Abstract
Anthocyanins are natural bioactive pigments in plants that play important roles in many physiological functions. They are found in various tissues and can protect plants against different stress conditions. Anthocyanins are synthesized and accumulate in nutritional organs, which is crucial for plants to adapt to and resist adverse environmental conditions, including high exposure to light, ultraviolet light, low temperatures, drought, pests and disease. Some progress has been made in understanding the adaptability of anthocyanin to the external environment. Begonia semperflorens is an excellent model for studying the function and regulation of anthocyanin synthesis. To investigate the biosynthesis and regulation of anthocyanins, RNA sequencing techniques were employed to investigate anthocyanin biosynthesis induced by low temperature in B. semperflorens leaves. A total of 74,779 unigenes with a mean length of 1249 bp were assembled. Functional annotations were implemented using five protein databases. Differentially expressed genes involved in the process of anthocyanin biosynthesis were identified. This study represents the first report of a broad-scale gene expression study on B. semperflorens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthocyanins are a group of colourful bioactive pigments that contribute to the display of red-to-purple colours in various plant tissues, including leaves, stems, roots, fruit and tissues relating to the flower (Lingua et al. 2013). Anthocyanins also play important physiological and ecological roles in plants by attracting pollinators and seed dispersers, protecting plants from irradiation due to intense light exposure and scavenging free radicals produced in cells under stressful conditions (Zhang et al. 2011a, b). Anthocyanins are usually biosynthesized in specific developmental stages (e.g. the juvenile or senescent phase) and in specific plant tissues (e.g. in flowers, fruits and seeds). Anthocyanin biosynthesis is induced by many biotic and abiotic stressors, including nutrient deficiency, low temperature, intense light exposure, wounding and pathogen infection (Zhang et al. 2010a, b; Xie et al. 2012; Van den Ende and El-Esawe 2014; Fogelman et al. 2015; Sivankalyani et al. 2016).
During recent decades, numerous studies have focused on the biosynthesis of anthocyanin in various plants. To date, all known pathways and genes involved in anthocyanin biosynthesis have been characterized in the model plant Arabidopsis thaliana using molecular, genetic and biochemical data. Researchers have revealed that the WD40-bHLH-MYB (WBM) transcription factor complexes regulate the biosynthesis of anthocyanin under light conditions in this model (Stracke et al. 2010; Petroni and Tonelli 2011).
Begonia semperflorens is native to South America and has naturalized elsewhere in the tropics and subtropics (Golding and Wasshausen 2002). Since it is a perpetual perennial plant, it is widely used in gardening. The leaves of B. semperflorens vary between different varieties, from copper and green to variegated colours. The copper leaf phenotype has been found to be rich in anthocyanin and thrives with bright colour in sunny conditions. The leaf edge of the green leaf phenotype has been observed to turn red in the sun and is thought to easily accumulate anthocyanin under circumstantial conditions such as low temperature, high temperature and nitrogen deficiency. With such properties, B. semperflorens is an excellent model to study the function and regulation of anthocyanin synthesis (Zhang et al. 2013, 2016).
Until now, few studies have focused on B. semperflorens, especially at the molecular level. In earlier research, we found that surplus photosynthate in B. semperflorens could induce up-regulation of several critical enzymes (e.g. phenylalanine ammonialyase PAL, chalcone isomerase CHI, dihydroflavonol 4-reductase DFR, and flavonoid 3-O-glucosyltransferase UFGT) in the anthocyanin biosynthesis pathway and as a result could induce anthocyanin biosynthesis. Excess carbohydrate was also found to trigger the bioprocess (Zhang et al. 2013). In our later research, we observed that, during low-temperature conditions, the colours of B. semperflorens cultivars turn red, indicating that anthocyanin synthesis occurs in the plant in such circumstances (Zhang et al. 2013). We predict that low temperature is closely related to anthocyanin synthesis and the induction of anthocyanin synthesis can protect plant photosynthetic organs chloroplast from the damage of the relative excess light energy under low temperature. To understand the molecular mechanisms of this biosynthesis process in B. semperflorens, in this study the transcriptomes of B. semperflorens under low temperature (LT) and normal growing temperature (CK) were analysed. To the best of our knowledge, this represents the first report of the broad-scale transcriptome of B. semperflorens.
