Abstract
Taking functional scientific devices and metal-based nanoparticles into account, the present research was carried out to evaluate the plant (Capsicum annuum) responses to cold plasma and zinc oxide nanoparticle (nZnO) in in vitro and pot conditions. Seeds were exposed to plasma (0.84 W/cm2 surface power densities) with three exposure times (0, 60, and 120 s) and/or two concentrations of nZnO (0 and 100 mgl− 1). The treated seeds were cultured in hormone-free MS medium (MS) or supplemented with 2 mgl− 1 BA and 0.5 mgl− 1 IAA (MSH). The seed pre-treatment with plasma enhanced a germination process and plant early growth, in contrast with the nZnO treatment. The treatment of nZnO significantly decreased the total fresh mass and leaf area in the seedlings grown in both culture media, while its growth-delaying impact was mitigated by the plasma treatment. The chlorophyll a and carotenoid were increased to 39.35 and 32% for the plasma-treated seedlings, respectively, than the control. The plasma and/or nZnO treatments acted as effective elicitors to induce the peroxidase activities in both culture media. Similarly, the activities of phenylalanine ammonia-lyase and soluble phenols were found to be significantly higher in the plasma and/or nZnO groups in both roots and leaves. Interestingly, inhibiting effects of nZnO on xylem differentiation was alleviated by the plasma treatments. In the pot condition, soaking seeds before the plasma treatment was the most effective method to affect plant growth. This is a first report reflecting the potential benefits of the cold plasma treatment to improve plant growth and resistance to the nanoparticle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, there are many interests in applying diversities of the functional devices generating cold plasmas to facilitate treating various living organisms, thereby influencing cells, tissues, growth, differentiation, development, and metabolism (Iranbakhsh et al. 2018). There are various kinds of producing instruments designed for different biological and biomedical aims, like potential therapy in dentistry and oncology (Hoffmann et al. 2013), as well as food industry (Ulbin-Figlewicz et al. 2015). One of these vastly developing devices is called dielectric barrier discharge plasma (DBD). Microbial contamination is regarded as one of the most critical issues and a restricting factor in the food industry (Ulbin-Figlewicz et al. 2015) and in vitro culture. Considerable antimicrobial effects of plasma, mainly due to the produced free radicals, charged particles, excited compounds, ozone, and emitted UV, have been well documented. DBD leads to UV radiation and generations of different kinds of active materials, especially nitrogen and oxygen reactive species (Bußler et al. 2015) which had sufficiently long lifetimes to affect cells and organic compounds. Therefore, DBD may be introduced as an alternative possible instrument to treat seeds for different aims (Iranbakhsh et al. 2017, 2018; Safari et al. 2017), especially decontamination, germination, growth, and plant protection. There are some limited reports, focusing on reactions of different plants to cold plasma, like improvements in germination rates (Será et al. 2008; Chen et al. 2012), decreases in the microbial contamination of seed (Mitra et al. 2014), increases in plant growth (Mihai et al. 2014), changes in some physiological parameters (Wu et al. 2007; Ling et al. 2014; Stolárik et al. 2015; Iranbakhsh et al. 2017, 2018), modifications in differentiation process (Safari et al. 2017), and alterations in pattern of gene expression (Iranbakhsh et al. 2018). The plasma treatment may affect plant cell at molecular level and act as an effective elicitor to modifying plant physiology (Iranbakhsh et al. 2017, 2018). So, more exact research is required to elucidate the basic mechanisms implicated in plant–plasma interaction.
Nowadays, there is great interest in nanotechnology and novel utilizations of nanoparticles is rapidly developing in the various sciences and industries (Asgari-Targhi et al. 2018). The size and concentration of nanoparticle, the plant species (Yang et al. 2015; Sheteiwy et al. 2015), and a type of soil (Peralta-Videa et al. 2014) have been mentioned as three main factors that play critical roles in plant responses to nanoparticles. Considering the importance of metal oxide nano-particles, especially nZnO as the most extensively applied (Keller et al. 2013), more studies are required to characterize their exact influences on plant cell and physiology. It has been manifested that nZnO is cytotoxic, capable of provoking double-stranded DNA damage and affecting specific signaling pathways in the exposed mammalian cells (Ng 2011). Varieties of nanoparticles in the form of metal oxide have been utilized to improve plant growth and protection (Gogos et al. 2012). Furthermore, the beneficial effects of in vitro application of nZnO on the growth and production of steviol glycoside have been recorded in Stevia rebaudiana (Javed et al. 2016). However, the phytotoxicity of various kinds of metal oxide nanoparticles in different plant species has been recorded (Yang et al. 2015; Sheteiwy et al. 2015). The inhibiting impacts of nZnO on the root elongation have been reported in maize and rice (Yang et al. 2015). The application of nZnO had detrimental impacts on the growth of rice (Boonyanitipong et al. 2011). Phytoxicity of nZnO in Oryza sativa induced critical antioxidant enzymes, and up-regulated expression of the antioxidant-related genes which are partially mitigated by seed priming with polyethylene glycol (Sheteiwy et al. 2015). The toxic concentrations of nZnO in the exposed rice seedlings provoked oxidative stress, and led to inductions in activities of antioxidant enzymes, increases in abscisic acid (ABA) content, and decreases in gibberellic acid (GA) level (Sheteiwy et al. 2017). Also, the histochemical evidence confirmed the severe oxidative burst in roots of rice plants counteracted with nano-ZnO (Sheteiwy et al. 2017). Deposition of nano metal oxides, like nZnO on the cell surface and different cellular organelles, leads to oxidative stress and follow-up signaling (Buzea et al. 2007).
