moldavica L., which was determined to be different three times (i.e. June, July and August) during both the 2014 and 2015 growing season and approximately 20, 35 and 55 cm, respectively.
Readily available soil water (RAW) refers to the fraction of ASW that a plant can readily uptake from the entire root zone without enduring the consequences of drought stress. It was measured according to the procedure of Allen et al. (1998) as follows:
The value of p normally differs for various plants ranging from 0.3 to 0.7 based on root depth; however, the minimum p value is considered for shallow-rooted plants at maximum rates of evapotranspiration (ETc) (> 8 mm day− 1) and the maximum p value is designated for deep-rooted plants at minimum rates of ETc (< 3 mm day− 1). The p factor was applied to measure the required time of watering to avoid drought stress. A value of 0.50 for p is commonly used for many plants. Therefore, in this study the value 0.50 was used for D. moldavica L. The fraction p is specified as evaporation power of air according to the following equation:
where prec is the suggested factor for many field crops and p is the fitted factor for atmospheric evaporative requirement. Subsequently, the watering treatments/regimes were planned according to the procedure of Kramer and Boyer (1995) based on maximum allowable depletion (MAD) percentage of ASW in the root zone and then irrigation treatments were applied as follows:
[I1: irrigation after applying 30% of ASW (i.e., well watered), I2: irrigation after applying 60% of ASW (i.e., moderate water-deficit stress), I3: irrigation after applying 90% of ASW (i.e., severe water-deficit stress)] from the root zone. A TDR prob was used 2 days after irrigation to determine the soil water content and 1 day before the next irrigation.
The ASW depletion value related to the soil water potential (Ψ) was calculated via a soil water characteristic curve. The irrigation volume (Virrig) required for increasing the root zone soil water to FC was determined by the following equation as described by Tafteh and Sepaskhah (2012):
Here, f is the depletion fraction for soil water (30%, 60%, and 90%) from the root depth, and Ea is the efficiency of irrigation (%), which was supposed to be approximately 70% for the entire growing period. The water for irrigation treatments was distributed by a system of pipes and the value was determined by a flow meter. Furthermore, the cumulative values of soil water applied (mm) for different irrigation regime treatments (I1, I2 and I3) over the growing seasons in 2014 and 2015 are shown in Table 2.
Measurements
Determination of leaf relative water content (RWC)
At 60–70% flowering stage, leaf relative water content, soluble sugars and free amino acid proline contents, chlorophyll a, b and total chlorophyll, flower yield, essential oil percentage and yield, and IWUEEOY of three plants (n = 3) per experimental unit were determined. RWC was determined on leaf tissues obtained from the fourth fully developed leaf at the top of the plant. The fresh weights (FW) of leaves were measured immediately after sampling. Then, they were submerged in double distilled water in test containers at room temperature (22 °C) for 6 h and the turgid weight (TW) was estimated. Dry weight (DW) was measured after drying the samples in an electrical oven at 70 °C for 48 h. Finally, RWC was measured according to the following equation (Levitt 1980):
Determination of plastid pigments
For extraction of chlorophyll (Chl), fresh leaf tissue (0.5 g) was blended with acetone (10 ml 80% V/V). The absorption of mixture was spectrophotometerically recorded at 645 and 663 for Chl a and Chl b, respectively. Plastid pigment contents were calculated as previously described by Lichtenthaler (1987) as follows:
Measurement of free proline and soluble sugar contents
The proline content of the leaf extracts was measured according to Bates et al. (1973). Fresh leaf tissue (500 mg) was homogenized in 10 ml of 3% sulfosalicylic acid and the extract was filtered. Then, the mixture was centrifuged at 10,000×g for 10 min. The extract (2 ml) was added into ninhydrin reagent and glacial acetic (both in 2 ml). The reaction solution was heated in a water bath at 100 °C for 1 h. Then, the reaction mixture was extracted with toluene (4 ml). Finally, the absorbance was noted spectrophotometrically at 520 nm against toluene blank and expressed as (mg g− 1 fw− 1).
The total soluble sugars of leaves were determined following the procedure described by Irigoyen et al. (1992). Dried leaf (500 mg) was mixed in ethanol (95%) and then the homogenized extract (0.1 ml) was blended with freshly prepared anthrone (3 ml). The reaction mixture was heated for 10 min in a laboratory water bath. Finally, the absorption of the obtained samples was recorded spectrophotometrically at 625 nm. The glucose standard curve was used for determination of total leaf soluble sugars content.
Estimation of flower yield
At flowering stage (60–70%), plants were harvested from the middle portion in each plot (2.5 m2) for estimation of shoot top flower yield. The harvested herbs were air dried for ten continuous days and then flower dry weight was measured and expressed as kg ha− 1.
Quantification of essential oil content and yield
The air-dried shoot samples (100 g) of D. moldavica L. were powdered for isolation of essential oil. The essential oil of the drug fraction was extracted by the hydrodistillation technique for 3 h using a Clevenger-type apparatus based on the procedure suggested in British Pharmacopoeia (British Pharmacopoeia 1993). The resulting essential oils were dried over Na2SO4 and kept in tightly closed dark vials at 4 °C until further analysis.
Measurement of irrigation water use efficiency (IWUE)
The IWUE is defined as the ratio of the plant yield to the value of water used (Howell 1994). IWUE values for flower yield (IWUEFY) and essential oil yield (IWUEEOY) were estimated by dividing flower yield (kg ha− 1) and oil yield (kg ha− 1) by total water (m3) used in m− 2 for each treatment of the irrigation regime, respectively (Askari and Ehsanzadeh 2015).
Statistical analysis
The normality of the data set was tested using skewness and kurtosis before analysis of variance (ANOVA) and mean (ANOM). Proper data transformation was done for certain traits that showed non-normal distributions. Data were subjected to ANOVA and ANOM using the MSTAT-C statistical software. The Pearson’s correlation coefficient was used for determination of correlations among the various measured traits by SPSS software version 16 (SPSS Inc., Chicago, IL, USA). Means were compared by Duncan multiple range test (DMRT) at the 5% probability level (p ≤ 0.05).