Begonia semperflorens belongs to the Family: Begoniaceae, Order: Violales. There are many ornamental plants in Begoniaceae, and related research about species belonging to the Begoniaceae is rare. Studying the transcriptome of B. semperflorens will contribute to our current understanding of the genetic background of many plants in the Violales taxonomic order.
Materials and methods
Plant material
Seeds of B. semperflorens ‘Super Olympia’ were germinated in a mixture of peat and vermiculite (7:3, v/v) in a growth chamber (10-h day length, 100 mol m−2 s−1 photosynthetic photon flux density (PPFD), 25/15 °C day/night temperatures and 100% relative humidity). The seedlings were watered daily and fertilized weekly with Hoagland’s nutrient solution. Approximately 30 days after germination, seedlings were selected and transferred to pots with a growth medium of peat, vermiculite and perlite (6:3:1, v/v/v). Normal control and low-temperature-treated samples were named CK and LT, respectively. CK plants were cultured under 25/15 °C (day/night) and 300 µmol m−2 s−1 intensity light for 10 h with 95% relative humidity every day. LT plants were cultured under 15/5 °C (day/night) and 300 µmol m−2 s−1 intensity light for 10 h with 95% relative humidity every day. After 12 days, the second pair of leaves growing from the apex of the plant of each sample was picked. Four parallel samples from four separate plants for each condition were prepared. All samples were harvested at the same time. At least 0.3 g of leaves was prepared for each sample and immediately transferred and snap-frozen in liquid nitrogen. After that, they were stored at − 80 °C for RNA extraction.
Anthocyanin quantification
The anthocyanin content was determined according to the methods described by Mita et al. (1997) with minor modifications. Frozen leaf tissue (300 mg) was homogenized in 3 ml of 1% (v/v) HCl:MeOH at 4 °C for 1 day. Following centrifugation at 3500×g for 15 min, the supernatant was measured spectrophotometrically at 530 and 657 nm. One unit of anthocyanins equals one absorbance unit (A530–A657 × 1/4) per ml of extraction solution.
RNA isolation, library preparation and sequencing
Total RNA was isolated from plant cells with a Quick RNA isolation kit (Bioteke Corporation, Beijing, China) according to the manufacturer’s protocol, followed by RNA purification using RNase-free DNase I (Takara, Dalian, China) in order to remove the genomic DNA contaminant. Thereafter, RNA degradation and contamination were monitored through electrophoresis on a 1% agarose gel. The NanoPhotometer® spectrophotometer (IMPLEN, Westlake Village, CA, USA) was used to check RNA purity. RNA concentration was measured using Qubit® RNA Assay Kit in Qubit®2.0 Fluorometer (Life Technologies, CA, USA). RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, Palo Alto, CA, USA). Three micrograms of total RNA per sample was pooled and used for subsequent RNA-Seq. Sequencing libraries were generated using the NEBNext®Ultra™ RNA Library Prep Kit for Illumina® (NEB, Ipswich, MA, USA) following the manufacturer’s recommendations, and index codes were added to attribute sequences to each sample. We purified mRNA from total RNA using poly-T oligo-attached magnetic beads. Fragmentation was carried out using divalent cations under elevated temperature in NEB Next First Strand Synthesis Reaction Buffer (5×). First strand cDNA was synthesized using a random hexamer primer and M-MuLV Reverse Transcriptase (RNase H−). Second strand cDNA synthesis was subsequently performed using DNA Polymerase I and RNase H. Remaining overhangs were converted into blunt ends via exonuclease/polymerase digestion. After adenylation of 3′ ends of DNA fragments, NEB Next Adaptor with hairpin loop structure was ligated to prepare for hybridization. To select cDNA fragments preferentially 150–200 bp in length, the library fragments were purified using the AMPure XP system (Beckman Coulter, Beverly, MA, USA). Then, 3 μl of USER Enzyme (NEB) was used with the size-selected adaptor-ligated cDNA at 37 °C for 15 min followed by 5 min at 95 °C before PCR. Then, PCR was performed with Phusion High-Fidelity DNA polymerase, Universal PCR primers and Index (X) Primer. At last, the PCR products were purified using the AMPure XP system, and library quality was assessed on the Agilent Bioanalyzer 2100 system.