Capsicum annuum L. belongs to Solanaceae family and is of importance, especially because of Capsaicin, an active component of chili peppers, which has several valuable properties, including antioxidant, antibacterial and anticarcinogenic (Baytak and Aslanoglu 2017; Safari et al. 2017). Plant culture in in vitro condition is considered as a suitable method to elucidate various hypotheses, proposed in plant science. Seed-borne contamination is known as an important restricting factor in plant tissue culture. Despite good documentation of the antimicrobial effects of plasma, there are not many studies on the behavior of plasma-pretreated seedlings in in vitro condition. Various strategies have been employed to develop new alternative approaches to promote plant growth, physiology, and protection. There are limited studies on the potential benefits of seed priming with the plasma (especially in in vitro condition) and possible responses and the involved mechanisms are unknown. Considering the highlighted crucial importance of the plasma and nanosciences and technologies, this study was carried out to evaluate the possible effects of the cold plasma and/or nZnO on the various aspects of growth, anatomy, and physiology of Capsicum annuum Var. cayenne. The present research provides the valuable information about plant growth, behavior, morphogenesis, and differentiation, as well as modifications in physiological traits in pepper, triggered by the cold plasma and/or nZnO in in vitro and pot conditions, for the first time.
Materials and methods
Materials, experimental apparatus, treatments and culture conditions
Seeds of Capsicum annuum cayenne were purchased from a reliable industrial company (Nikandishan, Iran). In this research, nZnO of 10–30 nm was purchased from US research nanomaterials, Inc (3302 Twig Leaf Lane Houston, TX 77,084, USA).
The experimental apparatus was DBD. Plasma at atmospheric pressure is generated between two glass plates as dielectric barriers covering the two powered circular plate copper electrodes (radius = 5.5 cm). The gap between dielectrics is 3 mm. Argon was utilized as a functional gas between dielectrics. The dielectric acts as a stabilizing material when the potential across the gap reaches the breakdown voltage leading to the formation of a large number of micro-discharges. To generate DBD plasma, a modified AC high voltage power supply was used. Applied voltage was measured by a high voltage probe (Pintek HVP40) connected to an oscilloscope (Tektronix TDS1012B). During experiments throughout the plasma treatment, the frequency and the voltage were fixed at 23 kHz and 11 kV, respectively. The instrument power was 80 W, so for 94.98 cm2 plasma treatment areas, the surface power density was equal to 0.84 W/cm2. The plasma diagnostic data (Iranbakhsh et al. 2018) and schematic of the applied plasma system (Iranbakhsh et al. 2017) were presented in our previously published papers.
Seeds were surface sterilized with 3% sodium hypochlorite, containing two drops liquid detergent for 10 min. Then, seeds were thoroughly washed three times with sterile distilled water. Seeds were exposed to plasma with three different exposure times, including 0, 60 or 120 s, and/or two levels of nZnO (0 and 100 mgl− 1). Seed pretreatment with nZnO was done by their incubations in the nZnO solution of 100 mgl− 1 during 2.5 h.
To evaluate the possible effects of the different applied treatments of plasma and/or nZnO on the germination rates and plant early growth, plasma and/or nZnO-treated seeds were grown in a petri dish condition (three replications with five uniform intact seeds in each) and the results were recorded 7 days after treatments.
Murashige and Skoog (MS) medium (Murashige and Skoog 1962) was applied for in vitro experiments. For in vitro experiments, the sterilized treated seeds with plasma (0, 60, or 120 s) and/or nZnO (0 or 100 mgl− 1) were cultured in hormone-free MS medium (called as MS) or supplemented with hormones of 2 mgl− 1 BA and 0.5 mgl− 1 IAA (called as MSH). All cultures were incubated in a germinator at 25 °C under illumination of 28 µmol photon m− 2 s− 1 (16 h of light and 8 h of dark). Non-treated seeds grown in MS or MSH condition were used as control samples in each culture medium. Treatment groups were called as follows: C: control; nZnO: seeds treated with nZnO; P60: seeds treated with plasma of 60 s; P120: samples treated with plasma of 120 s; P60–nZnO: seeds treated with plasma of 60 s and nZnO; P120–nZnO: seeds treated with plasma of 120 s and nZnO.
In complementary experiments, the seeds (dry or 24 h-soaked) were treated with the plasma and/or nZnO (similar to the mentioned conditions for in vitro studies) and grown in pots, containing soil mixture of perlite and peat (1:1) under illumination of 33.75 µmol photon m− 2 s− 1.
Characteristics of nZnO
The characteristics of nZnO were determined by Fourier transform infrared spectroscopy (FTIR, Thermo Nicolet) and X-ray diffraction (XRD) (SEIFERT XRD 3003 PTS).
Growth and Histological studies
Thirty five days after seed pretreatments, seedlings were harvested for further analyses. Total leaf areas were measured by a digital leaf area meter (Leaf Area Meter- AM-200; ADC BioScientific Ltd, UK). Also, the total fresh weights were determined and expressed in g plant− 1. Handmade cross sections of the basal stems [Ethanol:glycerol (80:20) as a fixator solution] were prepared, stained by carmine and methylene blue, observed with the light microscope and photographed in two different magnifications, including 100× and 400×.
