Abstract
Soil salinity is mainly caused by excessive use of fertilizers and the use of poor quality water, and adversely affected crop growth especially when grown in protected environments. Soil salinity causes salt stress in plants, which inhibits plant growth, leading to morphological, metabolic and physiological changes. Though it is a major problem occurs more frequently, there is less information on the behavior of calla lily (Zantedeschia aethiopica) under these conditions, and most studies are conducted with other species of the genus Zantesdeschia. Therefore, this study aimed to evaluate ecophysiological, biochemical and anatomical growth responses of calla lily plants to salt stress. Rhizomes were grown in trays containing coconut fiber as a substrate and treated with 0, 25, 50, 75 and 100 mM NaCl to induce stress. A decrease in plant height was observed, as well as in the number of tillers and leaves, main root length, fresh and dry matter of the shoot and root system. A reduction in photosynthetic rate, stomatal conductance and transpiration rate was observed at 60 days. However, after 90 days, the photosynthetic rate was unchanged, with increased stomatal conductance and transpiration rate for plants exposed to 75 mM NaCl. Salt stress caused a higher accumulation of carbohydrates in shoots and roots. Thus, high concentrations of NaCl affect the development of calla lily, indicating that this species is susceptible to salt stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The main causes of salt stress in ornamental plants are the use of brackish irrigation water, fertilization required and cultivation in protected environments (Rasool et al. 2013). Calla lily is highly susceptible to salt stress in unsuitable growing conditions, although there is no record of its behavior under these conditions. The habitat of calla lily cultivation is in swamps and edges of rivers and streams, however, for commercial production, planting in these areas is not recommended due to the constant humidity that favors many diseases, especially bacteria such as Pectobacterium carotovorum. Thus, commercially, calla lily is suggested to a well-drained areas, with constant irrigation, maintaining high soil moisture (Paiva and Almeida 2012). However, poor quality water used to irrigation cause damage to plants.
In addition to causing damage to plants, the use of poor water or improper use of fertigation may also cause nutritional imbalance. Information on fertilization is still scarce for ornamental plants and the empirical treatment with high doses of fertilizers affects the production and quality of inflorescences, mainly by the salinity caused by excess fertilizers (Almeida et al. 2012; Furtini Neto et al. 2015).
In ornamental plants, excess salts cause, a stunting of plants, reduced chlorophyll rate, photosynthetic rate, quality of plants and flowers, due to imposed physiological drought and nutritional deficiency, particularly due to calcium (Ca2+) and potassium (K+) ions (Veatch-blohm et al. 2012). Therefore, changes in gas exchange occur because the osmotic stress in the root zone that reduces the availability of water for the plant. Furthermore, the excess salt also cause synthesis of abscisic acid, which is transported to the guard cells and induces the stomatal closure, then causes decrease in photosynthesis (Fraire-Velázquez and Balderas-Hernández 2013). The photosynthetic reduction is also caused by changes in the structures of chloroplasts due to the toxic effects caused by Na+ and Cl− ions, thus chlorophylls content decrease (Assis júnior et al. 2007; Veatch-Blohm et al. 2012).
Anatomically, salt stress may causes lignification of cell walls, the appearance of Caspary thickenings, calcium oxalate crystals storage, reduction of the vascular bundles cell and disruption in the deposition of cells. The tissue thickness, Na+ transport via apoplastic, number of stomata and epidermal cells, the distance of the vascular bundles and differentiation and the number of xylem vessels are also altered under the conditions of high concentrations of NaCl (Melo et al. 2011; Veatch-Blohm et al. 2012).
It was reported that salinity causes reduction in plant height in ornamental sunflower, carnation and roses, besides affecting the productivity and quality of flowers and plant dry matter (Travassos et al. 2011; Maciel et al. 2012; Navarro et al. 2012; Cai et al. 2014). Moreover, it was examined how salinity applied either pre- or post-emergence altered shoot growth and flower production in four Zantedeschia K. Koch (Z. elliotiana xremannii, Z. rehmannii Engl, Z. albomaculata, Z. rehmannii violacea and Z. elliotiana xmaculata). In these conditions, salinity applied post-emergence did not significantly affect shoot growth in either the 25 or 50 mM NaCl treatments, however, irrigation with a 50 mM NaCl solution reduced dry weight and flower production. In this way, results indicate that use of irrigation water salinized by 25 mM NaCl can be used without a loss, enabling its cultivation with poor quality water (Veatch-Blohm and Morningstar 2011).