The clustering of the index-coded samples was performed on a cBot Cluster Generation System using TruSeq PE Cluster Kit v3-cBot-HS (Illumina) according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina Hiseq 2000 platform and paired-end reads were generated.
Quality control, transcriptome assembly and gene functional annotation
Raw data (raw reads) in fastq format were first processed through in-house Perl scripts. In this step, clean data (clean reads) were obtained by removing reads containing adapter, reads containing poly-N and low-quality reads from raw data. At the same time, Q20, Q30, GC-content and sequence duplication level of the clean data were calculated. All the downstream analyses were based on clean data with high quality. The left files (read1 files) from all libraries/samples were pooled into one left.fq file, and the right files (read2 files) into one right.fq file. Transcriptome assembly was accomplished based on the left.fq and right.fq using Trinity (Grabherr et al. 2011) with min_kmer_cov set to 2 by default and all other parameters set to default. Contig is the seq extended by k-mer with k − 1 overlap. The k is 25. The filtered data were deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under the Accession Numbers SRS1381787 and SRS1396330. BLASTX alignment (Altschul et al. 1997) with an E value ≤ 10−5 was used to implement functional annotation between unigenes and protein databases. Protein databases, including the Nr (ftp://ncbi.nih.gov/blast/db/nr), Swiss-Prot (http://www.ebi.ac.uk/swissprot/), KEGG (http://www.genome.jp/kegg/), COG (http://www.ncbi.nlm.nih.gov/COG/) and GO (http://geneontology.org/) databases, were used for BLAST search and annotation.
Expression annotation
Gene expression levels were determined by RSEM (RNA-Seq by Expectation Maximization) (Li and Dewey 2011) for each sample: clean data were mapped back onto the assembled transcriptome by bowtie software with parameters of “-q –phred33-quals -n 2-a -m 300 s”, and the read count for each gene was obtained from the mapping results. Prior to differential gene expression analysis, for each sequenced library, the read counts were adjusted by the edgeR program package through one scaling normalized factor. Differential expression analysis of two samples was performed using the DEGseq (Anders and Huber 2010) R package. P-values were adjusted using qvalue (Broberg 2005). The false discovery rate (FDR) ≤ 0.01 coupled with the absolute value of log2 (fold change) ≥ 1 was set as the threshold for differential expression, and FDR ≤ 0.01 coupled with the absolute value of log2 (fold change) ≥ 2 was set as the threshold for significantly differential expression.
GO enrichment analysis of the differentially expressed genes (DEGs) was implemented by the GOseq R packages-based Wallenius non-central hyper-geometric distribution (Young et al. 2010), which can adjust for gene length bias in DEGs. KEGG (Kanehisa et al. 2008) is a resource database that utilizes molecular information, especially large-scale molecular datasets generated by genome sequencing and other high-throughput experimental technologies, for understanding high-level functions and utilities of biological systems, such as the cell, the organism and the ecosystem (http://www.genome.jp/kegg/). We used KOBAS (Mao et al. 2005) software to test the statistical enrichment of differentially expressed genes in KEGG pathways.
Quantitative real-time reverse transcription PCR (qRT-PCR)
Using qRT-PCR, we selected three anthocyanin-related genes (BsF3H, BsPAL and BsANS) from the DEGs to convince our analysis on the DEGs. The primers were shown in Table A1.
To verify the effect of low temperature on anthocyanin biosynthesis, we treated B. semperflorens seedlings with low temperature and dichlorophenyl dimethylurea (DCMU, a diuron, significantly inhibits light-dependent anthocyanin accumulation in Arabidopsis) based on the previous studies.