Photosynthetic pigments
Photosynthetic pigments, including chlorophyll a (Chla), chlorophyll b (Chlb) and carotenoids, were extracted using 80% acetone. The contents of these pigments were calculated based on the equation presented by (Lichtenthaler and Wellburn 1983).
Enzyme extraction
The frozen fresh organs were powdered by liquid nitrogen. Then, enzymes were extracted at 4 °C in a mortar and pestle using 0.1 M phosphate buffer at pH of 7.5, containing 0.5 mM Na2-EDTA and 0.5 mM ascorbic acid as an extraction buffer. The homogenates were centrifuged for 15 min at 4 °C and the supernatants were applied as enzyme extracts.
Peroxidase activity
Peroxidase activity was measured according to the previously method described by Hemeda and Klein (1990). Peroxidase activity was expressed as ΔA min− 1 g− 1 fw.
Phenylalanine ammonia lyase
The reaction mixture for measuring activity of phenylalanine ammonia lyase (PAL) enzyme consisted of 6 µM phenylalanine, Tris–HCl buffer (0.5 M, pH 8), and 200 µL of enzyme extract. After 60 min at 37 °C, the reaction was ended by adding 50 µL of 5 N HCl. PAL activities were analyzed (the rate of conversion of L-phenylalanine to trans-cinnamic acid) at 290 nm. PAL activity was determined by measuring the amount of cinnamic acid produced and expressed in µgCin min− 1 g− 1 fw, according to the method described by Beaudoin-Eagan and Thorpe (Beaudoin-Eagan and Thorpe 1985).
Soluble phenols
Total soluble phenolics were extracted from the leaves and roots of seedlings grown in MS medium using ethanol 80% (v/v) as a solvent. Phenol contents were assessed using the Folin–Ciocalteu reagent procedure. Tannic acid was applied as a standard compound. Total soluble phenol contents were expressed in mgg− 1 fw.
Statistical analysis
The experimental design was completely randomized design (CRD) in factorial experiment. Data were analyzed using SPSS software. Mean separation was performed with Duncan’s multiple range test at the level of P ≤ 0.05.
Results and discussion
Characteristics of nZnO
The characterization of nZnO was obtained by the XRD patterns using X-ray diffraction. XRD analysis confirmed the nZnO presence (Supplementary Fig). The strong intense peaks ranging from 30 to 40 theta scales indicated the crystalline nature of the nanoparticles. The intense absorption band in the range of 3237–3565 cm− 1 is due to the O–H stretching (Gnanasangeetha and SaralaThambavani 2013). The peak at about 1425–1495 cm− 1 shows the carboxylate group (Chithra et al. 2015). The band at 881 is assigned to bending mode of carbonate. The peak in the region between 482 and 595 cm− 1 is allotted to Zn–O stretching mode. The peak observed at 570 is owing to the successful synthesis of nZnO (Chithra et al. 2015).
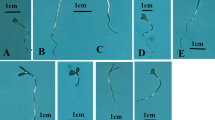
Early growth of seedlings in the petri dish condition
The individual and combined plasma treatments enhanced the growth of root system in the seedlings grown under the petri dish condition, over the untreated control (Fig. 1). This finding is in agreement with the previous studies, indicating the enhancing effects of the cold plasma on the early growth of seedlings (Sera et al. 2008, 2010, 2017; Mihai et al. 2014; Stolarik et al. 2015; Iranbakhsh et al. 2017, 2018; Safari et al. 2017). The plant responses to the plasma treatment may differ depending on the four key factors, including plant species and developmental stage, types of plasma producing instruments, time of treatment, and feed gases (Sera et al. 2017; Iranbakhsh et al. 2018). The plasma-triggered reactions have been mainly attributed to the different mechanisms, including the modifications in seed coat structure (Filatova et al. 2014; Stolarik et al. 2015), changes in water uptake rates (Ling et al. 2014), some other physiological changes (Iranbakhsh et al. 2017), and differentiation process (Safari et al. 2017). The plasma-induced sharp modifications in the surface structure of seeds in maize, wheat, and lupines have been confirmed in the scanning electron microscope images recorded by Filatova et al. (2014). Interestingly, the growth-delaying impact of nZnO was mitigated by the plasma treatment. Recently, the evidence has been provided that the cold plasma modified the expression pattern of heat shock factor A4A (HSFA4A; known as a hydrogen peroxide sensor, anti-apoptosis agent, and crosslink agent with vital signaling cascades) and improved wheat growth, physiology, and resistance to salt stress (Iranbakhsh et al. 2018). The vital elicitor and signaling agents (especially nitric oxide (NO), ozone, and/or UV) which are generated during the plasma production may contribute to the induced changes (Iranbakhsh et al. 2018). NO may determine the growth of root system, mainly through regulating auxin transport, cell division process in a meristem region, and cellular differentiation (Fernández-Marcos et al. 2011). In addition, the cold plasma changed the hormonal status in Pisum sativum (Stolárik et al. 2015). On the other hand, there is strong evidence that nZnO may change the hormonal balances (Sheteiwy et al. 2017). The inhibiting impact of nZnO has been attributed to the reductions in α-amylase activities (an important enzyme implicated in the starch hydrolysis process) which in turn lead to a lack of the required energy for seedling early growth (Sheteiwy et al. 2016). Therefore, it seems that the plasma-originated agents, especially NO, may be responsible for the observed results, probably via triggering a specific signaling, altering cellular metabolism, and modifying the hormonal balances.