Results
All examined traits of D. moldavica L. in the present study such as leaf relative water content, chlorophyll a and b and total chlorophyll contents, leaf proline and soluble sugars, flower yield, essential oil content, essential oil yield, irrigation water use efficiencies for flower yield (IWUEFY) and essential oil yield (IWUEEOY) were significantly (p < 0.05) influenced by the experimental treatments (i.e. zeolite, biofertilizers and soil moisture) and their main and interaction effects (two-way or double, and three-way or triple interactions) in both years (Table 3). The main mean values for the aforesaid characteristics are presented first (Table 4) and the interaction means are explained afterward (Tables 5, 6, 7, 8).
Relative water content (RWC)
Averaged over years, mean RWC decreased significantly with increase in water-deficit stress (Table 4). The effect of zeolite application was significant for RWC, and the highest RWC value was observed at 8 t ha− 1 zeolite. Also, RWC was significantly influenced by biofertilizer treatments; however, the maximum value was obtained from nitroxin × phosphate barvar-2 (73.778%) followed by nitroxin (73.528%) and phosphate barvar-2 (73.250%), and the minimum was recorded for the control (72.139%). The mean of RWC for double interaction effects is shown in Table 7. In addition, combined treatment of nitroxin × phosphate barvar-2 caused the maximum value of RWC under 30% depletion of ASW (Table 8). Furthermore, application of fertilizers increased RWC in the presence of zeolite in comparison with control. There was no significant difference among fertilizer treatments (nitroxin, phosphate barvar-2 and nitroxin × phosphate barvar-2) in both years. In triple interaction effects, nitroxin × phosphate barvar-2 with zeolite application under 30% depletion of ASW caused maximum percentage (91.5%) of RWC (Table 8).
Chlorophyll contents
Mean comparison showed that the maximum content of chlorophyll a and chlorophyll b was obtained in 30% depletion of ASW (Table 4). Application of zeolite had a significant effect only on chlorophyll a content. The maximum value of chlorophyll a and chlorophyll b was observed in plants treated with zeolite compared to the control.
Also, combined application of nitroxin and phosphate barvar-2 fertilizers showed maximum value of chlorophyll a and chlorophyll b contents. Furthermore, the two-way interactions of water deficit × either zeolite, and/or biofertilizers showed that the 30% depletion of ASW with zeolite and biofertilizer application caused the maximum amount of chlorophyll a and b (Table 6). In 2015, the highest value of chlorophyll a and b were related to plants subjected to the first level of irrigation regime (I1) and nitroxin × phosphate barvar-2, respectively (Table 5). The treatment combinations (triple interactions) of zeolite, nitroxin × phosphate barvar-2 and irrigation after depleting 30% of the ASW resulted in the highest chlorophyll a and b (1.682 and 1.705 mg g− 1 fw− 1) contents, respectively (Table 8).
Total chlorophyll content was significantly impacted (p < 0.01) by all employed treatments, the two and three interactions between them in both years. Over both years, total chlorophyll content was increased with application of zeolite and biofertilizers under all irrigation treatments; however, the highest value of total chlorophyll (3.195 mg g− 1 fw− 1) was obtained from plants exposed to nitroxin × phosphate barvar-2, 8 t ha− 1 zeolite and 30% depletion of ASW, while the lowest value (1.33 mg g− 1 fw− 1) was observed without application of zeolite and biofertlizers under severe water-deficit stress conditions (Table 8).
Proline
The mean leaf proline content was increased by 47.5% and 78.9% in 2014, and 64.07% and 86.5% in 2015, respectively, when dragonhead was grown under I2 and I3 irrigation regimes compared to I1 (Table 5). The effects of zeolite and biofertilizers were not statistically significant on free amino acid proline content; however, the highest proline content was obtained in plots without applying zeolite and also in combined application of nitroxin and phosphate barvar-2 (Table 4). Although, Table 8 presents the significant differences in triple interaction effects on proline content obtained by applying combined fertilizers without zeolite under stress caused by 90% depletion of ASW.
Soluble sugars
According to Table 3, the interaction of Z × B and I × Z × B on the content of soluble sugars was statically insignificant. Mean comparison of main effects of zeolite application and different biofertilization showed that the highest total soluble sugars were observed in control plants untreated with zeolite and in combined treatment of nitroxin × phosphate barvar-2, respectively (Table 4). In addition, in 2015, the highest value of total soluble sugars was observed under 60% depletion of ASW (Table 5). According to Table 6, soluble sugars increased with increasing severity water deficit stress, and zeolite application recorded minimum soluble sugar content compared to the respective control. The highest (2.678 mg g− 1 fw− 1) soluble sugar accumulation was observed in combined treatment of biofertilizers and 90% depletion of ASW without zeolite application (Table 8).
Flower yield
The flower yield was decreased by 10.26 and 47.4% with increasing drought intensity from 60 to 90% depletion of ASW compared to the first level of water availability (I1), respectively (Table 4). Mean comparison of zeolite treatment showed that the highest yield of flower belonged to the zeolite application.
The treatment combinations of nitroxin and phosphate barvar-2 resulted in the highest flower yield in both years, 2014 and 2015 by 12.9 and 13.2% compared to the non-fertilized control, respectively (Table 5). However, analysis of interaction effects showed that flower yield increased in all levels of soil moisture with zeolite application compared to the respective control (Table 6). In addition, combination of nitroxin and phosphate barvar-2 produced the highest yield of flower in all levels of water deficit over the respective control (Table 6). Also, combined application of biofertilizers (nitroxin and phosphate barvar-2) along with zeolite caused the maximum flower yield (by 16.2%) compared to non-fertilized control without zeolite (Table 7). In the case of three interactions, the maximum amount of flower yield (4.369 kg ha− 1) was achieved by application of zeolite and a combination of nitroxin and phosphate barvar-2 in 30% depletion of ASW (Table 8).