B. semperflorens seedlings with four leaves were divided into three groups: CK (normal temperature and light; 25/15 °C, 10 h, 300 μmol m−2 s−1); LT (low temperature and light; 16/6 °C, 10 h, 300 μmol m−2 s−1); LT + DCMU (10 µM DCMU was applied to leaves 6 h before low-temperature treatment). Leaves were harvested at 0, 9 h, 1, 4 and 6 days after the start of each treatment, and then used for performing QRT-PCR. Gene expression was measured under different conditions with the StepOne and StepOnePlus Real-Time PCR Systems (Applied Biosystems, Foster City, CA, USA) using SYBR® Premix Ex Taq™ II (TaKaRa). The B. semperflorens 18S rRNA gene (Genebank Accession No. KJ959633) was used as an internal reference to identify differences in each cDNA templates. The QRT-PCR cycling conditions were as follows: 95 °C for 10 s, followed by 40 cycles of 95 °C for 10 s and 60 °C for 45 s. We employed the \(2^{{ - \Delta \Delta C_{\text{T}} }}\) method (Livak and Schmittgen 2001) to analyse relative gene expression based on three biological replicates.
Results and discussion
Anthocyanin was triggered by low temperature
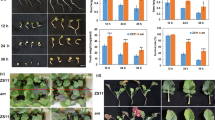
Many adverse environmental factors, such as ultraviolet radiation, high or low temperature, drought and pest invasion, can induce anthocyanin biosynthesis. Low temperature can induce anthocyanin biosynthesis in many plants (Xie et al. 2012; Huang et al. 2012; Schulz et al. 2015) by decreasing nutrient absorption and photosynthetic rates, two important triggers of anthocyanin biosynthesis (Peng et al. 2008; Zhang et al. 2010a, b). Anthocyanin biosynthesis is known to be triggered by excess sucrose (Zhang et al. 2013; Solfanelli et al. 2006), which usually occurs in plants at low temperatures. In this study, two different conditions, low temperature (LT) and normal control temperature (CK), were used to build libraries using Illumina sequencing technology (Fig. 1a). As shown in Fig. 1b, LT-seedlings showed significant higher content of anthocyanin, which is consistent with our observation that leaves of LT-seedlings turned red, and the colour deepened with time. Based on the induction of anthocyanin biosynthesis by low temperature (Zhang et al. 2013), this results proved the casual correlation between anthocyanin biosynthesis and low temperature from transcriptome.
RNA-Seq and sequence assembly
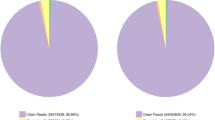
After quality checks, data with low quality were filtered out, and 25,022,374 and 22,506,321 clean reads were placed in the LT and CK libraries, respectively. The phred quality score > 30 (Q30) was 92.10 and 94.07% for the two samples, respectively. The GC content of the LT and CK libraries was 46.81 and 47.49%, respectively (Table 1).
Short clean reads were then assembled into 9,984,407 contigs in the total library, which were then further assembled into 174,352 transcripts and subsequently 74,779 unigenes. N50 of the transcripts and unigenes was 2200 and 1249 bp, respectively. The mean length of the transcripts and unigenes was 1312 and 684 bp, respectively (Table 2).
Another CK seedling was used as a biological replicate with the former one. RNA-seq was done, and their correlation was analysed. The correlation coefficients of the expression of the two duplicate samples are shown in Fig. A1. The duplicate showed linear correlation with its corresponding sample. The r 2 value (r represents Pearson r values) was 0.9779.
Transcriptome functional annotation
Since there is no genome of a closely related species available, we employed the transcriptome of B. semperflorens to study the molecular mechanisms of the anthocyanin biosynthesis process. All unigenes were aligned using databases including the non-redundant (Nr) protein database, Swiss-Prot database, Kyoto Encyclopedia of Genes and Genomes (KEGG) database, Cluster of Orthologous Groups (COG) database and Gene Ontology (GO) protein database. A total of 42,027 unigenes were identified and annotated; 41,161, 28,154, 17,165, 12,577 and 25,415 unigenes were identified in the five databases, respectively (Table 3).