Growth and biomass of seedlings grown in in vitro conditions
The morphological differences between the plasma and/or nZnO-treated seedlings cultured in a hormone-free MS medium or supplemented with the hormones are depicted in Fig. 2. In both MS and MSH media, the nZnO treatments led to the delay or reductions in the growth and biomass production, indicated by a fewer total fresh mass and leaf area found in the nZnO groups, compared with the control. Plasma significantly (p ≤ 0.05) increased the total fresh mass by 12.5, 40.6, 46.9, and 18.7% for the P60, P60–nZnO, P120, and P120–nZnO treatments, respectively, relative to the control in MS medium (Table 1). Interestingly, the significant (p ≤ 0.05) increase in the fresh mass was also observed in the plants treated with the plasma than the control in MSH medium (Table 1). The maximum fresh mass was noticed with the P120 treatment, compared with the control. Despite the observed inhibiting or delaying impacts of nZnO, no necrotic and/or chlorosis symptoms of nZnO toxicity were found. In the both MS and MSH media, the leaf area was significantly (p ≤ 0.05%) decreased at the nZnO treatment alone compared to the control (Table 1). However, the leaf area was found to be significantly increased to 6.3, 13.8, 31.4, and 24.9% for P60, P60–nZnO, P120, and P120–nZnO groups, respectively, in MS medium, while it was promoted by 53.3, 7.3 and 62.4% for P60, P60–nZnO, and P120 treatments, respectively, in the MSH medium, compared to the untreated control (Table 1).
The morphological differences in the plasma and/or nZnO-treated seedlings (35 days after seed treatment) cultured in hormone-free MS medium (a–f) or supplemented with the hormones, including 2 mgl− 1 BA and 0.5 mgl− 1 IAA (MSH) (G–L). a Control in MS; b the plasma treatment of 60 s in MS; c the plasma treatment of 120 s in MS; d nZnO in MS; e the plasma treatment of 60 s and nZnO in MS; f the plasma treatment of 120 s and nZnO in MS; g control in MSH; H- the plasma treatment of 60 s in MSH; i the plasma treatment of 120 s in MSH; j nZnO in MSH; k the plasma treatment of 60 s and nZnO in MSH; l the plasma treatment of 120 s and nZnO in MSH
It seems that nZnO had a detrimental impact on the shoot and/or root meristem zones, thereby delaying or inhibiting the growth rates and differentiation process. It has been well hypothesized that plant sensitivities to various environmental factors are dependent on the plant species and developmental stage. The application of nZnO may disrupt membranes and damage DNA (Ma et al. 2013). It had the damaging impacts on the root system and early growth rates in rice plant (Boonyanitipong et al. 2011). Moreover, nZnO provoked oxidative burst and increased.
malondialdehyde (MDA) contents as a sign of lipid peroxidation of cellular membrane in rice seedlings (Sheteiwy et al. 2017). Also, the adverse effects of nZnO on the growth and production of secondary metabolites have been recorded in Stevia rebaudiana (Javed et al. 2016). The deleterious impacts of nano-metal oxides have been attributed to the oxidative stress (Buzea et al. 2007; Sheteiwy et al. 2017). The improving effects of the cold plasma on the plant early growth have been reported in various species, including Chenopodium album (Sera et al. 2008), wheat (Sera et al. 2010; Iranbakhsh et al. 2017, 2018), radish (Mihai et al. 2014), pea (Stolarik et al. 2015), pepper (Safari et al. 2017), and Cannabis sativa (Sera et al. 2017). Interestingly, the plasma treatments relieved the reducing effects of nZnO on the plant growth, probably via inducing plant defense-related responses by UV radiation and/or signaling molecules generated during the plasma. The obtained results showed that the presence of the applied hormones in a culture medium amplified the effects of plasma of 60 s on the total biomass accumulation, suggesting that the cold plasma treatments may probably alter the hormonal balances and/or cellular sensitivities to the hormones. Therefore, it seems that the composition of the culture medium may modify the plasma–plant interactions. The possible provoked changes in the meristem zones, cell cycle, differentiation, and/or hormonal balances may be responsible for the observed morphological differences between the treatment groups. The modifications in the growth and differentiation process provoked by the cold plasma might be attributed to the different bioactive signaling molecules [especially ozone and nitric oxide (NO)] originated from the plasma treatments as well as UV radiation, generated during the plasma. NO, known as a bioactive signaling compound, can modify cell division reaction in the meristem, cell differentiation process, and polar auxin transport via affecting the PIN1 protein levels (Fernández-Marcos et al. 2011). There is evidence that the phytohormonal balances were altered by the cold plasma in pea plants (Stolárik et al. 2015). Up-regulations and down-regulations of transcripts were found in Arabidopsis thaliana exposed to nZnO (Landa et al. 2015). Also, nZnO changed the pattern expression of some critical genes in rice (Sheteiwy et al. 2015).
