Abstract
Iron (Fe) is a key element for all living systems, especially for photosynthetic organisms because of its important role in the photosynthetic electron transport chain. Fe limitation in cyanobacteria leads to several physiological and morphological changes. However, the overall metabolic responses to Fe limitation are still poorly understood. In this study, we integrated elemental, stoichiometric, macromolecular, and metabolomic data to shed light on the responses of Synechocystis sp. PCC 6803, a non-N2-fixing freshwater cyanobacterium, to Fe limitation. Compared to Synechocystis growing at nutrient replete conditions, Fe-limited cultures had lower growth rates and amounts of chlorophyll a, RNA, RNA:DNA, C, N, and P, and higher ratios of protein:RNA, C:N, C:P, and N:P, in accordance with the growth rate hypothesis which predicts faster growing organisms will have decreased biomass RNA contents and C:P and N:P ratios. Fe-limited Synechocystis had lower amounts Fe, Mn, and Mo, and higher amount of Cu. Several changes in amino acids of cultures growing under Fe limitation suggest nitrogen limitation. In addition, we found substantial increases in stress-related metabolites in Fe-limited cyanobacteria such antioxidants. This study represents an advance in understanding the stoichiometric, macromolecular, and metabolic strategies that cyanobacteria use to cope with Fe limitation. This information, moreover, may further understanding of changes in cyanobacterial functions under scenarios of Fe limitation in aquatic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metabolomics aims to characterize the metabolome of organisms, and it is gaining importance in fields such as microbiology, plant physiology, and more recently in ecology (Macel et al. 2010; Jones et al. 2013; Rivas-Ubach et al. 2014, 2016a). The metabolome consists of all the low-molecular-weight compounds (metabolites) present in an organism at a given time (Fiehn 2002) constituting all of the compounds involved in physiological processes allowing cells to function, grow, and respond to environmental stressors (Peñuelas and Sardans 2009). The metabolome can be considered, thus, as the “chemical phenotype” of an organism (Fiehn 2002).
Any fine-scale shifts in metabolites may underpin higher order shifts in cellular macromolecular composition (e.g., changes in overall protein and nucleic acid composition) and stoichiometry (Rivas-Ubach et al. 2012) and can thus, in turn affect the whole-organism requirement for key nutrient elements such as C, N, and P, forming the basis of what is now known as “biological stoichiometry” (Sterner and Elser 2002; Elser et al. 2008). Furthermore, environment-induced metabolic shifts can also affect the profile of less-abundant elements, such as the suite of essential metals that make up an organism’s metallome (da Silva and Williams 2001). Indeed, emerging studies have shown how metabolomes can be related to biomass stoichiometry and growth rate. For example, Rivas-Ubach et al. (2012) found that changes in foliar C:N:P:K stoichiometry of the Mediterranean shrub Erica multiflora were related to shifts in the metabolome of leaves during growing seasons and across soil moisture regimes. Such relationships between metabolomes, biomass stoichiometry, and growth rate may also occur in other organisms. In this study, we integrated metabolomics, macromolecular, and stoichiometric analyses of a model freshwater unicellular cyanobacterium, Synechocystis sp. PCC 6803, grown under iron (Fe) limited conditions.
Fe is required for many enzymes necessary for physiological processes, such as respiration (Richardson 2000), nitrogen reduction and assimilation (Bellenger et al. 2011), and especially photosynthesis (Ferreira and Straus 1994; Raven et al. 1999; Behrenfeld 1999; Shcolnick and Keren 2006). In cyanobacteria, photosystem I (PS I) and photosystem II (PS II) complexes of the photosynthetic electron transport chain (PETC) are Fe-rich with 12 and 3 Fe atoms, respectively (Ferreira and Straus 1994), and 6 Fe atoms in the cytochrome b6/f complex (Baniulis et al. 2008). This results in a high cellular Fe quota with ~ 4 × 106 Fe atoms per cell for Synechocystis sp. PCC 6803 (Keren et al. 2004). The high content of Fe in the PETC and its low availability make this element a key limiting nutrient for photoautotrophs in many ecosystems (Behrenfeld et al. 1996, Behrenfeld 1999; Vrede and Tranvik 2006; Mackey et al. 2015). In addition, Fe bioavailability is further influenced by physico-chemical properties of water such as acidity which is expected to increase in many aquatic ecosystems in the forthcoming decades (Shi et al. 2010, 2012).
Cyanobacteria exhibit several morphological and physiological responses to Fe deficiency including the release of intracellular Fe from storage complexes (Keren et al. 2004; Shcolnick et al. 2009), induction of high-affinity Fe uptake systems (Katoh et al. 2001a, b; Kranzler et al. 2014), and decreases in photosynthetic capacity, phycobilisomes, chlorophyll and other pigments, cell yields, and growth rates (Ferreira and Straus 1994; Dang et al. 2012). Alterations in gene and protein expression underlie most of the morphological and physiological responses of cyanobacteria to Fe limitation (Castielli et al. 2009; Hernández-Prieto et al. 2012; Kopf et al. 2014).
Metabolomic changes in cyanobacteria and especially in Synechocystis sp. PCC 6803, under low Fe availability are not well-documented. Since metabolomes are the final product of genomes (Fiehn 2002), we expect that transcriptomic and proteomic changes of cyanobacteria in response to Fe deprivation should be reflected in their metabolomes. More specifically, the reduced photosynthetic capacity of Fe-limited cyanobacteria should trigger changes in C and N metabolism that are detectable as differences in the abundance of metabolites. We used metabolomics approaches in combination with macromolecular and elemental analyses to characterize the metabolomes and the C, N, P, trace metal, DNA, RNA, and protein contents to obtain a better overall understanding of the effects of Fe limitation on the physiological status of wild-type Synechocystis sp. PCC 6803. Data obtained are interpreted with the aim of integrating metabolomics with biological stoichiometry to provide important information for understanding the impacts of Fe limitation on communities and on biogeochemical cycling in marine and freshwater ecosystems.
Materials and methods
Growth of Synechocystis sp. PCC 6803
Synechocystis sp. PCC 6803 (hereafter Synechocystis) was grown in 1.8 L of BG-11 medium pH 7.8 (Allen 1968) in 2 L trace metal clean polycarbonate bottles at 24 °C under continuous aeration with 0.2 μm filtered air and illumination (50 μmol photons m−2 s−1 irradiance). During the exponential phase of growth, cells were harvested and transferred to normal composition BG-11 (N:P 100, 18 μM Fe) or modified BG-11 to assess the effect of Fe limitation (N:P 100, 1.8 μM Fe) on physiological processes in Synechocystis. Six replicates were grown for Synechocystis cultures growing at complete media conditions and for the cultures growing at Fe-limited conditions. Upon transfer of Synechocystis to each bottle, the growth rate of the cultures was monitored via absorbance at 730 nm, and once cultures reached exponential growth (7 days for complete, and 10 days for Fe-limited cultures), cells were harvested for the following analyses: (1) absorbance and chlorophyll a, (2) cell counts, (3) elemental composition (C, N, P, S, and trace metals), (4) biological macromolecule composition (DNA, RNA, and protein), and (5) metabolomic profiling. Three cultures from each treatment were also selected for transcriptomic analyses in a separate study (Kellom et al. 2018).