Differentially expressed genes
Differentially expressed genes (DEGs) were screened with the criteria of false discovery rate (FDR) ≤ 0.01 coupled with absolute value of log2 ratio ≥ 2. A total of 4507 DEGs (6.03% of the all unigenes) were identified between the two samples, among which 2333 were up-regulated and 2174 were down-regulated in the comparison of LT and CK (Table A2). Of the 4507 DEGs, 4032 could be identified and annotated in the five protein databases (Table A3). A total of 2574 DEGs were annotated by GO classification, and they were assigned to 49 subgroups: 14 in the “cellular component” category, 15 in “molecular function” category and 20 in “biological process” category. The subgroups containing the highest numbers of DEGs were “metabolic process”, “cellular process”, “cell part”, “cell” and “catalytic activity” (Fig. 2). In the COG annotation, 1980 DEGs were identified as being involved in 25 categories (Fig. 3). In the KEGG annotation, 1531 DEGs were identified as being involved in 113 pathways (Fig. 4). “Carbon metabolism”, “biosynthesis of amino acids” and “ribosome” were the highest three levels with the most unigenes (Table A4), which are consistent with our previous results that excess carbohydrate contents accumulated under low temperature triggered anthocyanin biosynthesis (Zhang et al. 2013).
Manual identification of anthocyanin synthesis and anthocyanin transporting genes
To identify anthocyanin synthesis and anthocyanin transporting genes in B. semperflorens, we used the unigenes in BLAST searches of five protein libraries. Differentially expressed genes (DEGs) were screened with the criteria of false discovery rate (FDR) ≤ 0.01 coupled with absolute value of log2 ratio ≥ 1. A total of 85 unigenes had sequence similarities to known anthocyanin synthesis genes from other plants, including twenty (4-Coumarate: Coenzyme A Ligase) 4CL encoding genes, five anthocyanidin synthase (ANS) encoding genes, nine CHI encoding genes, nine F3H encoding genes, 22 encoding CHS genes, eight encoding DFR genes, nine glutathione S-transferase (GST) encoding genes and six PAL encoding genes (Table A5). Of all the DEGs, five 4CL encoding genes, three ANS encoding genes, three CHI encoding genes, one F3H encoding gene, 14 encoded chalcone synthase (CHS) genes, one DFR encoding gene, four GST encoding genes, three PAL encoding genes and another 26 anthocyanin-related genes were found (Table 4). Unigenes involved in anthocyanin metabolism (141 unigenes), ascorbic acid (45 unigenes), glutathione (184 unigenes), NADP+ (107 unigenes), NADPH (168 unigenes) and oxidoreduction (20 unigenes) were also retrieved (Table A6). Of these, 11 ascorbic acid encoded genes, 38 glutathione encoded genes, 17 NADP+ encoded genes, 19 NADPH encoded genes and three oxidoreduction encoded genes were found (Table A7). Sixty-seven DEPs in the phenyl propionate synthesis pathway and 31 DEPs in the flavonoid biosynthetic pathway were identified in the comparison of LT and CK. The process of anthocyanin synthesis is implemented by these two pathways. Fifty-eight DEPs participating in starch and sucrose metabolism were also identified in the KEGG analysis.
DEGs of the biosynthesis of anthocyanin in this study can be divided into two categories: (1) structure genes, which directly encode enzymes in anthocyanin synthesis and exist in different plants, and (2) regulating genes, which regulate the expression patterns of structure genes, spatially and temporally affecting pigment accumulation. That is, low temperature may not only directly trigger the anthocyanin biosynthesis genes to accumulate anthocyanins in plants; it may also regulate the process using transcription factors. Moreover, low-temperature-induced anthocyanin synthesis enhanced the related enzymes in anthocyanin biosynthesis pathways and GST enzymes in anthocyanin transport pathways, resulting in the accumulation of anthocyanin in plant leaves and stems. At the same time, more callose was accumulated in the phloem of leaf and stem, resulting in a blockage of carbohydrates, one of the substrates for anthocyanin biosynthesis. Two methods, cutting off downward transported carbohydrates with ring cutting and adding exogenous sucrose to promote the accumulation of carbohydrates in plants, are used to verify the hypothesis (Zhang et al. 2013). The results support the conclusion that carbohydrates directly trigger anthocyanin synthesis under low temperatures.