Photosynthetic pigments and tissue differentiation
In the present study, the photosynthetic pigment contents were increased by nZnO and plasma treatments over the control (Table 1). Interestingly, the photosynthetic pigments were increased significantly (p ≤ 0.05) to 34.8, 28, 31.8, 40% (for chlorophyll a) and 43.9, 36, 36.3, and 36% (for carotenoid), respectively, for the P60, P60–nZnO, P120, and P120–nZnO treated plants than the control (Table 1). The level of Chlorophyll b was also enhanced significantly (p ≤ 0.05) by 33.3 and 23.8% for P120 and P120–nZnO treatments, respectively, compared to the control, whereas the Chlorophyll b contents in the nZnO, P60, and P60–nZnO treated plants did not change significantly over the untreated plants (Table 1). The observed differences in the tissue patterns of the basal stems of the nZnO and/or plasma-treated seedlings cultured in MS medium supplemented with the hormones are presented in Fig. 3. The patterns of the vascular system clearly varied between the treatments, among which the widest vessels were found in seedlings treated with plasma of two min (Fig. 3). The inhibiting impacts of nZnO on the differentiation of the vascular tissues were considerably relieved by the seed pre-treatments with plasma. According to the above-described changes in the growth, physiology, and anatomy, the beneficial effects of the plasma treatment could be attributed to the underlying mechanisms, like improvements in the vascular system, plant nutritional status, and/or possible enhances in the photosynthesis process. While, increases in the reactive oxygen species (ROS), lipid peroxidation of membranes, and nutritional disturbance appear to be responsible for the toxicity of nZnO. Disturbing potential of nZnO on the plant nutritional status, especially S and P, has been reported (Peralta-Videa et al. 2014). High levels of nZnO in suspensions completely inhibited the root elongation of some crop including radish, rape, ryegrass, lettuce, corn and cucumber (Lin and Xing 2007). The overaccumulations of ROS in Spirodela punctuta (Thwala et al. 2013) and rice (Chen et al. 2015; Sheteiwy et al. 2017) plants exposed to nZnO have been reported. Also, nZnO altered hormonal balances (ABA and GA), mainly via modifying the expression patterns of the key involved genes (OsABA8ox2 and OsNCED1) (Sheteiwy et al. 2017). It is obvious that changes in the hormonal balances may affect differentiation process and the relation between sources and sink organs. Interestingly, the ultra-structural analysis provided valuable evidence showing that the high doses of nZnO led to severe damage in the leaf mesophyll cells in rice cultivars (Sheteiwy et al. 2015). Also, increases in the number of osmiumphobilic granule and starch grains, the disappearance of various organelles, and damages in cell structure were found in leaf cells of rice counteracted with nZnO (Sheteiwy et al. 2015).
The effects of plasma and/or nZnO treatments on the tissue patterns of the basal stem of Capsicum annuum cayenne grown in MS medium supplemented with 2 mgl− 1 BA and 0.5 mgl− 1 IAA (MSH) (35 days after seed treatment). 1: Cortex region; 2: xylem tissues; 3: pith parenchyma. a Control; b nZnO; c the plasma treatment of 120 s; d the plasma treatment of 120 s and nZnO (a–d photographed at magnification of ×100). e Control; f nZnO; g the plasma treatment of 120 s; h the plasma treatment of 120 s and nZnO (e–h photographed at magnification of ×400)
Peroxidase, PAL and soluble phenols
In MS condition, the pre-treatment with plasma resulted in the significant promotions in the peroxidase activities of the leaves and roots. The leaf peroxidase activities of seedlings grown in MS were significantly (p ≤ 0.05) increased by 42.7, 33.4, and 55.6% for P60–nZnO, P120, and P120–nZnO treatments, respectively, compared to the control (Table 2). The root peroxidase activity was found to be significantly (p ≤ 0.05) induced by the nZnO and/or plasma treatments in comparison with the control (Table 2). The P120 treatment alone caused the drastic rise in the root peroxidase activity, over the untreated control (Table 2). The significant inductions in the peroxidase activities were found in the plasma and/or nZnO-treated seedlings grown in MSH, among which the highest amounts were recorded in the nZnO treatments (Table 2). The plasma and/or nZnO treatments led to the significant considerable inductions in the activities of PAL enzymes in leaves and roots of the seedlings cultured in the both culture media (Table 3). The results showed that the PAL activity in the leaves of seedlings cultured in MS was found to be increased significantly (p ≤ 0.05) by 35.1, 30.8, 67, 63.3, and 43.1% for the nZnO, P60, P60–nZnO, P120, and P120–nZnO treatments, respectively compared to that of the control (Table 3). In the roots of seedlings grown in MS medium, increases in the PAL activity were significantly (p ≤ 0.05) raised from the plasma and/or nZnO treatments (Table 3). In the plants grown in MSH, the nZnO treatment alone did not change significantly (p ≤ 0.05) the leaf PAL activity compared to the untreated control, while the PAL activity was enhanced by 33, 44.4, 27.9, and 25.9% for the P60, P60–nZnO, P120, and P120–nZnO treatments respectively, compared to the control (Table 3). In comparison to the control, the significantly higher amounts of the root PAL activity were found in the plasma and/or nZnO-treated seedlings (Table 3). Furthermore, the leaf soluble phenols were also significantly (p ≤ 0.05) enhanced by 87, 48.2, 91.8, 82.3, and 52.9% for the nZnO, P60, P60–nZnO, P120, and P120–nZnO treatments, respectively, over the control. Interestingly, the highest concentration of the root soluble phenols was noticed with the P120–nZnO treatment (Table 3). There was a positive correlation between the PAL activities and total soluble phenol contents in the leaves and roots of the treated seedlings (r = 0.753 and r = 0.882, respectively p = 0.001).