Growth rate, absorbance spectra, and chlorophyll a measurements
The growth rate and final yield of Synechocystis for each treatment was assessed via absorbance at 730 nm and chlorophyll a measurements, respectively. Absorbance spectra from 350 to 800 nm on 0.5 mL of culture were also obtained using a Beckman Coulter DU®730 UV/Vis Spectrophotometer (Beckman Coulter, Indianapolis, IN, USA). The growth rate (GR) of cultures was calculated as follows:
Chlorophyll a was measured on 2 mL of culture harvested via centrifugation at 21,000×g for 10 min at 4 °C. Supernatant of all tubes was removed and cell pellets were extracted with 1 mL of 100% methanol for 5 min in the dark at room temperature (24 °C). After centrifugation (21,000×g; 5 min; 4 °C) to collect cell debris, the supernatant was transferred into a polystyrene cuvette to measure the absorbance of cell extracts at 665 nm. Methanol (100%) was used as blank. Chlorophyll a (Chl a ) concentration (µg mL−1) was calculated as follows (Bennette et al. 2011):
Cell imaging and cell count measurements
Synechocystis cells were imaged via epifluorescence microscopy to check for contamination and final cell counts were performed using an automated particle counter. Epifluorescence microscopy was performed on 10 μL culture aliquots fixed with 4% formaldehyde, deposited onto PTFE-coated microscope slides, and DAPI stained. Cells were imaged at 1000× magnification. Cell counts were conducted using the Multisizer™ 3 Coulter Counter (Beckman Coulter, Indianapolis, IN, USA) equipped with a 20 μm aperture tube (0.4–12.0 μm dynamic range for determination of particle size) on 50–1000-fold dilutions of culture.
After transcriptomic analysis, we found 16S rRNA gene sequences from Hymenobacter, a rod-shaped bacterium ranging in length from 2 to 6 μm (Krieg et al. 2010). Visual inspection of cultures via epifluorescence microscopy revealed the presence of a few rod-shaped cells (approximately 2–4 μm in length) in some fields. However, all cultures displayed a single particle size peak of approximately 1.63 ± 0.08 µm using the Coulter counter suggesting minor contamination by this rod-shaped bacterium.
Elemental composition measurements
The concentrations of C, N, P, and trace metals (Ca, Co, Cu, Fe, Mg, Mn, Mo, Ni, Zn, and V) in Synechocystis were measured following incubation. For C and N analyses, 30 mL of cells from each culture bottle were harvested and centrifuged at 5000×g for 10 min at 4 °C and washed with 0.85% NaCl to remove excess media. Samples (200 mL) for P, S, and trace metal analyses were equally harvested and washed three times with an oxalic acid solution (pH 7.8, 50 mM Na2EDTA, 100 mM oxalic acid, 304 μM MgSO4.7H2O, 245 μM CaCl2.2H2O, and 189 μM Na2CO3) to remove extracellular Fe and other metals (Tovar-Sanchez et al. 2003). Cell pellets were stored at -80 °C until analyses.
For C, N, P, and trace metal determination, samples were dried for 24 h at 60 °C. C and N were measured on 1–3 mg dry weight samples using a Costech Elemental Analyzer coupled to a Finnigan DeltaPlus Isotope Ratio Mass Spectrometer (EA-IRMS; Thermo-Finnigan MAT 253, West Palm Beach, FL, USA). C and N contents were calculated via comparison with a tomato leaves standard (NIST SRM 1573a).
P, S, and trace metals were measured on 50–120 mg dry weight of samples digested three times overnight at 100 °C with 5 mL of 16 M nitric acid. After digestion, samples were dried and resuspended in 5 mL of 0.32 M nitric acid. Samples were diluted 15- and 100-fold to measure the following elements: Ca, Co, Cu, Fe, Mg, Mn, Mo, Ni, P, S, V, and Zn. Calibration curves were constructed for the elements using a phosphorus-enriched black-shale standard of known elemental composition. Trace metals were measured via Inductively-Coupled Plasma Mass Spectrometry (ICP-MS; iCAP Q ICP-MS, Thermo Fisher Scientific, Waltham, MA, USA). EA-IRMS and ICP-MS measurements were carried out in the W. M. Keck Foundation Laboratory for Environmental Biogeochemistry at Arizona State University.
Biological macromolecule (DNA, RNA, and protein) composition analyses
DNA was extracted from 20 mL of cells using the FastDNA® Spin Kit and FastPrep®-24 homogenizer (MP Biomedicals, Solon, OH, USA) following the manufacturer’s protocol. DNA was quantified via fluorescence using the PicoGreen assay kit (Invitrogen, Carlsbad, CA, USA).
RNA was extracted from 20 mL of cells using the FastRNA® Pro Blue Kit (MP Biomedicals, Solon, OH, USA) following the manufacturer’s protocol with the following exception. A second chloroform extraction was performed to further purify nucleic acids and samples were precipitated overnight with isopropanol at – 20 °C. To remove gDNA, samples were treated with the RTS DNase (MO BIO Laboratories, Carlsbad, CA, USA) according to the manufacturer’s protocol. DNased RNA was purified via the RNeasy MinElute Cleanup Kit (Qiagen, Valencia, CA, USA) and RNA concentration was measured using the Qubit® RNA Assay Kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions.
Protein was extracted from 20 mL of cells. Briefly, cells were resuspended in 500 µL of 10 mM HEPES-NaOH (pH 7.2) amended with 1X Protease Inhibitor Cocktail (Promega, Madison, WI, USA). Resuspended cells were transferred to Lysing Matrix B tubes (MP Biomedicals, Solon, OH, USA) and homogenized for 45 s at a speed of 6.0 m s−1 in a FastPrep®-24 homogenizer. Samples were centrifuged at 5000×g for 5 min at 4 °C to pellet cellular debris and lysing beads. 250–300 µL of supernatant was transferred to another tube and the protein concentration measured using the Qubit® Protein Assay Kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions.
Metabolite extraction
The remaining biomass (from ~ 800 mL of culture) was reserved for metabolomic analyses. Cell pellets were rapidly frozen in liquid nitrogen and lyophilized (Rivas-Ubach et al. 2013). Lyophilized cells were stored at − 80 °C until metabolite extraction.
Metabolite extraction for NMR analyses
Polar and semi-polar metabolites were extracted by following Rivas-Ubach et al. (2013). Briefly, 30 mg of lyophilized cells from each sample was extracted in 6 mL of water/methanol (1:1) via vortexing and sonication. These procedures were repeated for two extractions of the same sample. Sample extracts were lyophilized and 1 mL of KH2PO4-buffered D2O + 0.01% TSP (trimethylsilyl propionic acid sodium salt) (pH 6.0) was added. TSP was used as an internal standard. Extracts were transferred to NMR sample tubes and analyzed as detailed in Rivas-Ubach et al. (2013).
