Abstract
Ruscus hypophyllum L. is a rare Mediterranean plant which is used in the traditional medicine. We studied its phenolic content and in vitro toxicity and genotoxicity using the neutral red uptake (NRU) test, the bacterial Vitotox test, and the comet assay in human C3A hepatic cells. Aqueous leaf and fruit extracts were investigated. Antigenotoxicity against 4-nitroquinoline-oxide (4NQO, 0.4 µg/mL) and Benzo(α)pyrene (BaP, 800 µg/mL) was also investigated with the Vitotox test. The extracts appeared to be genotoxic only at high exposure levels in the comet assay. There was no indication of a genotoxic activity in the Vitotox test and also no indication of antigenotoxicity. The moderate polyphenol content may provide an explanation for the absence of antigenotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medicinal plants have been used throughout human history. They have the ability to synthesize a wide variety of chemical compounds that may be used to perform or modify important biological functions. Many extracts prepared from plants have biological activities in vitro and in vivo. In recent years, there has been great interest in investigating compounds from plants and their effects on DNA. This is because preparations from the traditional medicinal plants were seldom tested the way our modern pharmaceutical compounds are, and hence, they may potentially have unknown adverse effects. Such effects may be mutation induction and carcinogenicity. Compounds from plant origin can be mutagenic but also antimutagenic, and hence, they can contribute to as well increases as reductions in the incidence of cancer in the population (Ames 1983). It may, therefore, be interesting to investigate not only genotoxicity but also potential antigenotoxic properties of plant extracts with alleged medicinal properties.
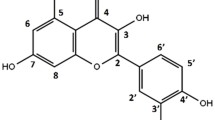
Ruscus hypophyllum L. belongs to the Liliacea family. It is a rare and endangered species which is especially found in the Northern region of Tunisia. It is native in N. Africa, as far east as Tunisia, but extends to the northern littoral of the Mediterranean (Yeo 1968). It is a perennial, dioecious, evergreen rhizomatous geophyte. The mature plant shrub may reach about 46 cm in height. It has a creeping rootstock and leaf-like phylloclades. True leaves are small green appendages around the flowers. The bracts are located in the middle of the upper surface of leaves, and flowers develop in their axils. Fruit is a rarely produced 0.6–1.3 cm-wide red globose berry (Mabberley 1997). Ruscus hypophyllum L. generally resembles R. hypoglossum, but can readily be distinguished by the shorter and broader cladodes and the tiny bracts. Ruscus species contain flavonoids, ruscogenins, and phenolic acids (Taruscio et al. 2004). Traditionally, decoctions of this plant are applied externally for boils and warts (Tuzlaci and Aymaz 2001) and fresh leaves are used in Turkey for livestock against cold and mastitis (Kültür 2007). Hadžifejzović et al. (2013) investigated the antimicrobial and antioxidant activities of R. aculeatus L. and R. hypoglossum L. and found that both had strong antimicrobial activities as well as antioxidant activities which were strongly correlated with the total phenolic content. However, overall, their biological activities are not well known. The in vitro toxicity and genotoxicity of aqueous fruit and leaf extracts of R. hypophyllum has not been studied yet. We here report the results of a genotoxicity evaluation using the in vitro bacterial Vitotox test and comet assay in human C3A hepatic cells. Antigenotoxicity against 4-nitroquinoline-oxide (4NQO, 0.4 µg/mL) and Benzo(α)pyrene (BaP, 800 µg/mL) was also investigated with the bacterial Vitotox test. The neutral red uptake test in C3A cells was used for evaluation of the in vitro toxicity and as a dose-finding test for the comet assay. The total phenolic and flavonoid contents were also determined.
Materials and methods
Plant material
Leaf and fruit were collected in 2015 in Tabarka (North Tunisia). The plant was identified according to the flora of Tunisia. A voucher specimen was deposited in the herbarium of our laboratory.
Extraction
3 g of dried leaf and fruit of R. hypophyllum were used to prepare infusions with 100 mL deionized water at 80 °C. Samples were filtered and sterilized with 0.45 μm Millipore filters. The water extracts were stored at −4 °C prior to experimentation.
Total phenolic content
The phenolic content was determined by the Folin–Ciocalteau colorimetric method with minor modifications (Singleton and Rosi 1965). To 100 µL of extract, 7.9 mL of deionized water and 0.5 mL of Folin–Ciocalteau reagent (F9252, Sigma Aldrich, St Louis, MO) were added, mixed by vortex, and 1.5 mL of 1.85 M Na2CO3 was added after 15 min. The absorbance was measured at 765 nm after 2 h. Gallic acid was used as a standard and results were expressed as µg of catechin equivalents per mg.