The obtained findings indicated that the independent factors of the present study considerably influenced the activities of the peroxidase (an important antioxidant enzyme) and PAL (a key enzyme in phenylpropanoid pathway) as well as the soluble phenol contents in both leaves and roots of the treated seedlings. The observed alterations in the evaluated physiological traits seem to be elicited by UV, and the vital signaling bioactive compounds, especially NO and ozone, generated during the plasma treatments. Treatments of wheat seedlings with cold plasma (DBD) led to the significant improvements in peroxidase and PAL activities (Iranbakhsh et al. 2018), as well as increases in phenols and biomass accumulations (Iranbakhsh et al. 2017). Cold plasma-induced activities of PAL, in combination with antioxidant enzymes in tomato plants, have been proposed as key crucial mechanisms, thereby counteracting against Ralstonia solanacearum, pathogen (Jiang et al. 2014). The cold plasma treatment provides a complicated situation in which cells are exposed to UV and critical signaling bioactive molecules, among which NO is the most important. In our opinion, the eliciting possible compound may be oligosaccharins, mainly originated from the plasma-caused structural changes in the cell wall. Some oligosaccharins are known as elicitors which exert signaling effects and induce various physiological mechanisms by which defense system is activated (Fry et al. 1993) and, hence, plant resistance against different stress factors is improved. The found inductions in the peroxidase and PAL activities in the seedlings treated with plasma may be considered as one of the important mechanisms involved in alleviating the inhibitory impacts of nZnO on the plant growth. It is obvious and well documented that the antioxidant enzymes, like peroxidase, play critical roles in the scavenging process of active oxygen species and regulating cellular redox status in plants exposed to the stress conditions. A peroxidase activity is considered as a critical index of the plant resistance against the various abiotic stresses (Iranbakhsh et al. 2018). The achieved results clearly showed that plant secondary metabolism and systemic modification in the plant biochemistry were triggered by the mentioned independent variables of this research (plasma, nZnO, and their interactions). NO as an active nitrogen species exerts potential of reacting with different extra/intracellular targets (Groß et al. 2013). It has been stated that NO induces the accumulations of cytoprotective proteins and the synthesis of some antioxidant enzymes in the plant cell (Wang et al. 2013). In addition to NO, ozone and UV radiation, generated during the plasma treatment, also have high potential for affecting plant physiology. Hence, the plasma-triggered accumulations cyto-protectant enzymes or compounds may be regarded as the possible potent mechanisms involved in relieving the toxicity signs of nZnO in the treated seedlings. In addition, bioactive compounds like ozone generated during plasma treatment may cause physiological changes. Four-hundred and sixteen up-regulated transcripts, as well as 961 down-regulated transcripts (particularly, the genes contribute to cell organization and DNA or RNA metabolism) have been found in Arabidopsis thaliana exposed to nZnO (Landa et al. 2015). There is evidence that nZnO up-regulated the expression of various vital genes, including APXa, APXb, CATa, CATb, CATc, SOD1, SOD2, and SOD3 (Sheteiwy et al. 2015) and GR1, GR2, Amy2A, and Amy3A (Sheteiwy et al. 2016) in rice.
Complementary pot experiment
The plasma treatments (especially plasma of 2 min) had the desirable enhancing effects on the growth rates of seedlings grown in the pot experiment. Moreover, the plasma treatments not only had the stimulatory effects on the plant growth but also mitigated the inhibitory impacts of nZnO (Figs. 4, 5). The evaluations based on the pot experiments indicated that the plasma treatments of soaked seeds were the most effective method to trigger the plant growth and development. It seems that soaking before the seed pretreatment considerably enhances the efficiency of the plasma treatment to improve the plant growth, most probably due to the existence of a positive and close correlation between water uptake and metabolism rates. It is obvious that the water uptake triggers the cellular metabolism in embryo, thereby amplifying and accelerating the effects of the bioactive signaling molecules generated during the plasma treatment. The observed plasma-induced modifications in the different characteristics related to the growth, differentiation, and physiology could be regarded as a supporting data and mechanisms implicated in alleviating the adverse impacts of nZnO.
The effects of the nZnO and/or Plasma treatments on the growth of Capsicum annuum Var. cayenne in the pot experiment, 50 days after plasma treatment where the plasma treatments were done on water soaked seeds for 24 h before the plasma treatment. a Control; b the plasma treatment of 60 s; c the plasma treatment of 120 s; d nZnO; e the plasma treatment of 60 s and nZnO; f the plasma treatment of 120 s and nZnO
The effects of the nZnO and/or plasma treatments on the growth of Capsicum annuum cayenne in the pot experiment, 35 days after the treatments. The plasma treatments were done on the dry seeds. a Control; b the plasma treatments of 60 s; c the plasma treatment of 120 s; d nZnO; e the plasma treatment of 60 s and nZnO; f the plasma treatment of 120 s and nZnO
Conclusion
In summary, the pot and in vitro experiments confirmed that the plasma treatment had the considerably growth promoting and protective effects. Also, the inhibiting and delaying impacts of nZnO on the growth and differentiation, especially the vascular system, were relieved by the plasma treatments, which could be attributed to the plasma-induced changes in the cellular physiology and defense system, through the possible specific signaling triggered by the bioactive agents (especially NO), and UV generated during the plasma. This research represents the remarkable and novel findings, depicting that the plasma treatments have the potential benefits to trigger and modify the critical physiological characteristics, thereby improving plant resistance against stress condition. This is the first report, showing the considerable potency of the plasma to improve plant resistance against nanoparticles in the form of metal oxide. Our research could be path finding for studying the efficiency of the cold plasma treatment as a possible new alternative approach to plant science, especially in the fields of seed science and plant tissue culture for disinfecting seed-borne contaminations and eliciting the productions of secondary metabolites. The cold plasma generating devises may be employed to scale up for different agriculture goals. These findings may improve our knowledge regarding the plasma science and technology to develop a new alternative approach in seed sciences, taking into account sustainable agriculture and/or environmental issues. It is obvious that further researches are required for elucidating the key and main involved mechanisms, especially at genetic levels, in these kinds of plant responses to the cold plasma and nanoparticles.