Analysis of anthocyanin concentration under low temperature
To further convince the DEGs, we selected three core anthocyanin biosynthesis genes (BsPAL, BsF3H and BsANS) to verify the relationship between anthocyanin biosynthesis and low temperature using qRT-PCR.
As shown in Fig. 4, three genes expression were significantly up-regulated in seedlings after treatment with low temperature (16/5 °C) for 9 h or 1 day, which resulted in a significant increase in anthocyanin level in seedlings after treatment with low temperature for 4 days. Treatment of DCMU significantly decreased all the three genes expression, which resulted in no changes in anthocyanin level in turn.
Anthocyanin as a protectant in low-temperature environments
Anthocyanin synthesis induced by low temperatures may be a protective mechanism for plants. According to the cellular component of the GO analysis of the DEGs, the synthesis of anthocyanin in cells is mainly concentrated in the cytoplasm around the vacuole. Based on the KEGG pathway analysis, we can conclude that the process is implemented by the phenyl propionate synthesis pathway and flavonoid biosynthetic pathway and ends with transport into the vacuole.
Based on the KEGG enrichment analysis (Fig. 5 and Table A4), we can infer that anthocyanins play two roles under low temperature, first by functioning as antioxidants. The most different expression unigenes over-presented in photosynthesis and photosynthesis-antenna proteins. Low temperature inactivates enzymes and organisms activities, and plants respond to low temperature by reducing photosynthetic rate, which produce relative excess light energy produced in plants. Excess light energy will produce a large number of active oxygen free radicals (ROS), such as ·O2 −, H2O2 and ·OH. These free radicals can directly oxidize and damage DNA, proteins, lipids and other biological macromolecules. As a result, the light mechanism of a plant leaf can be destroyed, leading to photooxidation and even light damage, thereby hindering the normal physiological metabolism of plants. This is consistent with the study of Cheng et al. (2016). From the KEGG enrichment analysis (Fig. 5 and Table A4), LT-seedlings also showed higher Enrichment Factor in phenylpropanoid biosynthesis, phenylalanine metabolism and flavonoid biosynthesis. Anthocyanins are flavonoids, and they therefore have the physiological function of the antioxidant activity of flavonoids. On the one hand, the molecular structure of anthocyanins has a number of phenolic hydroxyl groups and can release electrons through their oxidation, directly removing various free radicals and inhibiting oxidation. Such functions were also reported by Amin et al. (2015). On the other hand, anthocyanins can also protect lipids from over-oxidation and other oxidative modification through chelating metal ions such as iron and copper, which act as catalysts in the free radical reactions, as verified in another study (Zhang and Chen 2000). In addition, anthocyanins can reduce the oxidation of low-density lipoprotein (LDL) by increasing the activities of superoxide dismutases and glutathione in cells. Second, anthocyanins accumulation under low temperature may function as a carbon sink to consume relative excess carbohydrate contents. As shown in Fig. 5 and Table A4, the most unigenes over-presented were in carbon metabolism. Meanwhile, increases in secondary metabolism, particularly flavonoid biosynthesis, indicated that anthocyanins are associated with carbon metabolism under low temperature. Under low temperature, relative excess carbohydrates accumulate in plants as a result of reduced respiratory rate and growth rate. Higher carbohydrate content has been found in many plants as a trigger of anthocyanin biosynthesis (Zhang et al. 2013; Scafidi et al. 2015). Anthocyanins are produced from a C6–C3–C6 skeleton using sugars as one substrate. Therefore, the induction of anthocyanin biosynthesis in Begonia can help consume excess carbohydrate, which otherwise will inhibit photosynthesis.
Conclusions and future perspectives
To understand the molecular mechanisms of the biosynthesis and regulation process in B. semperflorens, the transcriptomes of B. semperflorens leaves under normal and low-temperature conditions were analysed. To the best of our knowledge, this represents the first report of the broad-scale transcriptome of B. semperflorens and will also contribute to our current understanding of the genetic background of many plants in the Violales taxonomic order.