Total flavonoid content
Total flavonoids were measured using a colorimetric assay developed by Dewanto et al. (2002). An aliquot of diluted extract or standard solution of catechin was added to 75 µL of a 7% NaNO2 solution and mixed for 6 min, before adding 0.15 mL (10%) AlCl3. After 5 min, 0.5 mL of a 1 M NaOH solution was added. The final volume was adjusted to 2.5 mL, thoroughly mixed, and the absorbance of the mixture was determined at 510 nm. Total flavonoids were expressed as µg catechin equivalent (CAE/mg). All extracts were analyzed in three replications.
In vitro toxicity
The neutral red uptake test (NRU)
This test is a well-known in vitro toxicity test which measures Neutral Red dye Uptake (NRU) upon addition of the dye (Borenfreund and Puerner 1985). We performed the NRU test on human C3A cells according to Repetto et al. (2008). After exposure of the cells to the test agent, the cells were washed and the neutral red dye was added. The evaluation of the test is done by resolving the intracellular bound dye and measuring the intensity photometrically in comparison with an untreated control sample. Toxicity is expressed as percent inhibition of neutral red dye retention in the sample. The NI50 value (50% inhibition of NRU) is determined from the dose response curve of the mean OD values of the different concentrations. The positive control consisted of different concentrations of sodium dodecyl sulphate (SDS; 0–0.42 mM).
In vitro genotoxicity
The Vitotox assay
This test uses Salmonella typhimurium TA104 bacteria in which a luxCDABE operon is coupled to a modified recN gene. In this bacterium, expression of the lux operon is SOS-regulated, resulting in light production when strains harboring this construct are treated with genotoxins that induce SOS. This strain is also called the Genox strain. An agent is genotoxic when the signal-to-noise ratio (S/N = light production of ‘exposed’ bacteria over that of unexposed controls) shows a dose–response relationship and rises above 1.5. A so-called “pr1” or Cytox strain contains lux genes under control of a constitutive promoter, so that the light production is not influenced by genotoxic compounds. This strain is used in parallel with the Genox strain and is cultivated and treated in exactly the same way. Here, S/N should remain approximately 1. A significant increased light production in this strain indicates that the lux operon was directly influenced by the test agent and hence that increased light production in the Genox strain probably does not indicate genotoxicity (false-positive response). If light production is significantly reduced (≪0.8), this may indicate toxicity. The Vitotox test has the advantage that it requires only small amounts of a test agent and that it takes only 4 h to evaluate genotoxicity. A detailed description of the test is given elsewhere (e.g., Verschaeve 2005, 2013; Verschaeve et al. 1999).
The Comet assay
We used the alkaline comet assay on human C3A hepatic cells to measure DNA breakage effects in vitro. The method that we used is described in more detail in some of our previous publications (e.g., Verschaeve et al. 2013). In summary, cells were embedded in agarose on a microscope slide and lysed with detergent and high salt to form nucleoids containing supercoiled loops of DNA linked to the nuclear matrix. After electrophoresis at high pH structures, resembling comets are formed. DNA comets were analyzed with an Axio Imager.Z2 (Zeiss) fluorescence microscope with the Metacyte and Metafer 4 (version 3.8.5) software from Metasystems (Altlussheim, Germany). The percentage of DNA in the comet tail was used as a measure of DNA damage. Ethyl methane sulfonate (0.75 mM) was included as a positive control. Two slides were prepared per exposure, and a total of 100 cells (DNA comets) were measured (50 per slide). The Mann–Whitney U test was used to determine statistical deviations from the unexposed control cells.
Results and discussion
Table 1 shows that the highest total phenolic and flavonoid content of R. hypophyllum was found in the leaf extract (respectively, 25.15 and 4.12 µg CAE/mg). Yet, this was less than in other plants such as Marrubium alysson and Peganum harmala, where the polyphenol contents were much higher (Edziri et al. 2010, 2011a). These plants were found to possess antigenotoxic properties against 4-nitroquinoline-oxide (4-NQO) and benzo(a)pyrene in the VITOTOX test (Edziri et al. 2011b). Overall, phenolic compounds, particularly flavonoids (such as quercetin), were found to have antiproliferative and anticarcinogenic activities (e.g., Sghaier et al. 2011a) as well as antimutagenic, antigenotoxic, and antioxidant activities (e.g., Edziri et al. 2011b; Sghaier et al. 2011b). Mutagenic (Nagao et al. 1981; Elliger et al. 1984) and carcinogenic (Zhu and Lier 1994) properties were, however, also reported.