Essential oil content
As Table 3 shows, essential oil percentage/content was significantly (p < 0.01) impacted by refrence treatments in both years. The moderate (I2) and severe (I3) water-deficit stress led to increase in essential oil content by 23.4 and 26% compared to mild water stress (I1), respectively; however, there was no significant difference between I2 and I3 treatments (Table 4).
Application of zeolite did not positively affect the essential oil content of dragonhead, as the highest essential oil content was obtained without zeolite application (Table 4). Although ANOVA identified significant difference among biofertilizer treatments on essential oil content (Table 3), there were no significant differences among means and they were placed in a similar statistical group (Table 4). Nevertheless, the maximum percentage of essential oil was achieved from nitroxin × phosphate barvar-2 treatment. Mean comparison of interaction effects indicated that the highest essential oil percentage (0.245%) was observed by 90% depletion of ASW without zeolite and combined application of nitroxin and phosphate barvar-2 (Table 8).
Essential oil yield
ANOVA results for essential oil yield response to drought stress, zeolite and biofertilizers in dragonhead plants are given in Table 3. As shown, the oil yield also affected the biofertilizer application (Table 3). Depletion of 60% soil moisture increased essential oil yield by 16.9 and 43.7% compared to plots subjected to 30 and 90% depletion of ASW, respectively (Table 4). Similar to other measured parameters, a combination of nitroxin and phosphate barvar-2 caused the highest yield of essential oil; however, no significant effect was observed among biofertilizer treatments (Table 4). According to Tables 5, 6, 7 and 8, the maximum essential oil yield in association with two-way and/or three-way interactions was obtained in plants exposed to zeolite and 60% depletion of ASW, and those treated with nitroxin × phosphate barvar-2 and 60% depletion of ASW.
Irrigation water use efficiency for flower yield (IWUEFY) and essential oil (IWUEEOY)
Over both years, water-deficit stress decreased IWUEFY with increasing stress severity. The lowest and the highest values for IWUEFY were observed in 2014, when plots were irrigated at 90 and 30% depletion of ASW, respectively (Table 5). The treatment combinations of nitroxin × phosphate barvar-2 with zeolite under 30% depletion of ASW (mild water stress) led to increase in IWUEFY by 31.1% compared with the control (Table 8). In general, treatment with biofertilizers had a greater effect (13.8%) in increasing IWUEEOY than application of zeolite (4.3%) in both years (Table 4).
Mean IWUEEOY was increased by 22.2% with increase in water-deficit stress from 30 to 60% depletion of ASW, and then dropped down (up to 46%) with an increase in severity of stress from 60 to 90% depletion of ASW in 2014, and the same change trend was found for IWUEEOY under the employed irrigation regimes in 2015 (Table 5).
The two-way interactions between water-deficit stress and zeolite (Table 6), and between water-deficit stress and biofertilization (Table 6) as well as between zeolite and biofertilozer application (Table 7) showed that IWUEEOY increased through application of zeloite or biofertilizers in all irrigation treatments compared to the respective control, while the minimum value of IWUEEOY in each level of water-deficit stress was observed without application of bacterial biofertilizers and zeloite (Table 6). According to Table 8, second-order interaction (i.e. three-way interaction) among treatment groups showed that the highest (3.117 ml m− 3) IWUEEOY was observed in interaction among nitroxin × phosphate barvar-2 and zeolite treatments with moderate water stress, while the lowest (1.264 ml m− 3) IWUEEOY was produced without zeolite and fertilizer application in plots exposed to severe water stress conditions (Table 8).
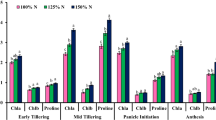
Figure 2 shows the relative comparison of the effects of reference treatments, zeolite and biofertilizer application under deficit irrigation, on some morpho-physiological characteristics of dragonhead plants. The maximum essential oil yield and IWUEEOY were observed in the plants inoculated with biofertilizer consortium (combined inoculation with nitroxin and phosphate barvar-2) under 60 depletion of ASW compared to the control (without zeolite and biofertilizers, and irrigation after 30% depletion of ASW).
The relative comparison of the effects of zeolite [0 (− Zeolite) and 8 t ha− 1 (+ Zeolite)] and biofertilizers [nitroxine, phosphate barvar-2, and their mixture (nitroxine × phosphate barvar-2)] under different irrigation regimes (I1, I2 and I3 refer to 30, 60 and 90% depletion of available soil water, respectively) on some morpho-physiological characteristics of Dracocephalum moldavica L. (the values were averaged of 2 years). RWC relative water content, T Chl total chlorophyll, FY flower yield, EOY essential oil yield, IWUESY irrigation water use efficiency of flower yield
Correlation coefficients between measured traits
There was a statistically significant positive correlation between leaf relative water content and flower yield (r0.01 = 0.78) and essential oil yield (r0.05 = 0.61) under experimental treatments (Table 9). Moreover, the flower yield and the irrigation water use efficiency of flower yield were negatively correlated with the proline content (r0.05 = 0.54 and r0.05 = 0.69, respectively). A positive and highly significant correlation (r0.01 = 0.85 and 0.98) was obtained between essential oil content and essential oil yield and IWUEEOY in dragonhead plants under he employed treatments, respectively (Table 9).
Discussion
Generally, most of the examined traits were higher in 2014 than in 2015, which can be ascribed, to some extent, to the differences between weather in terms of temperature, precipitation, relative humidity and evapotranspiration. According to previous reports (Mokhtassi-Bidgoli et al. 2013; Baghbani-Arani et al. 2017), higher agronomic performance, physiological functions and crop yield were obtained from higher precipitation and lower temperature during the critical growth stage.
It appears from our results that RWC, chlorophyll (Chl a, Chl b and total Chl) contents, flower yield and IWUEFY decreased with increase in water stress intensity. Some of the basic/and or adaptive mechanisms in terrestrial plants to combat osmotic stress effects are known to be related to the stimulation of physiological responses such as changes in water and osmolyte status, soluble sugars, proline, photosynthetic pigments and secondary metabolite levels in cell and subcellular structures.