Metabolite extraction for LC–MS analyses
Metabolites were extracted following t’Kindt et al. (2008) with minor modifications. Two sets of 2 mL centrifuge tubes were labeled: set A for extractions of samples and set B for keeping the extracts from set A. 30 mg of lyophilized cells of each sample was transferred to set A tubes and 1 mL of methanol/water (80:20) was added to each tube. Set A was vortexed for 15 min, sonicated for 5 min at room temperature and centrifuged at 23,000×g for 5 min. After centrifugation, 0.6 mL of the supernatant from each tube was transferred to the corresponding set B tubes. This procedure was repeated again for two extractions of the same sample and the second aliquot was combined with the previous one. The set B tubes were then centrifuged at 23,000×g for 5 min. The supernatants were collected by crystal syringes, filtered through 0.22 µm pore microfilters, and transferred to a labeled set of HPLC vials. The vials were stored at − 80 °C until LC–MS analysis.
Metabolomic measurements of cellular extracts
NMR-based metabolomics
NMR experiments were performed on a Bruker AVANCE 600 spectrometer (Bruker Biospin, Rheinstetten, Germany) working at a magnetic field of 14.1 T (1H and 13C NMR frequencies of 600.13 and 150.13 MHz, respectively) and equipped with an automatic sample changer, a multinuclear triple resonance TBI probe, and a temperature control unit. The temperature into the probe head was previously calibrated and maintained constant for all the experiments at 24.5 °C; for this purpose, an equilibration delay (2 min) is left once the tube is into the magnet and prior to the shimming process. All NMR sample handling, automation, acquisition, and processing were controlled using TopSpin 3.1 software (Bruker Biospin, Rheinstetten, Germany). Spectra were referenced to the internal reference TSP (1H and 13C at δ 0.00 ppm).
1H NMR fingerprinting
Extracts were analyzed through standard pulse-acquisition one-dimensional (1D) 1H-NMR experiments with suppression of the residual water resonance. Water resonance signal was presaturated at a power level of 55 dB, corresponding to an effective field of 30 Hz during a relaxation delay of 2 s. Samples were analyzed as a set of 32 k data points, over a spectral width of 16 ppm, as the sum of 128 transients and with an acquisition time of 1.7 s. The experimental time was ~ 8 min per sample. Fourier transformation was applied to the resulting interferograms (free induction decay, FID) and the spectra obtained were phased and baseline corrected. All FIDs of polar samples were multiplied by an exponential apodization function equivalent to 0.2 Hz line broadening prior to the Fourier transform.
NMR metabolite identification
Standard 2D NMR experiments [1H–1H correlated spectroscopy (COSY), 1H–1H total correlation spectroscopy (TOCSY), 1H–13C heteronuclear single quantum correlation (HSQC), and 1H–13C heteronuclear multiple bond correlation (HMBC)] and 1D selective 1H TOCSY experiments were performed in representative extract samples for the identification of the metabolites. Experiments were acquired with standard presaturation of the residual water peak during the relaxation delay using standard Bruker pulse sequences and routine conditions. All assigned metabolites were further confirmed by reported literature data (Rivas-Ubach et al. 2012) (see Table S1 of supporting information for identified metabolites with NMR.)
LC–MS analyses
LC–MS chromatograms were obtained using a Dionex Ultimate 3000 HPLC system (Thermo Fisher Scientific/Dionex RSLC, Dionex, Waltham, MA, USA) coupled to an LTQ Orbitrap XL high-resolution mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) equipped with an HESI II (heated electrospray ionization) source. A reversed-phase C18 Hypersil gold column (150 × 2.1 mm, 3-µm particle size; Thermo Scientific, Waltham, MA, USA) was used for chromatography at 30 °C. Mobile phases were filtered and degassed for 10 min in an ultrasonic bath prior to use, and consisted of 0.1% acetic acid in water (A) and acetonitrile (B). Sample injection volume was set at 5 µL. At a flow rate of 0.3 mL per minute, the elution gradient began at 90% A/10% B and was held for 5 min, and then, the elution changed linearly to 10% A/90% B during the next 15 min. The initial proportions (90% A/90% B) were thus linearly recovered over the next 5 min, and the column was washed and stabilized for 5 min. The Orbitrap operated in FTMS (Fourier Transform Mass Spectrometry) full-scan mode with a mass range of 50–1000 m/z and high-mass resolution (60,000). The resolution and sensitivity of the spectrometer were monitored by injecting a standard of caffeine after every ten samples, and the resolution was further monitored with lock masses (phthalates). All samples were injected twice, with the HESI operating in positive (+H) and (−H) ionization modes. Blank samples were also analyzed at the beginning of the sequence. (see Rivas-Ubach et al. (2016b) for details of LC–MS methods.)
Metabolomic data processing
NMR bucketing
The processing of 1H NMR spectra is detailed in Rivas-Ubach et al. (2013). First, all 1H NMR spectra were phased, baseline corrected, and referenced to the resonance of the internal standard (TSP) at δ 0.00 ppm with TOPSPIN 3.1. A variable-size bucketing was thus applied to all 1H NMR spectra with AMIX software (Bruker Biospin, Rheinstetten, Germany), scaling the buckets relative to the internal standard (TSP). The output was a data set containing the integral values of each 1H NMR peak in the described pattern. The buckets corresponding to the same molecular compound were summed.
LC–MS chromatograms
The LC–MS RAW data files were processed by MZmine 2.17 (Pluskal et al. 2010). LC–MS chromatograms were baseline corrected, and a list of ions was generated according to retention time and exact mass. Ion chromatograms were thus deconvoluted and posteriorly aligned, and auto-assigned, and numerical values of the area of the peaks were finally exported in CSV format (see Table S2 of supporting information for details). Metabolites were assigned by exact mass and retention time from the measurements of the standards in the spectrometer (see Table S3 of supporting information for details). Assignation of metabolites was based in two independent orthogonal data; exact mass of compounds and retention time (RT) relative to the standards. According to the Metabolomics Standards Initiative (Sumner et al. 2007), our LC–MS metabolomic results are thus based on putative identifications. However, the high-mass accuracy of the Orbitrap technology (< 10 ppm) and the use of RT reduce considerably the number of false positives (Rivas-Ubach et al. 2016b). As in NMR bucket tables, the different variables corresponding to the same molecular compounds were summed.
The area values obtained by the integration of the LC–MS chromatograms and 1H-NMR spectra are directly related to the concentration of the corresponding variable, even though they do not represent the real concentration in the sample. However, the use of those values is suitable for metabolomic comparative analyses as previously demonstrated in other studies (Lee and Fiehn 2013; Mari et al. 2013; Leiss et al. 2013; Gargallo-Garriga et al. 2014, 2015; Rivas-Ubach et al. 2016c, 2017). In this study, we use the term concentration when referring to changes in the relative amount of metabolites between the tested treatments.