It is assumed that the cytoprotective anticancer effect of flavonoids is due to interaction with the cytochrome P-450 mixed function oxidase system which results in a decreased metabolic activation of potential carcinogens (Hertog et al. 1993). Interference with tyrosine kinase (Ferry et al. 1996) or cyclin-dependent kinases activities (Casagrande and Darbon 2000), induction of apoptosis (Constantinou et al. 1998), and the regulation of mutant p53 levels (Avila et al. 1994) may also contribute to the antiproliferative activity of flavonoids.
In this study, we used the Vitotox test and comet assay to assess the genotoxicity/antigenotoxicity of the water extracts of R. hypophyllum. Both tests are indicator tests rather than mutation tests. They were chosen based on previous observations showing that a combination of both tests provided complementary data which was sufficient to allow a relatively rapid genotoxicity screening of many plant extracts and fractions, without the need to use other more time consuming tests (Verschaeve 2015).
The Vitotox test was conducted in the absence and presence of S9 so as to allow genotoxicity testing of the pure compound and its metabolites. The results are given in Fig. 1.
The figure shows that all S/N ratios were approximately “1” in both the Genox and Cytox strains and for both samples (leaves and fruits). This means that there was no indication of cytotoxicity or genotoxicity in the Vitotox test at the applied concentrations. Figure 2 gives the results of the antigenotoxicity investigation. Here, 0 mM corresponds to the positive control only (resp. 4NQO or BaP in cultures without or with S9). The other given concentrations represent the same concentration of the corresponding positive control + the indicated concentration of the plant extract.
Antigenotoxicity study in the Vitotox test investigated against 4NQO (without S9) and benzo(α)pyrene (with S9). NR4 and NR5 as in Fig. 1; 0 µg/mL is mutagen alone; indicated concentrations are extract concentrations in addition to the mutagen
We see that the positive controls behaved as expected: S/N in the Genox strain increased above 1.5 and there was no corresponding increase (or decrease) in S/N in the Cytox strain. Both extracts hardly decreased S/N of the positive controls indicating that they also had no significant antigenotoxic effect. This was so as well in the absence and presence of S9. The Vitotox test is an indicator test based on SOS induction (Verschaeve 2013) and is despite its high predictivity for bacterial mutagenicity (Westerink et al. 2009), therefore, not sufficient to decide about an agent’s genotoxicity. As indicated above, the alkaline comet assay in mammalian/human cells is considered an important additional test. Both tests are complementary. Here, human C3A cells were used. These cells are still able to perform phase I and II biotransformation processes. That is why we did not use S9 in the comet assay. In this test, we used the % tail DNA as a measure of DNA damage as this was in our hands, and also according to literature data (e.g., Kumaravel and Jha 2006) the most suitable parameter. A preliminary in vitro toxicity and dose-finding test was conducted using the NRU test in C3A cells.
Figure 3 gives the NI50 values according to this test. Investigated concentrations were the highest possible (5 mg/mL, solubility limit concentration and highest recommended dose; Tice et al. 2000) and two further dilutions. All tested doses, including the highest possible exposure level, were found not toxic (NI50 >90%). These doses were, therefore, also investigated in the comet assay. Figure 4 gives the % tail DNA for a few concentrations of 4NQO. It can be seen that all investigated concentrations showed statistically significant increases of the % tail DNA (p < 0.001). Ethyl methane sulfonate (EMS, 0.5 mM) showed even more DNA damage.
Statistically significant increases in DNA damage were also found for the plant leaves (here designated as NR4; p < 0.05) and fruit extracts (designated as NR5; p < 0.001), at least at the high concentration of 5 mg/mL (Fig. 5).
DNA damage was nevertheless much less important than that of the well-known mutagens (4NQO and EMS), and at lower concentrations, no significant effect was found. The results yet indicate a genotoxic potential and, therefore, encourage some caution and further testing. According to the Vitotox test, there was no antigenotoxic effect. This is somewhat in contradiction with literature data on antigenotoxicity of phenolic-enriched plant extracts which can be explained by the presence of flavonoids and phenolic compounds as antioxidant constituents (e.g., Ribeiro et al. 2010; Mihailovic et al. 2013; Ehtesham-Gharaee et al. 2015). However, it should again be remembered that antigenotoxicity was only studied in the bacterial Vitotox test and that the mechanisms involved may not be revealed in this test. Phenolic contents were furthermore moderate and may explain lack of antigenotoxicity.