Leaf water content is positively associated with the tolerance of plants to stressors such as water deficit stress. The prolonged exposure to water-deficit stress reduces leaf water potential, which adversely affects plant growth processes such as leaf elongation, stem extension and root proliferation (due to reduction in the rate of expansion and cell division), limits photosynthesis (due to decreases in stomatal conductance), hampers plant water relations, and decreases water use efficiency and yield (Farooq et al. 2009; Askari and Ehsanzadeh 2015). In the present study, lower RWC in control plants and higher RWC in those treated with zeolite and biofertilizers were associated with lower and higher chlorophyll contents and flower yield, respectively.
However, application of zeolite duo to a particular structure plays a crucial and efficient role in modifying both the physico-chemical and biological features of the soil and can reduce leaching of nitrogen and increase the value of water availability and maintain soil fertility in the root zone through improvement of cation exchange capacity (Ippolito et al. 2011). In the current study, the maximum value of the examined physiological and phytochemical characteristics were obtained by application of 8 t ha− 1 zeolite under 30% depletion of ASW (e.g. RWC, Chl a and b, and total chlorophyll values), under 60% depletion of ASW (e.g. essential oil content and yield) and under 90% depletion of ASW (e.g. proline and soluble sugars contents). Zeolites with three-dimensional, microporous and framework structures containing silicon, aluminium, oxygen, etc. absorb high humidity that could be efficient in reducing water stress effects on plants. Our results of interaction effects of zeolite × water-deficit stress were similar to the previous findings reported by Goksey et al. (2004), Mohammad et al. (2005), and Afsharmanesh (2009).
Chlorophyll content is an important physiological indicator that is strongly related to the photosynthetic efficiency and plant productivity (Askari and Ehsanzadeh 2015). The degree of drought-induced reduction in chlorophyll content is assumed to depend on the severity/intensity and duration of the water shortage stress. In the current work, Chl a and b, and total Chl of dragonhead were diminished when plots were depleted by 60–90% ASW compared to 30%. The decrease in chlorophyll content upon exposure to drought stress may be attributed to extra generation of free radicals (ROS), alterations in the ratio of lipid to protein of light-harvesting complexes, enhanced expression of chlorophyllase activity and chlorophyll degradation, as well as inhibition of photosynthetic pigments biosynthesis (Haider et al. 2017; Meher et al. 2018).
According to the results, in contrast to decreases in RWC, proline and soluble sugar contents of the leaves were improved in dragonhead plants when grown under deficit water stress. A high correlation between RWC and proline and soluble sugars has been previously identified in other species such as Glycine max and Aeluropus lagopoides (Porcel and Ruiz-Lozano 2004; Mohsenzadeh et al. 2006). Organic solutes and their alterations in plant organs may be of potential application in elucidating plants productivity under different environmental perturbations. Osmotic adjustment induced by different types of compatible solutes and amino acids such as proline helps plant to maintain turgor and water potential, therefore, considered as a vital adaptive and protective approach by which plants combat adverse environmental conditions (Schutz and Fangmeier 2001). In addition, results showed that plots under 90% depletion of ASW without zeolite application imposed severe stress in dragonhead; nevertheless, enhancing soluble sugars and proline contents through osmotic adjustment process make vital contribution to maintain physiological functions of the plants for avoiding of water-deficit stress effects. It is noteworthy that the significant and positive correlation between RWC and proline is indicative of osmotic adjustment status in the water-stressed dragonhead; however, in our study a higher proportional increase in proline content is found under severe water-deficit stress compared to other conditions. Plants have improved a series of mechanisms for adaptation and survival under severe environmental perturbations. The capability of a plant to tolerate water-deficit stress is affected by several metabolic networks that intensify continuous water absorption, support cell organelles and structures, and regulate the ion homeostasis in cells (Mohammadi et al. 2018). The most common biochemical pathways are those that cause the biosynthesis of specific proteins and accumulation of cellular osmoprotectants or low molecular weight compounds (e.g. proline and SS), and activation of antioxidant defense enzymes, which subsequently maintain ion and water flux across the membrane and support highly efficient ROS-detoxifying machinery under various environmental stresses (Gholami Zali and Ehsanzadeh 2018). The same authors reported that drought stress causes significant changes in specific activities of antioxidant enzymes such catalase (CAT) in the absence and presence of proline. Interestingly, there was significant negative correlation between proline and malondialdehyde (MDA) and H2O2 contents in Thymus vulgaris plants under drought stress conditions; however, in well-watered treatments, positive and significant correlation was obtained between proline and H2O2 and MDA contents (Mohammadi et al. 2018).
Biosynthesis of essential oils and or other bioactive compounds in medicinal plants is influenced by several factors such as genetics, physiological variations, edaphic and climatic conditions, geographic situations, environmental biotic and abiotic stresses and agronomical practices (Ramakrishna and Ravishankar 2011; Keshavarz Afshar et al. 2014). Also, the highest value of essential oil percentage (or content) was in the severe water-deficit stress conditions together with either zeolite and or biofertilizers (Nitroxin and phosphate barvar-2). These results are in accordance with Munne-Bosch and Alegre (2000); and Dadrasan et al. (2015), where they observed that the percentage of essential oil in Melissa officinalis L. and Rosmarinus officinalis L. (Lamiaceae), and in Trigonella foenum-graecum L. (Fabaceae), was increased under mild drought stress. In this study, the yield of essential oil was decreased with increase in water stress levels from 60% (I2) to 90% (I3) depletion of ASW, but the essential oil content was increased with increase in drought intensity from I1 to I2 (60% depletion of ASW).