Salt stress is a frequent problem in floriculture. However, physiological studies that show the effects of stress on ornamental plants are scarce and there are few dates for calla lily. Therefore, this study aimed to evaluate ecophysiological, biochemical and anatomical growth responses in the early development of calla lily plants subjected to salt stress, to understand better the tolerance levels of the species to salinity.
Materials and methods
Calla lily rhizomes with approximately 5.0 cm long were planted in 16 L polyethylene trays, with 10 rhizomes per tray. The substrate used was granulated coconut fiber which were fertilized every 15 days with 100 mM NPK 13-40-13, 90 mM potassium nitrate, 70 mM calcium nitrate, 50 mM urea, 80 mM magnesium sulfate and 1.42 mM micronutrients (adapted from Comissão de Fertilidade do Solo do Estado de Minas Gerais-CSFEMG 1999).
The experiment was conducted in a growth chamber at 25 °C, 16-h photoperiod and with average irradiance of 56 µmol m−2 s−1. The application of salinity treatments was performed by irrigation using distilled water, initiated 10 days after the rhizomes were planted. Irrigations with treatments were done twice a week to maintain soil moisture near field capacity, using 0.5 L per tray on each day of irrigation.
The experimental was conducted in a completely randomized (CRD) design, and the treatments consisted of different NaCl concentrations (0, 25, 50, 75 and 100 mM) diluted in distilled water, with the electrical conductivities of the solutions, 0.0052, 2.30, 4.58, 6.86 and 9.14 dS m−1, obtained by Richards equation (1954).
Non-destructive growth assessments were made at 60 days (when the first symptoms caused by salinity appeared) and 90 days after salt stress was imposed. Plant height, number of leaves and number of tillers were reckoned. Dry matter assessments of shoot and root, besides root length (measured from the lower end of the rhizome to the apex of the primary root), were evaluated at 90 days. For these evaluations, five replicates with two plants per replication were used.
Ecophysiological evaluations were performed at 60 and 90 days in the morning, between 9 and 11 AM, collecting a sample per plant, with seven replicates per treatment. Photosynthetic rate (A), stomatal conductance (gs) and transpiration rate (E) were evaluated using the Portable Infrared CO2 Analyzer (IRGA® LCA-4 ADC Hoddesdon, UK). The relative chlorophyll content was determined using the portable AtLeaf+® chlorophyll meter (FT Green LLC, Wilmington, DE).
Biochemical assessments were performed at the end of the experiment, and a sample per plant was collected, with three replicates per treatment. The analyses were performed in duplicate. For the quantification of total soluble sugars and starch, the anthrone method (Yemm and Willis 1954) was used; for reducing sugars, the DNS method (Miller 1959); the quantitation of non-reducing sugars was obtained by the difference between the concentration of soluble sugars and reducing sugars, and total protein by the Bradford method (1976).
Samples for anatomical analyses were collected at 90 days after planting. Fully expanded leaves and roots were stored in 70% alcohol (v v−1). Cross cuts were made on the leaves in both epidermis and roots, and staining was performed according to Kraus and Arduin (1997). Semi-permanent slides were prepared and observed under a microscope (Zeiss Scope AX10®) coupled to a digital camera, and photomicrographed using the AxioVision R.E.L. 4.8® software.
The parameters observed in the leaves were: polar (PD) and equatorial (ED) diameter of the stomata, stomatal density (number of stomata per mm2) and the ratio PD/ED. The evaluations of PD and ED were performed in 15 replicates per treatment and stomatal density, in five replicates.
Data were submitted to analysis of variance, when significant (P < 0.05) by the F test and the regression analysis; the choice of the equation was based on the highest coefficient of determination (R 2). When polynomial, after the derivation of the equation, the inflection points of the curves were determined. The software SISVAR® (Sistema de Análise de Variância para Dados Balanceados) (Ferreira 2014) was used.
Results
Perusal of the results revealed that increasing in NaCl dose from 25 to 100 mM in irrigation water progressively inhibited the development of calla lily plants. At a concentration of 100 mM necrosis in leaf tissues was observed and accelerated senescence of mature leaves was noted, due to the presence of toxic Na+ and Cl− ions, which prevented growth, biochemical and ecophysiological characters (Fig. 1).
Plant height decreased with increasing NaCl concentrations at both 60–90 days after the imposition of salt stress and plants that received 75 mM NaCl showed a reduction of 52.15% in height at 60 days and, at 100 mM, it was 69.73%, compared with those under control (without NaCl), and these concentrations had the highest decrease in growth rate (Fig. 2). At 90 days, treatments with 100 mM NaCl strongly limited plant height, not allowing any further assessments to record.