Author contribution statement
AI, ZOA, and MG designed the experiments. NOA and NS prepared materials and performed treatments. NOA, NS, ZOA, and AI carried out the biochemical analysis. AI carried out the statistical evaluation. AI, ZOA, and MG contributed to the interpretation of the results. AI took the lead in writing the paper. AI, ZOA, NOA, MG, and NS have contributed, seen, and approved the article.
References
Asgari-Targhi G, Iranbakhsh A, Ardebili ZO (2018) Potential benefits and phytotoxicity of bulk and nano-chitosan on the growth, morphogenesis, physiology, and micropropagation of Capsicum annuum. Plant Physiol Biochem 127:393–402
Baytak AK, Aslanoglu M (2017) Sensitive determination of capsaicin in pepper samples using a voltammetric platform based on carbon nanotubes and ruthenium nanoparticles. Food Chem 228:152–157
Beaudoin-Eagan LD, Thorpe TA (1985) Tyrosine and phenylalanine ammonia lyase activities during shoot initiation in tobacco callus cultures. Plant Physiol 78:438–441
Boonyanitipong P, Kositsup B, Kumar P, Baruah S, Dutta J (2011) Toxicity of ZnO and TiO2 nanoparticles on germinating rice seed Oryza sativa L. Int J Biosci Biochem Bioinform 1:282
Bußler S, Herppich WB, Neugart S, Schreiner M, Ehlbeck J, Rohn S, Schlüter O (2015) Impact of cold atmospheric pressure plasma on physiology and flavonol glycoside profile of peas (Pisum sativum ‘Salamanca’). Food Res Int 76:132–141
Buzea C, Pacheco II, Robbie K (2007) Nanomaterials and nanoparticles: sources and toxicity. Biointerphases 2:MR17–MR71
Chen HH, Chen YK, Chang HC (2012) Evaluation of physicochemical properties of plasma treated brown rice. Food Chem 135:74–79
Chen J, Liu X, Wang C, Yin S-S, Li XL, Hu WJ, Simon M, Shen ZJ, Xiao Q, Chu CC (2015) Nitric oxide ameliorates zinc oxide nanoparticles-induced phytotoxicity in rice seedlings. J Hazard Mat 297:173–182
Chithra MJ, Sathya M, Pushpanathan K (2015) Effect of pH on crystal size and photoluminescence property of ZnO nanoparticles prepared by chemical precipitation method. Acta Metall Sin 28:394–404
Fernández-Marcos M, Sanz L, Lewis DR, Muday GK, Lorenzo O (2011) Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proceed Nat Acad Sci 108:18506–18511
Filatova II, Azharonok VV, Goncharik SV, Lushkevich VA, Zhukovsky AG, Gadzhieva GI (2014) Effect of rf plasma treatment on the germination and phytosanitary state of seeds. J Appl Spectrosc 81(2):250–256
Fry SC, Aldington S, Hetherington PR, Aitken J (1993) Oligosaccharides as signals and substrates in the plant cell wall. Plant Physiol 103:1
Gnanasangeetha D, SaralaThambavani D (2013) One pot synthesis of zinc oxide nanoparticles via chemical and green method. Res J Mat Sci 2320:6055
Gogos A, Knauer K, Bucheli TD (2012) Nanomaterials in plant protection and fertilization: current state, foreseen applications, and research priorities. J Agric Food Chem 60:9781–9792
Groß F, Durner J, Gaupels F (2013) Nitric oxide, antioxidants and prooxidants in plant defence responses. Front Plant Sci 4:419
Hemeda HM, Klein BP (1990) Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci 55:184–185
Hoffmann C, Berganza C, Zhang J (2013) Cold atmospheric plasma: methods of production and application in dentistry and oncology. Medical Gas Res 3:1
Iranbakhsh A, Ghoranneviss M, Ardebili ZO, Ardebili NO, Tackallou SH, Nikmaram H (2017) Non-thermal plasma modified growth and physiology in Triticum aestivum via generated signaling molecules and UV radiation. Biol Plant 61(4):702–708. https://doi.org/10.1007/s10535-016-0699-y
Iranbakhsh A, Ardebili NO, Ardebili ZO, Shafaati M, Ghoranneviss M (2018) Non-thermal plasma induced expression of Heat Shock Factor A4A and Improved Wheat (Triticum aestivum L.) growth and resistance against Salt Stress. Plasma Chem Plasma Process 38:29–44
Javed R, Usman M, Yücesan B, Zia M, Gürel E (2016) Effect of zinc oxide (ZnO) nanoparticles on physiology and steviol glycosides production in micropropagated shoots of Stevia rebaudiana Bertoni. Plant Physiol Biochem. https://doi.org/10.1016/jplaphy201605032
Jiang J, Lu Y, Li J, Li L, He X, Shao H, Dong Y (2014) Effect of seed treatment by cold plasma on the resistance of tomato to Ralstonia solanacearum (bacterial wilt). Plos one 9:e97753
Keller A, McFerran S, Lazareva A, Suh S (2013) Global life cycle releases of engineered nanomaterials. J Nanopart Res 15:1692
Landa P, Prerostova S, Petrova S, Knirsch V, Vankova R, Vanek T (2015) The transcriptomic response of arabidopsis thaliana to zinc oxide: a comparison of the impact of nanoparticle, bulk, and ionic zinc. Environ Sci Technol 49:14537–14545