It is remarkable that the essential oil content would not be constantly enhanced with raising stress intensity, because plants utilize more photosynthetic carbon assimilates to produce osmotically active substances including sugar compounds and proline, providing the vital situations for survival in such natural environments they are dealing with. These metabolites are expensive for plants and, therefore, plants pay back these expenses through diminishing growth, development and reproduction (Munns 1993). It has been acknowledged that the yield of essential oil was decreased under drought, but the percentage of oil was improved following stress treatment (Farahani et al. 2009). However, Sing Sangwan et al. (1994) showed that the production of essential oil was unchanged or enhanced under limited water conditions. Here, the maximum essential oil percentage value was gained at 90% depletion of ASW and the highest shoot top flower yield was produced at 30% depletion of ASW, but the highest oil yield oil was observed at 60% depletion of ASW; it appears that the stress of water deficit enhances the percentage of essential oil in medicinal plants, because under stress conditions, more secondary active compounds are synthesized and these substances can prevent the oxidation of other chemicals of the cells (Selmar et al. 2017). However, essential oil yield reduced under severe water deficit stress, due to the fact that interaction between the essential oil content and vegetative yield are two prominent subjects in calculation of oil yield. Therefore, watering at 60% depletion of ASW is suitable for creating a good trade-off between the percentage of essential oil and aerial flower yield, leading to the maximum yield for essential oil.
The dual effects of water limitation on physiological and biochemical processes, yield attributes and essential oil production were previously reported in diverse species, for example in dragonhead (Rahbarian et al. 2010, 2014; Gholizadeh et al. 2010a, b), Balm (Abbaszadeh et al. 2008), Mint (Misra and Srivastava 2000), Basil (Hassani et al. 2004), Feverfew (Saharkhiz et al. 2007), and Peppermint (Alkire and Simon 1993).
According to Dadrasan et al. (2015) moderate and severe water deficit induction causes decrease (40 and 65%) in forage yield in fenugreek (Trigonella foenum-graecum L.), respectively. During water scarcity, plants tend to diminish water deprivation from leaves by closing the stomatal pores, which subsequently restricts the availability of carbon dioxide for photosynthetic process and biomass accumulation (Sun et al. 2013). Also, the mass overflow of nutrients occurs to the belowground part of plant and, therefore, acquisition of nutrient becomes restricted by severe stress which further reduces mass production (Diaz-Lopez et al. 2012). In the present work, nitroxin and phosphate barvar-2 can provide suitable nutritional conditions for dragonhead plants. Also, nitroxin and phosphate barvar-2 accompanied by zeolite increased the plant morpho-physiological traits, especially essential oil content and yield. Rahbarian et al. (2014) reported that the maximum RWC and oil yield in dragonhead were obtained under mild water-deficit stress via application of 30 t manure/ha. The minimum values of these traits were obtained under severe stress with manure application. Sharma et al. (2003) reported that combination of nitroxin and phosphate barvar-2 increased chlorophyll content and consequently photosynthesis potential and finally caused an increase in dragonhead yield. Mahfouz and Sharaf-Eldin (2007) pointed out that applying biological fertilizers such as Azotobacter, Azospirillum and Bacillus increased essential oil content in fennel plants. Reduction in RWC caused an increase in essential oil content. Similar findings related to increasing essential oil percentage under water stress were reported by Ranjbar et al. (2004) and Rahbarian et al. (2014). Furthermore, regarding significant interaction between biofertilizers and zeolite, it can be suggested that zeolite amendment with soil has a vital function in mitigating adverse impacts of deficit water stress and reducing soil fertilizer/nutrient leaching (Ippolito et al. 2011).
The IWUEFY was reduced with raising water stress intensity, but IWUEEOY was improved at mild deficit water stress compared to the other stress levels. On the hand, more favorable water availability conditions (30 and 60% depletion of ASW) were caused the maximum water use efficiency for IWUEFY and IWUEEOY, respectively. Our results are in agreement with the previous reports (Lima et al. 2013; Silva et al. 2010). According to Lima et al. (2013), castor (Ricinus communis L.) produced low yield and low water use efficiency in dryer years, showing that under low soil water availability the WUE of plants was very low. Furthermore, Silva et al. (2010), reported that the maximum WUE value for production of specific gel in Aloe vera (Aloe barbadensis Miller) was obtained in plants subjected to intermediate irrigation regime relative to extreme treatments. In contrary to the latter reports, however, Hazrati et al. (2017) demonstrated that the WUE of Aloe vera improved with water limitations. It may be due to the fact that A. vera L. plants (with CAM metabolism) are able to keep their stomata open under water scarcity conditions and continue carbon assimilation (Cousins and Witkowski 2012). The ability of plants to spend water effectively for the accumulation of dry weight extremely differs among different species. In our study, a reduction in WUE for flower yield shows that there was no desirable practical use of photo-assimilates by dragonhead reproductive parts under the prolonged water shortage stress conditions. Here, reduction in shoot top flower yield by water-deficit stress is a significant reason of reduced irrigation WUE of flower yield. In addition, deficiency of water during the reproductive growth stage affects early and late flower yield and dry matter.
The results of interaction means of different irrigation regimes and biofertilizers containing nitrogen stabilizing (nitroxin) and phosphat solubilizing (barvar-2) bacteria indicated that the maximum value of the examined traits recorded by combined application of nitroxin and phosphate barvar-2 under low (e.g. RWC, Chl a and b, and total Chl contents and flower yield) and medium (e.g. percentage and yield of essential oil) water-deficit stress conditions in both years. In the present study, the applied biofertilizers such as nitroxin (comprising Azetobacter sp., Azospirillum sp., and bacteria of Pseudomonas genus with phosphate-solubilizing ability) and phosphate barvar 2 (containing Pantoea agglomerans and Pseudomonas putida) significantly affected physiological functions and IWUE of flower and essential oil yield in dragonhead, which are in some parts comparable to those of Gharib et al. (2008), who obtained that inoculation of Majorana (Majorana hortensis) plants with biofertilizers significantly impacted the growth parameters as well as essential oil production.
Overall, the superiority of biofertilizers consortium inoculation over control or individual inoculation can be related to the positive and cumulative synergistic impacts of inoculation. These beneficial microorganisms enhance host plant growth and development via different direct and/or indirect mechanisms including bioavailability of P and N for plant absorption, production of antibiotics, secretion of vitamins, Fe sequestration by siderophores and chelates, synthesis of the enzymes and growth stimulators (auxins, cytokinins, and gibberellins), fixing atmospheric nitrogen and reduction in ethylene (an stress-accumulated hormone in plants) levels inside plant cells using ACC deaminase (Glick 1995; Ghorbanpour et al. 2015).