Statistical analyses
The data set for this study was analyzed as a function of the categorical independent variable (treatment with two levels: complete and Fe-limited cultures) and 1451 dependent continuous variables. Of these dependent variables, 24 were GR, cell density, elemental concentrations (C, N, P, S, Fe, Ca, Mg, Mn, Zn, Cu, Ni, Co, Mo, and V), stoichiometric variables (C:N, C:P, and N:P), macromolecular variables (Protein, DNA, and RNA), and macromolecular ratios (RNA:DNA and protein:RNA). The other 1427 were metabolomic variables, of which 34 were identified. First, variables that were not normally distributed and/or lacked equal variances, assessed via the Shapiro–Wilk and Levene’s tests, respectively, were log10-transformed before statistical inference.
To test for overall significant differences between metabolomes of the complete and Fe-limited cultures, the metabolome fingerprints including the identified and the non-identified metabolites (1427 variables) were subjected to PERMANOVA using the Euclidean distance with treatment (complete and Fe-limited cultures) as fixed factor and permutations set at 10,000. In addition, to understand how the metabolomic and physiological variables shifted with Fe limitation, the whole data set (1451 variables) was subjected to principal component analysis (PCA). For visualization purposes, only known metabolomic variables are represented in the PCA (Fig. 1). As expected, the first PC separated complete from Fe-limited cases and only PC1 is shown in Fig. 1. The score coordinates of the samples were subjected to t test to determine statistical significance in the case separation between treatments (Rivas-Ubach et al. 2013).
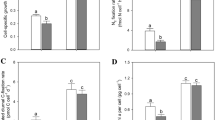
Principal component 1 (PC1) of the principal component analysis (PCA) of the elemental, stoichiometric, macromolecular, and metabolomic variables in Synechocystis sp. PCC 6803. a PC1 of the variables plot of complete versus Fe-limited cultures. Bars represent the loadings along the PC1 for each of the variables and have been represented in different colors. Growth rate (GR) and cell density are shown in orange. C, N, P, S, and C:N:P ratios are shown in red. Chlorophyll a, DNA, RNA, protein, and their ratios are shown in violet. Metals (Fe, Ca, Mg, Mn, Zn, Cu, Ni, Co, Mo, and V) are shown in grey. Different metabolite classes are also represented in different colors: blue: carbohydrates; cyan: nucleotides; green: amino acids; yellow: organic acids and brown: other secondary metabolites. Abbreviations have been used for several metabolites: adenosine monophosphate (AMP), uridine monophosphate (UMP), thymidine monophosphate (TMP), nicotinamide adenine dinucleotide (NAD+), alanine (Ala), arginine (Arg), methionine (Met), phenylalanine (Phe), leucine (Leu), proline (Pro), serine (Ser), threonine (Thr), tryptophan (Trp), tyrosine (Tyr), valine (Val), lysine (Lys), NMR overlapping signal of lysine and alanine (Lys.Ala), glutamine (Gln), glutamate (Glu), aspartic acid (Asp.ac), citric acid (Citric.ac), lactic acid (Lactic.ac), malic acid (Malic.ac), pyruvic acid (Pyruvic.ac), and succinic acid (Succinic.ac). b PC1 of the case plot categorized by treatment (blue: complete cultures; red: Fe-limited cultures). Variables marked with asterisks showed statistical significance (P < 0.05) and variables marked with crosses showed marginal statistical significance (P < 0.1) in t tests (Tables S4 and S5 of Supporting Information)
T tests were used to detect significant differences between treatments (complete versus Fe-limited) for each individual identified variable. The variables that did not meet the assumptions of t tests after log10 transformation were compared using a Mann–Whitney U test.
All statistical analyses were performed with R (R Core Team 2013). The Shapiro–Wilk tests, t tests, and Mann–Whitney U tests were performed with the functions shapiro.test, t.test, and wilcox.test, respectively, from the package “stats” (R Core Team 2013). Levene’s tests were performed with the leveneTest function in the “car” package (Fox and Weisberg 2011). The PERMANOVA analysis was conducted with adonis function in the package “vegan” (Oksanen et al. 2013). The PCA was performed by the pca function of the mixOmics package of R (Dejean et al. 2013).
Results
Growth of Synechocystis under Fe concentrations one-tenth of the normal composition of the BG-11 medium produced a blue shift in the chlorophyll absorbance peak from 679 to 673 nm (Figure S1), which is diagnosis of Fe limitation in Synechocystis (Ryan-Keogh et al. 2012).
PCA including all the elemental, macromolecular, and metabolomic variables showed clear separation between complete and Fe-limited cultures along the first principal component (PC1), which gathered 28.4% of the total variance (Fig. 1). This separation in the cases of each treatment along PC1 was statistically significant (t = 10.98; P < 0.001).
The growth rate, final cell density, and chlorophyll a content of cultures were significantly lower in Fe-limited than complete cultures (Fig. 1; Table S4). Fe limitation also resulted in significantly lower concentrations of C, N, P, S, Fe, Mg, Mn, Mo, RNA, and RNA:DNA ratios, and significantly higher Cu content, C:N, C:P, N:P and protein:RNA ratios (Fig. 1, Table S4).
The PERMANOVA indicated that the overall metabolomes of Fe-limited and complete Synechocystis varied significantly (P < 0.01) (Table 1). Several of the individual identified metabolites changed significantly between complete and Fe-limited cultures. Concentrations of α-glucose, leucine, lysine, phenylalanine, proline, valine, lactic acid, malic acid, succinic acid, and δ-tocopherol increased significantly under Fe limitation (P < 0.05) (Fig. 1; Table S5). Concentrations of disaccharides, hexoses, arginine, and aspartic acid showed marginally significant (P < 0.1) increases in Fe-limited cultures (Fig. 1; Table S5). AMP, glutamine, and threonine decreased significantly (P < 0.05) and β-glucose decreased marginally significantly (P < 0.1) in Fe-limited cultures.
We found significant effects on the metallome (Ca, Cu, Co, Fe, Mg, Mn, Mo, Ni, Zn, and V) of Synechocystis grown under Fe-limited conditions (Table 2; Table S4). Fe, Mg, Mn, and Mo contents and C:Fe, C:Mg, C:Mn, and C:Mo ratios decreased significantly under Fe limitation, while Cu content and C:Cu ratio increased significantly.
Discussion
Lowering the Fe concentration to one-tenth of the normal composition of BG-11 resulted in Fe limitation of Synechocystis, as diagnosed by a characteristic blue shift in the chlorophyll absorbance peak (Figure S1; Wilson et al. 2007). This shift likely indicates the association of the chlorophyll-binding protein, iron stress-inducible protein (IsiA) with PSI to increase the size of the light-harvesting complex and possibly compensate for phycobilisome degradation (Bibby et al. 2001; Ryan-Keogh et al. 2012).