In conclusion, our study can be considered as the first report on the polyphenolic content and in vitro toxicity and genotoxic activity of aqueous leaf and fruit extracts of Ruscus hypophyllum L. We have found that both leaf and fruit aqueous extracts displayed genotoxic activity at a high concentration (5 mg/mL) only. This plant possess moderate polyphenol contents which may explain the lack of antigenotoxicity in the Vitotox test. These results require further investigation.
Author contribution statement
LV is responsible for the in vitro genotoxicology tests and supervised the experiments. He fully contributed to the writing of the manuscript. HE is responsible for the other parts of the work. She fully contributed to the writing of the manuscript. RA conducted the genotoxicity tests described in this paper. DB provided technical support and revised the manuscript. FS identified the plants. AM provided technical support and revised the manuscript. MM provided technical support and revised the manuscript.
References
Ames BM (1983) Dietary carcinogens and anticarcinogens oxygen radical and degenerative diseases. Science 221:1256–1263
Avila MA, Velasco JA, Cansado J, Notario V (1994) Quercetin mediates the down-regulation of mutant p53 in the human breast cancer cell line MDA-MB468. Cancer Res 54:2424–2428
Borenfreund E, Puerner JA (1985) Toxicity determination in vitro by morphological alterations and neutral red absorption. Toxicol Lett 24:119–124
Casagrande F, Darbon JM (2000) Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: regulation of cyclin-dependent kinases CDK2 and CDK1. Biochem Pharmacol 61:1205–1215
Constantinou AI, Kamath N, Murley JS (1998) Genistein inactivates bcl-2, delays the G2/M phase of the cell cycle, and induces apoptosis of human breast adenocarcinoma MCF-7 cells. Eur J Cancer 34:1927–1934
Dewanto V, Wu X, Adom KK, Liu RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50:3010–3014
Edziri H, Mastouri M, Mahjoub MA, Patrich G, Matieu M, Ammard S, Alid SM, Laurent G, Zine M, Aouni M (2010) Antibacterial, antiviral and antioxidant activities of aerial part extracts of Peganum harmala growing in Tunisia. Toxicol Environ Chem 92:1283–1292
Edziri H, Mastouri M, Mahjoub MA, Ammar S, Zine M, Laurent G, Aouni M (2011a) Antiviral activity of leaves extracts of Marrubium alysson L. J Med Plant Res 5:360–363
Edziri H, Mastouri M, Mahjoub A, Anthonissen R, Mertens B, Cammaerts S, Gevaert L, Verschaeve L (2011b) Toxic and mutagenic properties of extracts from Tunisian traditional medicinal plants investigated by the neutral red uptake-, VITOTOX- and alkaline comet assays. S Afr J Bot 77:703–710
Ehtesham-Gharaee M, Eshaghi A, Shojaee S, Asili J, Emami SA, Behravan J, Mosaffa F (2015) Protective effects of Scutellaria lindbergii root extract against oxidative-induced cell and DNA damage in mouse fibroblast-like cells. Drug Chem Toxicol 38:293–299
Elliger GA, Henika PR, MacGregor JT (1984) Mutagenicity of flavones, chromones and acetophenones in Salmonella typhimurium. New structure–activity relationships. Mutat Res 135:77–86
Ferry DR, Smith A, Malkhandi J, Fyfe DW, deTakats PG, Anderson D (1996) Phase I clinical trial of the flavonoids quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res 2:659–668
Hadžifejzović N, Kukić-Marković J, Petrović S, Soković M, Glamočlija J, Stojković D, Nahrstedt A (2013) Bioactivity of the extracts and compounds of Ruscus aculeatus L. and Ruscus hypoglossum L. Ind Crop Prod 49:407–411
Hertog MG, Feskeens EJ, Hollman CH, Katan MB, Kromhout D (1993) Dietary antioxidant flavonoids and risk of coronary heart disease: de Zutphen elderly study. Lancet 342:100711
Kültür S (2007) Medicinal plants used in Kirklareli Province (Turkey). J Ethnopharmacol 111:341–364
Kumaravel TS, Jha AN (2006) Reliable comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat Res 605:7–16
Mabberley DJ (1997) The plant book: a portable dictionary of the vascular plants. Cambridge University Press, Cambridge