Moreover, plant growth-promoting rhizobacteria (PGPRs) have been successfully applied as biotic elicitors for enhancement of secondary metabolite contents in aromatic medicinal herbs (Ghorbanpour et al. 2013). Due to the significant increasing costs of water consumption and disposal, enhanced water treatment technologies/strategies make economic and social sense. In the present study, regarding significant interaction between biofertilizers and zeolite, the treatment combinations of zeolite and nitroxin × phosphate barvar-2 could improve physiological functions and essential oil yield of dragonhead plants following irrigation after 60% depletion of ASW (the best treatment). Therefore, there is no doubt there are financial benefits of getting high essential oil yield with lower water consumption. The high amount of the essential oil yield following employed treatments, particularly following biofertlizers application and upon combination of zelolite and biofertlizers, were due to enhanced dry matter and flower yield and enhanced essential oil content. These findings are in agreement with Tahami et al. (2011), who reported that the essential oil of Ocimum basilicum L. plants increased following inoculation with biofertilizers under an organic farming system. Similar results were reported by Jahan and Jahan (2010) on German chamomile exposed to organic fertilizers.
Conclusions
The results exhibit that the employed biofertilizers [nitroxin (containing free-living nitrogen fixing bacteria including Azetobacter sp., Azospirillum sp., and phosphate-solubilizing bacteria from Pseudomonas genus) and phosphate barvar-2 containing Pantoea agglomerans (p5) and Pseudomonas putida (p13)] significantly affect quantitative and qualitative production of D. moldavica L. under different watering conditions. Application of nitroxin × phosphate barvar-2 with zeolite under 30% depletion of ASW (mild water deficit stress) caused maximum percentage of RWC, total chlorophyll content, shoot top flower yield and IWUEFY. However, the highest essential oil percentage, proline and soluble sugars contents were observed when the available soil water depletion was reached at 90% (severe water deficit stress) without zeolite and combined application of nitroxin and phosphate barvar-2.
Author contribution statement
Designed and performed the experiment: KKA. Wrote the manuscript: KKA and Mh. Revised and approved the final manuscript: MH.
References
Abbaszadeh B, Ashourabadi ES, Lebaschi MH, Kandy MNH, Moghadami F (2008) The effect of drought stress on proline contents, soluble sugars, chlorophyll and relative water contents of balm (Melissa officinalis L.). Iran J Med Aromat Plants 23(4):504–512
Afsharmanesh G (2009) Study of some morphological traits and selection of drought resistance alfalfa cultivars (Medicago sative L.) in Jiroft, Iran. Plant Ecophysiol 1(3):109–118
Alkire BH, Simon JE (1993) Water management for Midwestern peppermint (Mentha Piperita L.) growing in highly organic soil, Indiana, USA. Acta Hortic 344:544–556
Allen RG, Pereira LS, Raes D, Smith M (1998) Crop evapotranspiration–guidelines for computing crop water requirements. FAO Irrigation and Drainage Paper, No. 56. FAO, Rome
Askari E, Ehsanzadeh P (2015) Osmoregulation-mediated differential responses of field-grownfennel genotypes to drought. Ind Crops Prod 76:494–508. https://doi.org/10.1016/j.indcrop.2015.07.010
Baghbani-Arani A, Modarres-Sanavy SAM, Mashhadi-Akbar-Boojar M, Mokhtassi-BidgoliA (2017) Towards improving the agronomic performance, chlorophyll fluorescence parameters and pigments in fenugreek using zeolite and vermicompost under deficit water stress. Ind Crops Prod 109:346–357. https://doi.org/10.1016/j.indcrop.2017.08.049
Baiazidi-Aghdam MT, Mohammadi H, Ghorbanpour M (2016) Effects of Nanoparticulate anatase titanium dioxide on physiological and biochemical performance of Linum usitatissimum (Linaceae) under well watered and drought stress conditions. Braz J Bot 39:139–146. https://doi.org/10.1007/s40415-015-0227-x
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
British Pharmacopoeia (1993) HMSO Publication, International edition, London
Clark RJ, Menary RC (1980) Environmental effect on peppermint (Mentha piperita L). II. Effect of temperature on photosynthesis, photorespiration and dark respiration in peppermint with reference to oil composition. Aust J Plant Physiol 7(6):693–697. https://doi.org/10.1071/PP9800693
Cousins SR, Witkowski ETF (2012) African aloe ecology: a review. J Arid Environ 85:1–17. https://doi.org/10.1016/j.jaridenv.2012.03.022
Dadrasan M, Chaichi MR, Pourbabaee AN (2015) Effects of temperature on photosynthesis, photorespiration and dark respiration in peppermint to oil composition. Aust J Plant Physiol 7(6):693–697. https://doi.org/10.1071/PP9800693
Díaz-López L, Gimeno V, Simón I, Martínez V, Rodríguez-Ortega WM, García-Sánchez F (2012) Jatropha curcas seedlings show a water conservation strategy under drought conditions based on decreasing leaf growth and stomatal conductance. Agric Water Manag 105:48–56. https://doi.org/10.1016/j.agwat.2012.01.001
Farahani HA, Valadabadi SA, Daneshian J, Shiranirad AH, Khalvati MA (2009) Medicinal and aromatic plants farming under drought conditions. J Hortic For 1(6):86–92
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–212. https://doi.org/10.1051/agro:2008021