The number of tillers and leaves per plant, however, showed no changes with increasing NaCl concentrations up to 60 days. A cumulative effect was noted and, at 90 days, the number of tillers per plant was found to increase that received up to the maximum estimated concentration of 32.5 mM NaCl (Fig. 3a). On the other hand, the number of leaves per plant and the length of main root were reduced with increasing NaCl concentrations (Fig. 3b, c).
Formation of lateral roots in plants was not observed at a concentration of 0, 25, 50 and 75 mM, but this occurred in 100 mM NaCl (Fig. 4).
As a result of these changes in the shoot and root system, there was also a decrease in the dry matter of shoots and root (Fig. 5a, b).
The ratio of dry matter of shoots/root system showed a decrease with increasing salt concentrations (Fig. 6a). In plants subjected to stress and treated with 25 mM NaCl, as well as in its absence, the allocation of dry matter was higher in the shoot. However, with increasing salt concentrations from 50 and to 75 mM NaCl, the opposite effect was noted, showing greater dry matter partitioning for the root system (Fig. 6b).
Analyzing the effect of stress imposed on physiological characteristics, we observed that after 60 days, the photosynthetic rate (A) decreased as there was an increase in NaCl concentrations, it is smaller in 75 mM (Fig. 7a). A decrease was also observed in stomatal conductance, transpiration rate and chlorophyll content. Stomatal conductance (gs) was higher at the estimated maximum concentration of 23.25 mM NaCl (Fig. 7b) which, in turn, reduced transpiration rate (Fig. 7c). However, at 90 days, the photosynthetic rate was not affected due to different NaCl concentrations. Nonetheless, stomatal conductance reduced up to the estimated minimum concentration of 39.13 mM, and the transpiration rate was increased (Fig. 7b, c).
Photosynthetic rate-A (a), stomatal conductance-gs (b), transpiration rate-E (c) and relative chlorophyll content (d) of calla lily plants submitted to concentrations of 0, 25, 50 and 75 mM NaCl after 60 and 90 days. a y = −0.0797x + 5.111, b 60 days: y = 0.000023x 2 – 0.001x + 0.045; 90 days: y = −0.000029x 2 + 0.001x + 0.013, c 60 days = −0.0007x 2 + 0.038x + 0.5016; 90 days: y = 0.0004x 2 − 0.0317x + 1.0118 and d 60 days: y = −0.0028x 2 + 0.14x + 51.589
After 60 days of salinity imposition, the relative chlorophyll content was lower at higher salt concentrations. However, after 90 days, this reduction with increased salt concentration did not occur (Fig. 7d).
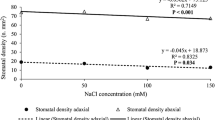
Observing the cross sections of calla lily leaves, there was an increase in stomatal density with stress induction (Fig. 8a, b). Under normal conditions, the number of stomata on the abaxial surface of the epidermis was approximately 32.5%, higher than on the adaxial surface for calla lily, as observed by Yiotis and Psaras (2011). However, with the increase in salinity, this difference was increased to 51% (Fig. 8a, b). In addition, a reduction in the polar (PD) and equatorial (ED) diameter of the stomata in the abaxial and adaxial epidermis was observed with the increase in salinity from 50 mM NaCl (Fig. 8a, b), as well as a parallel reduction in the ratio PD/ED in the adaxial and abaxial epidermis were also noted, reducing from 50 mM NaCl to the adaxial surface and linearly on the abaxial surface with increasing of salt concentration (Fig. 8c, d).
Stomatal density and polar and equatorial diameter of stomata on the adaxial (a) and abaxial (b) surface, PD/ED ratio of stomata in the adaxial (c) and abaxial (d) epidermis of calla lily plants treated submitted to concentrations of 0, 25, 50, 75 and 100 mM NaCl for 90 days. c y = −0.0009x 2 + 0.007x + 1.322 and d y = −0.0016x + 1.3861
Protein concentration in roots did not change due to different NaCl concentration. However, in shoots, with 75 mM NaCl, the protein concentration was almost five times higher than the control treatment (Fig. 9).
In the shoot, the concentrations of total soluble sugars and non-reducing sugars in plants treated with 50 mM NaCl were increased by 76.79% (Fig. 10a) and 73.94% (Fig. 10b), when compared with control. However, the concentration of reducing sugars did not differ due to different NaCl concentrations. The highest starch concentrations were observed in plants treated with 70.45 mM NaCl (Fig. 10c). In the roots, the concentrations of total soluble sugars, reducing and non-reducing sugars increased with increasing doses of NaCl (Fig. 10a, b, d). Moreover, was noted the minimum for starch with 35.08 mM NaCl (Fig. 10c).