Further research on the mechanism of the protection function of anthocyanins is needed to determine how environmental signals influence anthocyanin biosynthesis, how the signals are transduced, how anthocyanin functions in plant protection, the relationship between anthocyanin and phytochrome and the effects of anthocyanin on plant photosynthesis. These functions of anthocyanin are important for plants adapting to the environment. In addition, there are a variety of anthocyanins, and since different plant species contain different anthocyanins, their physiological functions to adapt to the environment are also distinctly different. In conclusion, anthocyanins play an important role in plant defence and adaptation to adverse environments, and further research is needed to understand and maximize their function.
Author contribution statement
KZ and BH conceived and designed the experiments; MG performed the experiments; WD analysed the data; JW contributed reagents/materials/analysis tools; KZ and BH wrote the paper.
Abbreviations
- WBM:
-
WD40-bHLH-MYB
- UFGT:
-
Flavonoid 3-O-glucosyltransferase
- LT:
-
Low temperature
- CK:
-
Control
- COG:
-
Clusters of orthologous groups
- GO:
-
Gene ontology
- KEGG:
-
The Kyoto Encyclopedia of Genes and Genomes
- DEGs:
-
Differentially expressed genes
- Nr:
-
Non-redundant
- FDR:
-
False discovery rate
- 4CL:
-
4-Coumarate: Coenzyme A Ligase
- ANS:
-
Anthocyanidin synthase
- CHI:
-
Chalcone isomerase
- CHS:
-
Chalcone synthase
- DFR:
-
Dihydroflavonol 4-reductase
- GST:
-
Glutathione S-transferase
- PAL:
-
Phenylalanine ammonialyase
- ROS:
-
Active oxygen free radicals
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped blast and psi-blast: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Amin HP, Czank C, Raheem S, Zhang Q, Botting NP, Cassidy A, Kay CD (2015) Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol Nutr Food Res 59(6):1095–1106
Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11:R106
Broberg P (2005) A comparative review of estimates of the proportion unchanged genes and the false discovery rate. BMC Bioinform 6:199
Cheng DD, Zhang ZS, Sun XB, Zhao M, Sun GY, Chow WS (2016) Photoinhibition and photoinhibition-like damage to the photosynthetic apparatus in tobacco leaves induced by Pseudomonas syringae pv. Tabaci under light and dark conditions. BMC Plant Biol 16(1):29–39
Fogelman E, Tanami S, Ginzberg I (2015) Anthocyanin synthesis in native and wound periderms of potato. Physiologiaplantarum 153(4):616–626
Golding J, Wasshausen DC (2002) Begoniaceae, edition 2: part I: annotated species list: Part ii: Illustrated key, abridgment and supplement. Contrib U S Natl Herb 43:1–289
Grabherr MG, Haas BJ, Yassou M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, Palma F, Birren W, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–652
Huang ZA, Zhao T, Fan HJ, Wang N, Zheng SS, Ling HQ (2012) The upregulation of NtAN2 expression at low temperature is required for anthocyanin accumulation in juvenile leaves of Lc-transgenic tobacco (Nicotiana tabacum L.). J Genet Genom 39:149–156
Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y (2008) Kegg for linking genomes to life and the environment. Nucleic Acids Res 36:480–484
Li B, Dewey CN (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform 12:323
Lingua G, Bona E, Manassero P, Marsano F, Todeschini V, Cantamessa S, Copetta A, D’Agostino G, Gamalero E, Berta G (2013) Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads increases anthocyanin concentration in strawberry fruits (Fragaria × ananassa var. Selva) in conditions of reduced fertilization. Int J Mol Sci 14(8):16207–16225
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) Method. Methods 25:402–408
Mao X, Cai T, Olyarchuk JG, Wei L (2005) Automated genome annotation and pathway identification using the keggorthology (KO) as a controlled vocabulary. Bioinformatics 21:3787–3793
Mita S, Murano N, Akaike M, Nakamura K (1997) Mutants of Arabidopsis thaliana with pleiotropic effects on the expression of the gene for β-amylase and on the accumulation of anthocyanin that are inducible by sugars. Plant J 11(4):841–851