We found that our cultures contained cells of Hymenobacter sp. through transcriptomic sequencing (Kellom et al. 2018) representing approximately 10% of the biomass across the cultures. However, this contamination should not be a major concern in our study. Organisms’ metabolomes have proven to present large plasticity among biological replicates (Steuer et al. 2003; Houshyani et al. 2012; Rivas-Ubach et al. 2016a). Furthermore, all the identified metabolites have been previously reported in Synechocystis sp. and our analyses are exclusively based on relative quantification and never in presence/absence. Therefore, the main differences between complete and Fe-limited are primarily due to the predominant Synechocystis biomass and any potential variation produced by Hymenobacter sp. would be masked in the overall metabolome variability. In addition, the transcriptome of Synechocystis under Fe limitation was similar to the previous studies (Kellom et al. 2018) and was concordant with our observed results. Even so, we restricted our main conclusions to those bolstered by reference to the previous studies.
Fe limitation and the growth rate hypothesis
The growth rate hypothesis (GRH) is one of the central paradigms in ecology and proposes that fast growing organisms increase the allocation of P to RNA to meet the high protein synthesis demand (Elser et al. 1996). Our stoichiometric and macromolecular results are in accordance with the GRH; Fe-limited Synechocystis showed slower growth than complete cultures and this was accompanied by decreases in RNA content and RNA:DNA ratio as well as the increases in C:P, N:P and protein:RNA ratios (Fig. 1, Table S4). The GRH has generally focused on conditions of N and/or P limitation or excess; however, our study suggests that other potential limiting factors such as Fe can also induce lower growth rates through mechanisms involving changes in cellular N:P and protein:RNA ratios.
Indirect N starvation produced by limitation of Fe
We observed potential links between metabolite abundances and biomass stoichiometry and note several metabolomic changes under Fe limitation suggesting a situation of N limitation. The lower C content in Fe-limited cultures (Fig. 1; Table S4) strongly indicates a reduction in C fixation capacity under lower Fe availability (Sharon et al. 2014). Although no Calvin Cycle intermediates were identified in the metabolomes, Fe-limited Synechocystis exhibited decreased gene expression of rbcS, encoding the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase (Kellom et al. 2018). With reduced C flux through the Calvin Cycle under Fe limitation, there may have been a decrease in the direct precursor of purines, ribose-5-phosphate, resulting in the observed reduction in AMP and other purine metabolites (Lengeler et al. 1999) (Fig. 1) as observed in N-starved Synechocystis sp. (Osanai et al. 2014).
We found significant changes in the abundances of some carbohydrates and tricarboxylic acid cycle intermediates under Fe limitation (Fig. 1; Table S5) that are similar to responses to N limitation. It has been observed that Synechocystis sp. and other cyanobacteria accumulate carbohydrates and precursors of glycogen (i.e., glucose and other hexoses increased in Fe-limited cultures in this study) under N deprivation (Gründel et al. 2012; Hasunuma et al. 2013; Osanai et al. 2014). Succinate is both a respiratory substrate and a C substrate for biosynthesis. The decreased C content of Fe-limited along with higher concentrations of succinate and malate (Fig. 1; Table S5) suggests that succinate was oxidized to malate via fumarate under limitation of Fe (Cooley and Vermaas 2001; Knoop et al. 2013). N starvation has proven to elicit a similar response in Synechocystis sp. with substantial increases in succinate, malate, and fumarate (Osanai et al. 2014).
The significant reduction in N content and the increases in C:N ratio in Fe-limited cultures indicate a state of N limitation, despite the high nitrate availability (17.6 mM). The negative effect of Fe limitation on N uptake is feasible, as Synechocystis sp. and other cyanobacteria down-regulate metabolites associated with N assimilation under C limitation (Huege et al. 2011). The concentrations of many amino acids were significantly reduced in Fe-limited cultures (Fig. 1; Table S5) which also observed under N starvation (Osanai et al. 2014). Although Fe-limited stoichiometric and metabolomic responses suggest a status of N limitation, macromolecular analyses indicated that protein synthesis did not decrease under Fe limitation (Fig. 1; Table S4). More focused research is necessary to better comprehend how N is allocated to different physiological functions under Fe limitation and how this could consequently impact the N cycle.
Fe limitation and metabolites indicative of oxidative stress
Oxygen radicals produced as a byproduct of photosynthesis and reduced Fe can interact leading to oxidative stress through the Fenton Reaction (Latifi et al. 2009). Transcriptomic studies in Synechocystis sp. have previously linked Fe and oxidative stress (Singh et al. 2004; Shcolnick et al. 2009). Oxidative stress has also been described under Fe limitation because of the redox imbalance between PS I and PS II and the reduced availability of Fe as a cofactor for superoxide dismutase and catalase (Latifi et al. 2009). Fe-limited cultures showed significant concentration increases of metabolites with antioxidant function (δ-tocopherol, choline, and proline) (Fig. 1; Table S5) suggesting a state of oxidative stress under Fe limitation. Tocopherols concentration has already shown to be increased to cope with lipid peroxidation in Synechocystis sp. (Maeda et al. 2005). We also found increases in proline under Fe limitation which has been described to increase in other cyanobacteria and plants in response to oxidative stress induced by heavy metal toxicity and to cope with increased salinity (Singh et al. 1996; Szabados and Savouré 2010). However, to the best of our knowledge, increases in proline in Synechocystis sp. as response to oxidative stress produced by Fe limitation has not been previously reported yet. Choline accumulation has been described in salt acclimation responses of cyanobacteria (Hagemann 2011); however, choline is also a precursor of glycine betaine which stabilizes proteins of the oxygen-evolving complex of PS II (Papageorgiou and Murata 1995). In addition, Synechocystis sp. is genetically competent for D-lactate production via pyruvate reduction (D-lactate dehydrogenase encoded by slr1556) (Kaneko et al. 1996) and we found increases in lactate concentrations in Fe-limited cultures (Fig. 1; Table S5). With decreased C uptake and potentially less consumption of NAD(P)H, the NAD(P)H/NAD(P)+ ratio of cells could be extremely imbalanced under Fe limitation. Our metabolomic analyses suggest that the higher concentration of lactate in Fe-limited cultures could have been the consequence to consume excess NADH and regenerate NAD+ as in fermentative metabolism (Lengeler et al. 1999). The production of antioxidants and lactate production would allow Synechocystis sp. to cope with a potential imbalanced NAD(P)H/NAD(P)+ ratio, indicating that responses to Fe limitation and redox homeostasis overlap in Synechocystis sp. Complementary analyses to our metabolomics measurements would be required to experimentally measure the cellular NAD(P)H/NAD(P)+ to definitely verify and determine more precisely the redox status of cyanobacteria under Fe limitation.
Flexibility in the metal composition of Synechocystis sp
Several trace metals have biologically important roles in photosynthetic and other metabolic processes, such as N assimilation. The differences in metal content between Fe-limited and complete cultures support a view that the metal composition of Synechocystis sp. is highly responsive to environmental conditions.