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Lin D, Xing B (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150:243–250
Ling L, Jiafeng J, Jiangang L, Minchong S, Xin H, Hanliang S, Yuanhua D (2014) Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci Rep 4:5859. https://doi.org/10.1038/srep05859
Ma H, Williams PL, Diamond SA (2013) Ecotoxicity of manufactured ZnO nanoparticles—a review. Environ Pollut 172:76–85
Mihai A, Dobrin D, Magurenau M, Popa M (2014) Positive effect of non-thermal plasma treatment in radish seeds. Romanian Rep Phys 66:1110–1117
Mitra A, Li YF, Klämpfl TG, Shimizu T, Jeon J, Morfill GE, Zimmermann JL (2014) Inactivation of surface-borne microorganisms and increased germination of seed specimen by cold atmospheric plasma. Food Bioproc Technol 7:645–653
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Ng KW (2011) The role of the tumor suppressor p53 pathway in the cellular DNA damage response to zinc oxide nanoparticles. Biomaterials 32:8218–8225
Peralta-Videa JR, Hernandez-Viezcas JA, Zhao L, Diaz BC, Ge Y, Priester JH, Holden PA, Gardea-Torresdey JL (2014) Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiol Biochem 80:128–135
Safari N, Iranbakhsh A, Ardebili ZO (2017) Non-thermal plasma modified growth and differentiation process of Capsicum annuum PP805 Godiva in in vitro conditions. Plasma Sci Technol 19(5):055501
Sera B, Spatenka P, Sery M, Vrchotova N, Hruskova I (2010) Influence of plasma treatment on wheat and oat germination and early growth. IEEE Trans Plasma Sci 38:2963–2968
Sera B, Sery M, Gavril B, Gajdova I (2017) Seed germination and early growth responses to seed pretreatment by non-thermal plasma in hemp cultivars (Cannabis sativa L.). Plasma Chem Plasma Proc 37:207–221
Será B, Stranák V, Serý M, Tichý M, Spatenka P (2008) Germination of Chenopodium album in response to microwave plasma treatment. Plasma Sci Technol 10:506
Sheteiwy MS, Yajing G, Dongdong C, Jie L, Aamir N, Qijuan H, Weimin H, Mingyu N, Jin H (2015) Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Sci Rep 5:14278
Sheteiwy MS, Fu Y, Hu Q, Nawaz A, Guan Y, Li Z, Huang Y, Hu J (2016) Seed priming with polyethylene glycol induces antioxidative defense and metabolic regulation of rice under nano-ZnO stress. Environ Sci Poll Res 23(19):19989–20002
Sheteiwy MS, Dong Q, An J, Song W, Guan Y, He F, Huang Y, Hu J (2017) Regulation of ZnO nanoparticles-induced physiological and molecular changes by seed priming with humic acid in Oryza sativa seedlings. Plant Growth Regul 83(1):27–41
Stolárik T, Henselová M, Martinka M, Novák O, Zahoranová A, Černák M (2015) Effect of low-temperature plasma on the structure of seeds, growth and metabolism of endogenous phytohormones in pea (Pisum sativum L). Plasma Chem Plasma Process 35:659–676
Thwala M, Musee N, Sikhwivhilu L, Wepener V (2013) The oxidative toxicity of Ag and ZnO nanoparticles towards the aquatic plant Spirodela punctuta and the role of testing media parameters. Environ Sci Process Impacts 15:1830–1843
Ulbin-Figlewicz N, Jarmoluk A, Marycz K (2015) Antimicrobial activity of low-pressure plasma treatment against selected foodborne bacteria and meat microbiota. Annal Microb 65:1537–1546
Wang Y, Loake GJ, Chu C (2013) Cross-talk of nitric oxide and reactive oxygen species in plant programed cell death. Front Plant Sci 4:314
Wu Z, Chi L, Bian S, Xu K (2007) Effects of plasma treatment on maize seeding resistance. J Maize Sci 15:111–113
Yang Z, Chen J, Dou R, Gao X, Mao C, Wang L (2015) Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants, maize (Zea mays L) and rice (Oryza sativa L). Int J Environ Res Public Health 12:15100–15109
Acknowledgements
The authors would like to thank MSc. Hamed Nikmaram, MSc. Maryam Amini, and MSc. Gasem Asgari for their benevolent and professional collaborations in the research procedure. Corresponding author specially would like to acknowledge of Plasma Physics Research Center, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Bartosz.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iranbakhsh, A., Oraghi Ardebili, Z., Oraghi Ardebili, N. et al. Cold plasma relieved toxicity signs of nano zinc oxide in Capsicum annuum cayenne via modifying growth, differentiation, and physiology. Acta Physiol Plant 40, 154 (2018). https://doi.org/10.1007/s11738-018-2730-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2730-8