Geerts S, Raes D (2009) Deficit irrigation as an on-farm strategy to maximize crop water productivity in dry areas. Agric Water Manag 96:1275–1284. https://doi.org/10.1016/j.agwat.2009.04.009
Gharib FA, Moussa LA, Massoud ON (2008) The effects of compost and biofertilizers ongrowth, yield and essential oil of sweet marjoram (Majorana hortensis) plant. Int J Agric Biol 10(4):381–387
Gholami Zali A, Ehsanzadeh P (2018) Exogenously applied proline as a tool to enhance water use efficiency: case of fennel. Agric Water Manag 197:138–146. https://doi.org/10.1016/j.agwat.2017.11.023
Gholizade A, Amin MS, Anuar AR, Esfahani M, Saberioon MM (2010a) The study on the effect of different levels of zeolite and water stress on growth, development and essential oil content of Moldavian Balm (Dracocephalum moldavica L.). Am J Appl Sci 7(1):33–37
Gholizade A, Amin MSM, Anuar AR, Saberioon MM (2010b) In Balm (Dracocephalum moldavica L.). Aust J Basic Appl Sci 4(10):5184–5190
Ghorbanpour M, Hatami M, Khavazi K (2013) Role of plant growth promoting rhizobacteria on antioxidant enzyme activities and tropane alkaloids production of Hyoscyamus niger under water deficit stress. Turk J Biol 37:350–360. https://doi.org/10.3906/biy-1209-12
Ghorbanpour M, Hatami M, Kariman K, Kazem Khavazi K (2015) Enhanced efficiency of medicinal and aromatic plants by PGPRs. In: Egamberdieva D, Shrivastava S, Varma A (eds) Plant-growth-promoting rhizobacteria (PGPR) and medicinal plants, soil biology 42. Springer International Publishing, Basel, pp 43–70. https://doi.org/10.1007/978-3-319-13401-7_3
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41:109–117. https://doi.org/10.1139/m95-015
Goksoy AT, Demir AO, Turan ZM, Dagustu N (2004) Responses of sunflower (Helianthus annuus L.) to full and limited irrigation at different growth stages. Field Crops Res 87:167–178. https://doi.org/10.1016/j.fcr.2003.11.004
Haider MS, Zhang C, Kurjogi MM, Pervaiz T, Zheng T, Zhang C, Lide C, Shangguan L, Fang J (2017) Insights into grapevine defense response against drought as revealed by biochemical, physiological and RNA-Seq analysis. Sci Rep. https://doi.org/10.1038/s41598-017-13464-3 (Article number: 13134)
Hassani A, Omidbaigi R, Heidari Sharif Abad H (2004) Effect of different soil moisture levels on growth, yield and accumulation of compatible solutes in Basil (Ocimum basilicum). Iran J Soil Water Sci 17(2):211–219
Hatami M, Ghorbanpour M (2014) Defense enzymes activity and biochemical variations of Pelargonium zonalein response to nanosilver particles and dark storage. Turk J Biol 38:130–139
Hazrati S, Tahmasebi-Sarvestani Z, Mokhtassi-Bidgoli A, Modarres-Sanavy SAM, Mohammadi H, Nicola S (2017) Effects of zeolite and water stress on growth, yield and chemical compositions of Aloe vera L. Agric Water Manag 181:66–72. https://doi.org/10.1016/j.agwat.2016.11.026
Howell T (1994) Irrigation engineering, evapotranspiration. In: Arntzem CJ, Ritter EM (eds) Encyclopedia of agricultural science. Academic Press, New York, pp 591–600
Ibanez H, Ballester A, Munoz R, Quiles MJ (2010) Chlororespiration and tolerance to drought, heat and high illumination. J Plant Physiol 167:732–738
Ippolito AJ, Tarkalson DD, Lehrsch GA (2011) Zeolite soil application method affects inorganic nitrogen, moisture, and corn growth. Soil Sci 176(3):136–142
Irigoyen JJ, Einerich DW, Sanchez-Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in modulated alfalfa (Medicago sativa) plants. Physiol Plant 84(1):55–60
Jahan M, Jahan A (2010) Organic production of German chamomile (Matricaria recutita L.) intercropped with pot marigold (Calendula officinalis L.). Planta Med 76:P122. https://doi.org/10.1055/s-0030-1264420
Keshavarz Afshar R, Chaichi MR, Assareh MH, Hashemi M. Liaghat A (2014) Interactive effect of deficit irrigation and soil organic amendments onseed yield and flavonolignan production of milk thistle (Silybum marianum L. Gaertn.). Ind Crops Prod 58:166–172
Kramer PJ, Boyer JS (1995) Water relation of plants and soils. Academic Press, New York
Levitt J (1980) Response of plants to environmental stresses: water, radiation, salt and other stresses. Academic Press, New York
Lichtenthaler HK (1987) Chlorophylls and carotenoids: the pigments of photosynthetic biomembranes. Method Enzymol 148:350–382
Lima JRS, Antonino ACD, Souza ES, Lira CABO, Silva IF (2013) Seasonal and interannual variations of evapotranspiration, energy exchange, yield and water use efficiency of castor grown under rainfed conditions in northeastern Brazil. Ind Crops Prod 50:203–211
Mahfouz SA, Sharaf-EldinMA (2007) Effect of mineral vs. biofertilizer on growth, yield and essential oil content of fennel (Foeniculum vulgare Mill.). Int Agrophys 21(4):361–366
Manivannan P, Jaleel CA, Kishorekumar A, Sankar B, Somasundaram R (2007) Changes in antioxidant metabolism of Vigna unguiculata (L.) Walp. by propiconazole underwater deficit stress. Colloids Surf B Biointerfaces 57(1):69–74
Meher, Shivakrishna P, Reddy AK, Rao DM (2018) Original article Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi J Biol Sci 25(2):285–289. https://doi.org/10.1016/j.sjbs.2017.04.008
Misra A, Srivastava NK (2000) Influence of water stress on Japanese mint. J Herbs Spices Med Plants 7:51–58
Mohammad MJ, Karam NS, AL-Lataifeh NK (2005) Response of croton grown in a zeolite-containing substrate to different concentration of fertilizer salutation. Commun Soil Sci Plant Anal 35:2283–2297. https://doi.org/10.1081/LCSS-200030637