Peng MS, Hudson D, Schofield A, Tsao R, Yang R, Gu HL et al (2008) Adaptation of Arabidopsis to nitrogen limitation involves induction ofanthocyanin synthesis which is controlled by the NLA gene. J Exp Bot 59:2933–2944
Petroni K, Tonelli C (2011) Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci 181:219–229
Scafidi P, Maria G, Barbagallo, Downey MO (2015) Influence of light exclusion on anthocyanin composition in ‘cabernet sauvignon’. Front Plant Sci 06:107–110
Schulz E, Tohge T, Zuther E, Fernie AR, Hincha DK (2015) Natural variation inflavonol and anthocyanin metabolism during cold acclimation in Arabidopsis thaliana accessions. Plant Cell Environ 38:1658–1672
Sivankalyani V, Feygenberg O, Diskin S, Wright B, Alkan N (2016) Increased anthocyanin and flavonoids in mango fruit peel are associated with cold and pathogen resistance. Postharvest Biol Technol 111:132–139
Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140(2):637–646
Stracke R, Jahns O, Keck M, Tohge T, Niehaus K, Fernie AR, Weisshaar B (2010) Analysis of production of flavonol glycosides-dependent flavonol glycoside accumulation in Arabidopsis thaliana plants reveals myb11-, myb12- and myb111-independent flavonol glycoside accumulation. New Phytol 188:985–1000
Van den Ende W, El-Esawe SK (2014) Sucrose signaling pathways leading to fructan and anthocyanin accumulation: a dual function in abiotic and biotic stress responses? Environ Exp Bot 108:4–13
Xie XB, Li S, Zhang RF, Zhao J, Chen YC, Zhao Q, Yao YX, You CX, Zhang XS, Hao YJ (2012) The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ 35(11):1884–1897
Young MD, Wakefield MJ, Smyth GK, Oshlack A (2010) Gene ontology analysis for RNA-Seq: accounting for selection bias. Genome Biol 11:R14
Zhang HY, Chen DZ (2000) Theoretical characterization and application of free radical scavenging activity of phenolic antioxidants. Acta Biophys Sin 16:1–9
Zhang KM, Yu HJ, Shi K, Zhou YH, Yu JQ, Xia XJ (2010a) Photoprotective roles of anthocyanins in Begonia semperflorens. Plant Sci 179:202–208
Zhang KM, Yu HJ, Shi K, Zhou YH, Yu JQ, Xia XJ (2010b) Photoprotective roles of anthocyanins in Begonia semperflorens. Plant Sci 179:202–208
Zhang Y, Zheng S, Liu Z, Wang L, Bi Y (2011a) Both HY5 and HYH are necessary regulators for low temperature-induced anthocyanin accumulation in Arabidopsis seedlings. J Plant Physiol 168(4):367–374
Zhang YQ, Zheng SH, Liu ZJ, Wang LG, Bi YR (2011b) Both hy5 and hyh are necessary regulators for lowtemperature-induced anthocyanin accumulation in arabidopsis seedlings. J Plant Physiol 168:367–374
Zhang KM, Li Z, Li Y, Li YH, Kong DZ, Wu RH (2013) Carbohydrate accumulation may be the proximate trigger of anthocyanin biosynthesis under autumn conditions in Begonia semperflorens. Plant Biol 15:991–1000
Zhang KM, Guo ML, He D, Wu RH, Li YH (2016) The inhibition effect and excessive carbon flux resulting from blocking anthocyanin biosynthesis under darkness in Begonia semperflorens. J Plant Growth Regul 35:22–30
Acknowledgements
This research was supported by the National Science Foundation of China (Grant No. 31100562) and The Key Scientific Research Project of High Education in Henan Province (Grant No. 15A220006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Additional information
Communicated by R. Baczek-Kwinta.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11738_2017_2578_MOESM1_ESM.png
Fig. A1 Correlation coefficients of the expression of duplicate samples. The x-axis is the logarithmic value of T01 (one sample), and the y-axis is the logarithmic value of the corresponding duplicate sample (T02) (PNG 35 kb)
Rights and permissions
About this article
Cite this article
Bi, H., Guo, M., Wang, J. et al. Transcriptome analysis reveals anthocyanin acts as a protectant in Begonia semperflorens under low temperature. Acta Physiol Plant 40, 10 (2018). https://doi.org/10.1007/s11738-017-2578-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2578-3