Low availability of Fe impacts the photosynthetic machinery of cyanobacteria through phycobilisome degradation, decreased chlorophyll content, and changes in the PETC (Ferreira and Straus 1994; Behrenfeld and Milligan 2013). Under Fe-limited conditions, cyanobacteria may partially reduce their need for this metal by replacement of Fe-containing redox proteins (cytochrome c553 and ferredoxin) with non-Fe dependent carriers (Cu-containing plastocyanin and FMN-containing flavodoxin, respectively) (Ferreira and Straus 1994). We observed that Fe-limited cultures in this study increased the expression of the genes encoding plastocyanin and flavodoxin (Kellom et al. 2018). Our metallomic analyses showed significant decreases (approximately 3 times) in cellular Fe content and Fe:C ratio and increases in Cu content and Cu:C ratio under Fe limitation (Fig. 1, Table 2, Table S4), which is in agreement with the overexpression of plastocyanin and flavodoxin under reduced Fe availability. A previous study of metal contents in Synechocystis sp. under Fe limitation did not find a significant effect on cellular Cu content; however, Fe limitation in that study was achieved through addition of a Fe-chelating agent, deferoxamine B (Shcolnick et al. 2009), which may also bind to Cu (Farkas et al. 1997) and limit its bioavailability. These contrasting effects of Fe limitation on Cu uptake by Synechocystis sp. suggest that the method of lowering metal availability should be considered in the experimental design of trace metal limitation studies.
The lower cellular Mn content in Fe-limited cells is in line with the previous studies that show Fe bioavailability affects Mn uptake (Shcolnick et al. 2009; Sharon et al. 2014). The Mn concentration (9.2 μM) of normal composition BG-11 medium is sufficient for Synechocystis sp. (Ogawa et al. 2002); thus, Mn was not limiting under the growth conditions used in this study. Mn, as a cofactor in the oxygen-evolving complex of PS II is required for oxygenic photosynthesis (Shcolnick and Keren 2006) and the activity of PS II decreases under Fe limitation in Synechocystis sp. (Ryan-Keogh et al. 2012; Sharon et al. 2014). Genes encoding PSII components were strongly down-regulated in Fe-limited cultures (Kellom et al. 2018).
Other trace metals showed a response to Fe limitation. Fe-limited cultures also had lower chlorophyll a content (Fig. 1, Table S4), suggesting thus a lower cellular demand for Mg. In addition, Mo is a key metal cofactor of enzymes involved in N assimilation (e.g., nitrate reductase). Fe-limited Synechocystis showed significant reductions of Mo content and Mo:C ration (Fig. 1, Table S4) suggesting certain coordination between decreased Mo and N uptake (Flores et al. 2005). This could be explained by the reduced Mo demand due the slower growth under Fe limitation.
Conclusions and potential implications in natural ecosystems
The C:N:P stoichiometry and macromolecule composition of Synechocystis sp. changed significantly under Fe limitation in ways that are consistent with the predictions of the GRH (Elser et al. 1996) (Fig. 2). This shows that limiting factors other than macronutrients, such as Fe, may also induce lower growth rates through mechanisms involving changes in cellular N:P and protein:RNA ratios. More work is needed to define the range of elemental and biological macromolecule compositions that Synechocystis produces under various conditions of nutrient availability and growth rates as this would permit insight into how growth responses of cyanobacteria in natural environments might alter the coupling of major biogeochemical cycles (C, N, P, and Fe).
Our combined metabolomic, metallomic, stoichiometric, and macromolecular results show that Fe limitation responses of Synechocystis overlap with C and N limitation responses (Fig. 2). Fe-limited Synechocystis also exhibited responses at the metabolomic level that indicate a state of oxidative stress and imbalance in the redox state of the cells. These insights aid in diagnosing the nutritional state of Synechocystis sp. in natural ecosystems. Microbial physiology underlies many biogeochemical processes and plays a crucial role in aquatic ecosystems (DeLong and Karl 2005). Enhancements of Fe limitation in natural aquatic ecosystems may result in significant impacts on their communities. Increases in the dissolution of CO2 (Orr et al. 2005; Shi et al. 2012), nitric acid, and/or ammonium (Vitousek et al. 1997; Bowman et al. 2008) in natural waters are potential drivers of acidification that could lead to significant reductions of Fe bioavailability for cyanobacteria (Shi et al. 2010, 2012). The understanding of metabolic changes of cyanobacteria under Fe limitation thus provides crucial insights into how those shifts in cells potentially scale to changes in ecosystem function by cascade effects through trophic webs (Fig. 2).
Author contribution statement
AR-U and AP-P performed the research and performed the statistical analyses. AR-U and AP-P wrote the manuscript. AR-U, AP-P, JE, JP, and JS designed the research. AP-P performed the elemental analyses. MO, OU, MP-T, and CL-Q performed the metabolomic analyses with LC–MS and NMR. JP, JS, and JE funded the research. All authors have read, commented, and approved the final version of the manuscript.
References
Allen MM (1968) Simple conditions for growth of unicellular blue-green algae on plates. J Phycol 4:1–4
Baniulis D, Yamashita E, Zhang H, Hasan SS, Cramer WA (2008) Structure–function of the cytochrome b 6 f complex. Photochem Photobiol 84:1349–1358
Behrenfeld MJ (1999) Widespread iron limitation of phytoplankton in the south Pacific Ocean. Science 283:840–843
Behrenfeld MJ, Milligan AJ (2013) Photophysiological expressions of iron stress in phytoplankton. Annu Rev Mar Sci 5:217–246
Behrenfeld M, Bale A, Kolber Z et al (1996) Confirmation of iron limitation of phytoplankton photosynthesis in the equatorial Pacific-Ocean. Nature 383:508–511
Bellenger J-P, Wichard T, Xu Y, Kraepiel AML (2011) Essential metals for nitrogen fixation in a free-living N2-fixing bacterium: chelation, homeostasis and high use efficiency. Environ Microbiol 13:1395–1411
Bennette NB, Eng JF, Dismukes GC (2011) An LC–MS-based chemical and analytical method for targeted metabolite quantification in the model cyanobacterium Synechococcus sp. PCC 7002. Anal Chem 83:3808–3816
Bibby TS, Nield J, Barber J (2001) Iron deficiency induces the formation of an antenna ring around trimeric photosystem I in cyanobacteria. Nature 412:743–745