Concentration of total soluble sugars—TSS (a), non-reducing sugars—NRS (b), starch (c) and reducing sugars—RS (d) in the shoot and root system of calla lily plants submitted to concentrations of 0, 25, 50 and 75 mM NaCl for 90 days. a R.S.: y = −0.0319x 2 + 5.4586x + 313.2, b R.S.: y = −0.0318x 2 + 5.4485x + 312.85, c shoot: y = −0.0236x 2 + 3.2415x + 339.66, R.S.: y = 0.3274x 2 − 22.94x + 881.5 and d y = 3.0685x + 333.12
Discussion
Plants are often exposed to conditions of biotic and abiotic stress and, between abiotic stress, salinity is one of the main limiting factors of crop production worldwide (Munns and Tester 2008). Salt stress restricts water uptake, leading the cells to lose turgor, increasing the concentration of ions in the cells, causing nutritional and metabolic imbalance and disorders. These resulted a reduction in the expansion of leaf surface, loss of turgor, dehydration, decreased growth and elongation of roots, besides the increase in respiratory rate and a decrease in photosynthetic rate (Munns and Tester 2008; Dias and Blanco 2010; Nawaz et al. 2010).
In calla lily, the reduction in height in the first evaluation period is indicative of the effect of salinity, a result of the osmotic effect of salts, which limits cell expansion (Munns 2002). However, in the same period, there were no differences in the number of tillers and leaves formed, indicating that they are affected in the longer term. Thus, after 90 days, the increase in the number of tillers up to a concentration of 32.5 mM NaCl can be justified by the essentiality of chlorine as a micronutrient, necessary for a better plant development, when it is present in irrigation water at appropriate levels, but at higher concentrations the number of tillers decrease again. The reduction in the number of leaves may be associated with the accumulation of Na+ and Cl− in the cell wall and into the cytoplasm of older leaves. Moreover, the reduction in the number of leaves may also be an adaptive mechanism of the plant to minimize water loss by transpiration (Nawaz et al. 2010).
Analyzing the root system of the plants when subjected to salt stress, a reduction in the main root length was observed, which can be attributed to restrictions in cell division and elongation. This decrease may be a result of the ionic toxicity occurring in the rhizosphere (Galvan-Ampudia and Testerink 2011). However, changing effects on root architecture, such as the formation of lateral roots, are strategies of plants to mitigate the stress imposed by salinity, being seen as an adaptive mechanism, since it is possible to cover a larger soil volume, in an attempt to find suitable portions with lower salinity and higher nutrient availability, enabling normal plant development (Castro et al. 2009; Galvan-Ampudia and Testerink 2011).
Moreover, with increasing salt concentrations, the largest reduction in shoot dry matter may be due to the translocation of ions from the root system which, at higher concentrations, become cytotoxic and cause damage to plant development (Nawaz et al. 2010). The greater partitioning of dry matter in the root system can be observed by the reduction of 80.33% in the shoot, while the root system reduced only 44.43%, when compared to the control and application of 75 mM NaCl. Therefore, it is possible to prove the sensitivity of the species Z. aethiopica to salt stress, since plants with greater tolerance restrict the accumulation of ions Na+ and Cl− in the shoot through compartmentalization in the root system, with greater allocation of dry matter in the shoots, not in the roots (Cavalcanti et al. 2004). Similar results were reported for four Z. K. Koch cultivars (Z. elliotiana x rehmannii, Z. rehmannii Engl., Z. albomaculata (Hook.) Baille and Z. rehmannii violaceae), which showed that the shoot biomass was significantly reduced in 50 mM NaCl treatment (Veatch-blohm and Morningstar 2011).
To understand how salt stress affected the growth characteristics of the plants, we analyzed the effect of the treatments on physiological and anatomical factors. A reduction in photosynthetic rate at 60 days was observed, which caused lower plant growth after 60 days using NaCl. This effect may be related to low photosynthetic assimilation of CO2 (Silva et al. 2008) or decreased stomatal conductance, transpiration rate and chlorophyll content (Veatch-blohm et al. 2012; Rivero et al. 2014).
The reduction in stomatal conductance after 60 days allowed plants to maintain low levels of toxic ions, functioning as a protective mechanism, in addition to the economy and efficient use of water. This is considered an adaptive mechanism of the plant (Parida and Das 2005; Chaves et al. 2009; Debez et al. 2013; Fernández-García et al. 2014). However, with the reduction in stomatal conductance, the photosynthetic assimilation of CO2 was also reduced, parallel to the production of photoassimilates, which limits plant growth (Feijão et al. 2011). These effects were distinctly observed in calla lily, when height, number of tillers and leaves were analyzed.