Mohammadi H, Ghorbanpour M, Brestic M (2018) Exogenous putrescine changes redox regulations and essential oil constituents infield-grown Thymus vulgaris L. under well-watered and drought stress conditions. Ind Crops Prod 122:119–132. https://doi.org/10.1016/j.indcrop.2018.05.064
Mohsenzadeh S, Malboobi MA, Razavi K, Farrahi-Aschtiani S (2006) Physiological and molecularresponses of Aeluropus lagopoides (Poaceae) to water deficit. Environ Exp Bot 56:314–322. https://doi.org/10.1016/j.envexpbot.2005.03.008
Mokhtassi-Bidgoli A, AghaAlikhani M, Nassiri-Mahallati M, Zand E, Gonzalez-Andujar JL, Azari A (2013) Agronomic performance, seed quality and nitrogenuptake of Descurainia sophia in response to different nitrogen rates and water regimes. Ind Crops Prod 44:583–592
Munne-Bosch S, Algere L (2000) The significance of beta carotene, alpha, tocopherol and the xanthophyll cycle in droughted Melissa officinalis plant. Aust J Plant Physiol 27(2):139–146
Munns R (1993) Physiological processes limiting plant growth in saline soil: some dogmas and hypotheses. Plant Cell Environ 16:15–24. https://doi.org/10.1111/j.1365-3040.1993.tb00840.x
Omidbaigi R (2005) Production and processing of medicinal plants, vol 2. Astan Quds Razavi Publications, Behnashr, Mashhad, p 438
Omidbeigi R (1997) vol 2. Tarahane Nashr Publication, Tehran, p 424
Porcel R, Ruiz-Lozano JM (2004) Arbuscular micorrizal influence on leaf waterpotential, solute accumulation and oxidative stress in soybean plantssubjected to drought stress. J Exp Bot 55:1743–1750. https://doi.org/10.1093/jxb/erh188
Rahbarian P, Afsharmanesh G, Shirzadi MH (2010) Effects of drought stress and manure on relative water content and cell membrane stability in dragonhead (Dracocephalum moldavica). Plant Ecophysiol 2:13–19
Rahbarian P, Sardoei AS. Gholamshahi S, Jandi GKJ (2014) Relative water content, cell membrane stability, essential oil and morphology of Dracocphalum moldavica L. are influenced by drought stress and manure. Int J Biosci 5(1):421–428
Ramakrishna A, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6(11):1720–1731. https://doi.org/10.4161/psb.6.11.17613
Ranjbar M, Esfahani M, Kavousi M, Yazdani MR (2004) Effects of irrigation and natural zeolite application on yield and quality of tobacco (Nicotiana tabaccum var. Coker 347). J Agric Sci 1(2):71–84
Saharkhiz MJ, Omidbaigi R, Sefidkon F (2007) The effect of phosphorus and irrigation treatments on the essential oil content and composition of Feverfew (Tanacetum parthenium) cv. Zardband). J Essent Oil Bear Plants 10(5):391–398. https://doi.org/10.1080/0972060X.2007.10643572
Schutz M, Fangmeier A (2001) Growth and yield responses of spring wheat (Triticum aestivum L. cv. Minaret) to elevated CO2 and water limitation. Environ Pollut 114(2):187–194. https://doi.org/10.1016/S0269-7491(00)00215-3
Selmar D, Kleinwächter M, Abouzeid S, Yahyazadeh M, Nowak M (2017) The impact of drought stress on the quality of spice and medicinal plants. In: Ghorbanpour M, Varma A (eds) Medicinal plants and environmental challenges. Springer, Cham. https://doi.org/10.1007/978-3-319-68717-9
Sharma A, Johri BN, Sharma AK, Glick BR (2003) Plant growth promoting bacterium Pseudomonos sp. Strain GRP3 influences iron acquisition in mung bean (Vigna radiate L. Wilczek). Soil Biol Biochem 35:887–894. https://doi.org/10.1016/S0038-0717(03)00119-6
Silva H, Sagardia S, Seguel O, Torres C, Tapia C, Franck N, Cardemil L (2010) Effect of water availability on growth and water use efficiency for biomass and gel production in Aloe Vera (Aloe barbadensis M.). Ind Crops Prod 31:20–27. https://doi.org/10.1016/j.indcrop.2009.08.001
Sing Sangwan N, Farooqi AHA, Sing Sangwan R (1994) Effect of drought stress on growth and essential oil metabolism in lemongrasses. New Phytol 128(1):173–179. https://doi.org/10.1111/j.11469-8137.1994.tb04000.x
Sun XP, Yan HL, Kang XY, Ma FW (2013) Growth, gas exchange, and water-use efficiency response of two young apple cultivars to drought stress in two scion-one rootstock grafting system. Photosynthetica 51(3):404–410. https://doi.org/10.1007/s11099-013-0040-3
Tafteh A, Sepaskhah AR (2012) Yield and nitrogen leaching in maize field under different nitrogen rates and partial root drying irrigation. Int J Plant Prod 6:93–114. https://doi.org/10.22069/ijpp.2012.672
Tahami SMK, Jahan M, Rezvani Moghaddam P (2011) Effects of organic and biological fertilizers on yield and essential oil of basil (Ocimum basilicum L.) under organic production system. In: The 17th IFOAM OWC. Namyangju, Korea
Tahami MK, Jahan M, Khalilzadeh H, Mehdizadeh M (2017) Plant growth promoting rhizobacteria in an ecological cropping system: a study on basil (Ocimum basilicum L.) essential oil production. Ind Crops Prod 107:97–104. https://doi.org/10.1016/j.indcrop.2017.05.020
Zhang P, Avudzega DM, Bowman RS (2007) Removal of perchlorate from contaminated waters using surfactant-modified zeolite. J Environ Qual 36(4):1069–1075. https://doi.org/10.2134/jeq2006.0432
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by P. Wojtaszek.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karimzadeh Asl, K., Hatami, M. Application of zeolite and bacterial fertilizers modulates physiological performance and essential oil production in dragonhead under different irrigation regimes. Acta Physiol Plant 41, 17 (2019). https://doi.org/10.1007/s11738-018-2801-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2801-x