Bowman WD, Cleveland CC, Halada Ĺ et al (2008) Negative impact of nitrogen deposition on soil buffering capacity. Nat Geosci 1:767–770
Castielli O, De la Cerda B, Navarro JA et al (2009) Proteomic analyses of the response of cyanobacteria to different stress conditions. FEBS Lett 583:1753–1758
Cooley JW, Vermaas WF (2001) Succinate dehydrogenase and other respiratory pathways in thylakoid membranes of Synechocystis sp. strain PCC 6803: capacity comparisons and physiological function. J Bacteriol 183:4251–4258
da Silva JJRF, Williams RJP (2001) The biological chemistry of the elements: the inorganic chemistry of life. Oxford University Press, New York, pp 1–583. ISBN: 0198508484
Dang TC, Fujii M, Rose AL et al (2012) Characteristics of the freshwater cyanobacterium Microcystis aeruginosa grown in iron-limited continuous culture. Appl Environ Microbiol 78:1574–1583
Dejean S, Gonzalez I, Le Cao K (2013) mixOmics: Omics Data Integration Project. R package version 5.0-1. http://CRAN.R-project.org/package=mixOmics
DeLong EF, Karl DM (2005) Genomic perspectives in microbial oceanography. Nature 437:336–342
Elser JJ, Dobberfuhl DR, MacKay NA, Schampel JH (1996) Organism size, life history, and N:P stoichiometry. Bioscience 46:674–684
Elser JJ, Sterner RW, Gorokhova E et al (2008) Biological stoichiometry from genes to ecosystems. Ecol Lett 3:540–550
Farkas E, Csóka H, Micera G, Dessi A (1997) Copper(II), nickel(II), zinc(II), and molybdenum(VI) complexes of desferrioxamine B in aqueous solution. J Inorg Biochem 65:281–286
Ferreira F, Straus NA (1994) Iron deprivation in cyanobacteria. J Appl Phycol 6:199–210
Fiehn O (2002) Metabolomics - the link between genotypes and phenotypes. Plant Mol Biol 48:155–171
Flores E, Frías JE, Rubio LM, Herrero A (2005) Photosynthetic nitrate assimilation in cyanobacteria. Photosynth Res 83:117–133
Fox J, Weisberg S (2011) An R companion to applied regression. R package, 2nd edn. Sage, Thousand Oaks CA. http://socserv.socsci.mcmaster.ca/jfox/Books/Companion
Gargallo-Garriga A, Sardans J, Pérez-Trujillo M et al (2014) Opposite metabolic responses of shoots and roots to drought. Sci Rep 4:6829
Gargallo-Garriga A, Sardans J, Pérez-Trujillo M et al (2015) Warming differentially influences the effects of drought on stoichiometry and metabolomics in shoots and roots. New Phytol 207:591–603
Gründel M, Scheunemann R, Lockau W, Zilliges Y (2012) Impaired glycogen synthesis causes metabolic overflow reactions and affects stress responses in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology 158:3032–3043
Hagemann M (2011) Molecular biology of cyanobacterial salt acclimation. FEMS Microbiol Rev 35:87–123
Hasunuma T, Kikuyama F, Matsuda M et al (2013) Dynamic metabolic profiling of cyanobacterial glycogen biosynthesis under conditions of nitrate depletion. J Exp Bot 64:2943–2954
Hernández-Prieto MA, Schön V, Georg J et al (2012) Iron deprivation in Synechocystis: inference of pathways, non-coding RNAs, and regulatory elements from comprehensive expression profiling. G3 (Bethesda) 2:1475–1495
Houshyani B, Kabouw P, Muth D et al (2012) Characterization of the natural variation in Arabidopsis thaliana metabolome by the analysis of metabolic distance. Metabolomics 8:131–145
Huege J, Goetze J, Schwarz D et al (2011) Modulation of the major paths of carbon in photorespiratory mutants of synechocystis. PLoS One 6:e16278
Jones OAH, Maguire ML, Griffin JL et al (2013) Metabolomics and its use in ecology. Austral Ecol 38:713–720
Kaneko T, Sato S, Kotani H et al (1996) Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res 3:109–136
Katoh H, Hagino N, Grossman AR, Ogawa T (2001a) Genes essential to iron transport in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol 183:2779–2784
Katoh H, Hagino N, Ogawa T (2001b) Iron-binding activity of FutA1 subunit of an ABC-type iron transporter in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Cell Physiol 42:823–827
Kellom M, Poret-Peterson AT, Rivas-Ubach A et al (2018) Transcriptomics of iron limitation without growth media chelation in the cyanobacterium Synechocystis sp. PCC 6803 (Submitted)
Keren N, Aurora R, Pakrasi HB (2004) Critical roles of bacterioferritins in iron storage and proliferation of cyanobacteria. Plant Physiol 135:1666–1673
Knoop H, Gründel M, Zilliges Y et al (2013) Flux balance analysis of cyanobacterial metabolism: the metabolic network of Synechocystis sp. PCC 6803. PLoS Comput Biol 9:e1003081
Kopf M, Klähn S, Scholz I et al (2014) Comparative analysis of the primary transcriptome of Synechocystis sp. PCC 6803. DNA Res 21:527–539
Kranzler C, Lis H, Finkel OM et al (2014) Coordinated transporter activity shapes high-affinity iron acquisition in cyanobacteria. ISME J 8:409–417
Krieg N, Parte A, Ludwig W et al (2010) Bergey’s manual of systematic bacteriology, vol 4. The Bacteroidetes, Spirochaetes, Tenericutes (Mollicutes), Acidobacteria, Fibrobacteres, Fusobacteria, Dictyoglomi, Gemmatimonadetes, Lentisphaerae, Verrucomicrobia, Chlamydiae, and Planctomycetes. Springer, New York
Latifi A, Ruiz M, Zhang C-C (2009) Oxidative stress in cyanobacteria. FEMS Microbiol Rev 33:258–278
Lee DY, Fiehn O (2013) Metabolomic response of Chlamydomonas reinhardtii to the inhibition of target of rapamycin (TOR) by rapamycin. J Microbiol Biotechnol 23:923–931
Leiss KA, Cristofori G, van Steenis R et al (2013) An eco-metabolomic study of host plant resistance to Western flower thrips in cultivated, biofortified and wild carrots. Phytochemistry 93:63–70
Lengeler J, Drews G, Schlegel H (1999) Biology of the prokaryotes. Wiley-Blackwell, Hoboken, pp 1–984
Macel M, Van Dam NM, Keurentjes JJB (2010) Metabolomics: the chemistry between ecology and genetics. Mol Ecol Resour 10:583–593
Mackey KRM, Post AF, McIlvin MR et al (2015) Divergent responses of Atlantic coastal and oceanic Synechococcus to iron limitation. Proc Natl Acad Sci USA 112:9944–9949
Maeda H, Sakuragi Y, Bryant DA, Dellapenna D (2005) Tocopherols protect Synechocystis sp. strain PCC 6803 from lipid peroxidation. Plant Physiol 138:1422–1435
Mari A, Lyon D, Fragner L et al (2013) Phytochemical composition of Potentilla anserina L. analyzed by an integrative GC–MS and LC–MS metabolomics platform. Metabolomics 9:599–607
Ogawa T, Bao DH, Katoh H et al (2002) A two-component signal transduction pathway regulates manganese homeostasis in Synechocystis 6803, a photosynthetic organism. J Biol Chem 277:28981–28986
Oksanen J, Guillaume-Blanchet F, Kindt R et al (2013) vegan: community ecology package. R package version 2.0-9. http://CRAN.R-project.org/package=vegan
Orr JC, Fabry VJ, Aumont O et al (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437:681–686