Mihailovic V, Matic S, Mišic D, Solujic S, Stanic S, Katanic J, Mladenovic M, Stankovic N (2013) Chemical composition, antioxidant and antigenotoxic activities of different fractions of Gentiana asclepiadea L. roots extract. EXCLI J 12:807–823
Nagao M, Morita N, Yahagi T, Shimizu M, Kuroyanag M, Fukuoka M (1981) Mutagenicities of 61 flavonoids and 11 related compounds. Environ Mutagen 3:401–419
Repetto G, Del Peso A, Zurita JL (2008) Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3:1125–1131
Ribeiro JC, Antunes LM, Aissa AF, Darin JD, De Rosso VV, Mercadante AZ, Bianchi MDL (2010) Evaluation of the genotoxic and antigenotoxic effects after acute and subacute treatments with açai pulp (Euterpe oleracea Mart.) on mice using the erythrocytes micronucleus test and the comet assay. Mutat Res 695:22–28
Sghaier MB, Skandrania I, Nasra N, Dijoux FM-G, Chekir-Ghedira L, Ghedira K (2011a) Flavonoids and sesquiterpenes from Tecurium ramosissimum promote anti proliferation of human cancer cells and enhance antioxidant activity: a structure–activity relationship study. Environ Toxicol Pharmacol 32:336–348
Sghaier MB, Boubaker J, Skandrania I, Bouhlel I, Limen I, Ghedira K, Chekir-Ghedira L (2011b) Antimutagenic, antigenotoxic and antioxidant activities of phenolic-enriched extracts from Teucrium ramosissimum: combination with their phytochemical composition. Environ Toxicol Pharmacol 32:220–232
Singleton VL, Rosi JA (1965) Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Taruscio G, Barney DL, Exon J (2004) Content and profile of flavonoids and phenolic acid compounds in conjunction with antioxidant capacity for a variety of Northwest Vaccinium berries. J Agric Food Chem 52:3169–3176
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu J-C, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
Tuzlaci E, Aymaz PE (2001) Turkish folk medicinal plants, Part IV: Gönen (Balike-sir). Fitoterapia 72:323–343
Verschaeve L (2005) The VITOTOX® genotoxicity test. In: Pandali SG (ed) Recent research developments in applied microbiology and biotechnology. Research Signpost, Trivandrum, pp 33–49
Verschaeve L (2013) High-throughput bacterial mutagenicity testing: Vitotox™ assay. In: Steinberg P (ed) High throughput screening methods in toxicity testing. Wiley, Hoboken, pp 213–232
Verschaeve L (2015) Investigations of plant-derived products with the in vitro comet assay. In: Front Genetic conference abstract: ICAW 2015—11th international comet assay workshop. doi:10.3389/conf.fgene.2015.01.00073
Verschaeve L, Van Gompel J, Regniers L, Van Parys P, van der Lelie D (1999) VITOTOX® genotoxicity and toxicity test for the rapid screening of chemicals. Environ Mol Mutagen 33:240–248
Verschaeve L, Mertens B, Nhlala AR, Anthonissen R, Gorissen B, Van Staden J (2013) Investigation of the genotoxicity of water extracts from Hypoxis species and a commercially available hypoxis preparation. Phytother Res 27:350–356
Westerink WMA, Stevenson JCR, Lauwers A, Griffioen G, Horbach GJ, Schoonen WGEJ (2009) Evaluation of the Vitotox™ and RadarScreen assays for the rapid assessment of genotoxicity in the early research phase of drug development. Mutat Res 676:113–130
Yeo PF (1968) A contribution to the taxonomy the genus Ruscus. Notes R Bot Garden Edinburgh 28:237–269
Zhu BT, Lier JG (1994) Quercetin increases the severity of estradiol-induced tumorigenesis in hamster kidney. Toxicol Appl Pharmacol 125:149–158
Acknowledgements
This work was supported by the faculty of Pharmacy (Laboratory of Transmissible Diseases and biological Active Substances) of the University of Monastir.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by MJ Reigosa.
Rights and permissions
About this article
Cite this article
Verschaeve, L., Edziri, H., Anthonissen, R. et al. In vitro toxicity and genotoxic activity of aqueous leaf and fruit extracts of Ruscus hypophyllum L.. Acta Physiol Plant 39, 206 (2017). https://doi.org/10.1007/s11738-017-2505-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2505-7