Osanai T, Oikawa A, Shirai T et al (2014) Capillary electrophoresis-mass spectrometry reveals the distribution of carbon metabolites during nitrogen starvation in Synechocystis sp. PCC 6803. Environ Microbiol 16:512–524
Papageorgiou GC, Murata N (1995) The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygen-evolving Photosystem II complex. Photosynth Res 44:243–252
Peñuelas J, Sardans J (2009) Ecological metabolomics. Chem Ecol 25:305–309
Pluskal T, Castillo S, Villar-Briones A, Orešič M (2010) MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform 11:395
R Core Team (2013) R: a language and environment for statistical computing. R package
Raven JA, Evans MCW, Korb RE (1999) The role of trace metals in photosynthetic electron transport in O2-evolving organisms. Photosynth Res 60:111–150
Richardson DJ (2000) Bacterial respiration: a flexible process for a changing environment. Microbiology 146(Pt 3):551–571
Rivas-Ubach A, Sardans J, Pérez-Trujillo M et al (2012) Strong relationship between elemental stoichiometry and metabolome in plants. Proc Natl Acad Sci 109:4181–4186
Rivas-Ubach A, Pérez-Trujillo M, Sardans J et al (2013) Ecometabolomics: optimized NMR-based method. Methods Ecol Evol 4:464–473
Rivas-Ubach A, Gargallo-Garriga A, Sardans J et al (2014) Drought enhances folivory by shifting foliar metabolomes in Quercus ilex trees. New Phytol 202:874–885
Rivas-Ubach A, Barbeta A, Sardans J et al (2016a) Topsoil depth substantially influences the responses to drought of the foliar metabolomes of Mediterranean forests. Perspect Plant Ecol Evol Syst 21:41–54
Rivas-Ubach A, Sardans J, Hódar JA et al (2016b) Similar local but different systemic metabolomic responses of closely related pine subspecies to folivory by caterpillars of the processionary moth. Plant Biol 18:484–494
Rivas-Ubach A, Hódar JA, Sardans J et al (2016c) Are the metabolic responses to folivory of closely related plant species linked to macroevolutionary and plant-folivore coevolutionary processes? Ecol Evol 6:4372–4386
Rivas-Ubach A, Sardans J, Hódar JA et al (2017) Close and distant: contrasting the metabolism of two closely related subspecies of Scots pine under the effects of folivory and summer drought. Ecol Evol. https://doi.org/10.1002/ece3.3343
Ryan-Keogh TJ, Macey AI, Cockshutt AM et al (2012) The cyanobacterial chlorophyll-binding-protein IsiA acts to increase the in vivo effective absorption cross-section of psi under iron limitation. J Phycol 48:145–154
Sharon S, Salomon E, Kranzler C et al (2014) The hierarchy of transition metal homeostasis: iron controls manganese accumulation in a unicellular cyanobacterium. Biochim Biophys Acta 1837:1990–1997
Shcolnick S, Keren N (2006) Metal homeostasis in cyanobacteria and chloroplasts. Balancing benefits and risks to the photosynthetic apparatus. Plant Physiol 141:805–810
Shcolnick S, Summerfield TC, Reytman L et al (2009) The mechanism of iron homeostasis in the unicellular cyanobacterium Synechocystis sp. PCC 6803 and its relationship to oxidative stress. Plant Physiol 150:2045–2056
Shi D, Xu Y, Hopkinson BM, Morel FMM (2010) Effect of ocean acidification on iron availability to marine phytoplankton. Science 327:676–679
Shi D, Kranz SA, Kim J-M, Morel FMM (2012) Ocean acidification slows nitrogen fixation and growth in the dominant diazotroph Trichodesmium under low-iron conditions. Proc Natl Acad Sci USA 109:E3094–E3100
Singh AK, Chakravarthy D, Singh TPK, Singh HN (1996) Evidence for a role for l-proline as a salinity protectant in the cyanobacterium Nostoc muscorum. Plant Cell Environ 19:490–494
Singh AK, Li H, Sherman LA (2004) Microarray analysis and redox control of gene expression in the cyanobacterium Synechocystis sp. PCC 6803. Physiol Plant 120:27–35
Sterner R, Elser J (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton, pp 1–439. ISBN: 0691074909
Steuer R, Kurths J, Fiehn O, Weckwerth W (2003) Observing and interpreting correlations in metabolomic networks. Bioinformatics 19:1019–1026
Sumner LW, Amberg A, Barrett D et al (2007) Proposed minimum reporting standards for chemical analysis. Metabolomics 3:211–221
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
t’Kindt R, De Veylder L, Storme M et al (2008) LC–MS metabolic profiling of Arabidopsis thaliana plant leaves and cell cultures: optimization of pre-LC–MS procedure parameters. J Chromatogr B Analyt Technol Biomed Life Sci 871:37–43
Tovar-Sanchez A, Sañudo-Wilhelmy SA, Garcia-Vargas M et al (2003) A trace metal clean reagent to remove surface-bound iron from marine phytoplankton. Mar Chem 82:91–99
Vitousek PM, Aber JD, Howarth RW et al (1997) Human alteration of the global nitrogen cycle: sources and cosequences. Ecol Appl 7:737–750
Vrede T, Tranvik LJ (2006) Iron constraints on planktonic primary production in oligotrophic lakes. Ecosystems 9:1094–1105
Wilson A, Boulay C, Wilde A et al (2007) Light-induced energy dissipation in iron-starved cyanobacteria: roles of OCP and IsiA proteins. Plant Cell 19:656–672
Acknowledgements
The authors thank Laia Mateu-Castell, Laura Steger, Zarraz Lee, Jessica Corman, Krist Rouypirom, Zureyma Martinez, Matthew Kellom, Wei Deng, and Jennifer Learned for their laboratory support. Thanks to Ravi Vannela for providing Synechocystis sp. PCC 6803 and Wim Vermaas for the helpful discussion of the data. ARU appreciates the financial support of the research fellowship (JAE) from the CSIC. This research was supported by the Spanish Government Project CGL2013-48074-P and the Catalan Government Project SGR 2014-274, the European Research Council (Synergy Grant SyG-2013-610028, IMBALANCE-P), and the NASA Astrobiology Institute at Arizona State University (Follow the Elements; NAI5-0018). A portion of the research was performed using EMSL, a DOE Office of Science User Facility sponsored by the Office of Biological and Environmental Research at the Pacific Northwest National Laboratory. MO and OU were supported by the Ministry of Education, the National Sustainability Program I (NPU I), grants LO1415 and LM2015061, and by CzeCOS ProCES project num. CZ.02.1.01/0.0/0.0/16_013/0001609. ATPP and JJE were supported by the ASU NASA Astrobiology Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Baczek-Kwinta.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rivas-Ubach, A., Poret-Peterson, A.T., Peñuelas, J. et al. Coping with iron limitation: a metabolomic study of Synechocystis sp. PCC 6803. Acta Physiol Plant 40, 28 (2018). https://doi.org/10.1007/s11738-018-2603-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2603-1