The unchanged photosynthetic rate at 90 days may be explained from the fact that even when other factors indicate the occurrence of stress, anatomical changes in leaves, such as greater density of chloroplasts and stomata, can result in increased stomatal conductance and transpiration rates (Shabala and Munns 2012). Thus, the lower PD/ED ratio of stomata at higher salt concentrations, the unchanged photosynthetic rate, the increase in stomatal conductance and transpiration rates can be related to increased stomatal density in the adaxial and abaxial epidermis. However, the changes were not sufficient for the adaptation of plants to salt stress, since all growth parameters were adversely affected by the increased concentration of NaCl. It was observed that the increase in salts in the soil prevented greater water absorption; therefore, the transpiration rate might have been higher than the water absorption, indicating an inefficient use of water.
The reduction in the relative chlorophyll content at higher salt concentrations after 60 days of salt stress is a result of the degradation of chlorophyll, mainly by chlorophyllase, at the beginning of salt stress (Veatch-blohm et al. 2012). However, after 90 days, this reduction did not occur with the increase in salt concentration, which justifies the unchanged photosynthetic rate found at 90 days, since a higher concentration of chlorophyll may indicate the highest chloroplast density (Shabala and Munns 2012; Tarchoune et al. 2012).
When evaluating biochemical parameters, we observed that there was a difference in the protein concentration only in shoots, possibly due to the translocation of ions from the roots, justifying the observations of changes in the relationship between the dry matter of shoots and root system and the reduction in growth parameters. Salinity caused an increase in total soluble sugars and non-reducing sugars in the shoot.
In roots, increase in concentrations of total soluble sugars, reducing and non-reducing sugars with increasing NaCl doses, is related to the maintenance of the osmotic balance of the cell (Lacerda et al. 2001). In the root system, the increase in carbohydrate contents and the unchanged protein synthesis may be an attempt to protect the rhizomes during salt stress (Prisco and Gomes Filho 2010), since this structure has a reserve function.
The change in protein synthesis, the increase in carbohydrate concentration and the reduction in photosynthetic rate are strongly related with growth inhibition of calla lily plants, when subjected to salt stress. Therefore, according to the observations on growth, ecophysiological, biochemical and anatomical analyses, it is possible to classify the species as sensitive to salt stress, since it responded negatively to the application of higher than 25 mM NaCl (2.30 dS m−1) concentrations according to the scale determined by Grieve et al. (2012).
Conclusions
Salt stress reduced the height, the number of leaves and the number of tillers in calla lily plants. This reduction, especially height, is correlated with lower rate of photosynthetic found in highest concentrations of NaCl and reducing carbohydrates in leaves, even though an adaptation attempt has occurred with increased roots.
In salt stress conditions occurred anatomical changes in the calla lily plants. The reduced diameter polar and equatorial and low stomatal functionality is indicative the poor adaptability of the species to salt stress. The increase of the stomata on the abaxial surface justifies the photosynthetic rate unchanged at 90 days of evaluation, because this date was reduced stomatal conductance and transpiration increased, leading to a decrease in photosynthetic rate.
Negative changes in growth analysis, ecophysiological and biochemical can classify the species as sensitive to salt stress, because it responded negatively to the application of higher than 25 mM NaCl.
Author contribution statement
Júnia Rafael Mendonça Figueiredo planned the study, performed the experiments, analyzed the results and wrote the manuscript. Patrícia Duarte de Oliveira Paiva, research supervisor, contributed with scientific advice, corrected and revised the final version of the manuscript. Michele Valquíra dos Reis assisted in the planning and supervision of the study, performed the experiments, analyzed the results and corrected the manuscript. Fernanda Carlota Nery contributed with scientific advice and with the correction of the manuscript. Samantha de Menezes Campos assisted performed the experiments and analyzed the results. Diogo Pedrosa Corrêa da Silva was responsible for the statistical analysis and contributed with scientific advice. Renato Paiva, responsible for coordinating the Tissue Culture Laboratory, contributed with scientific advice. All authors read and approved the final manuscript.
References
Almeida EFA, Paiva PDO, Frazão JEM, Santos FHS, Resende FA, Campos ML (2012) Produção de copo-de-leite em resposta à adubação com NPK e esterco bovino. Rev Bras Hort Ornam 18:129–134. doi:10.14295/rbho.v18i2.684
Assis Júnior JO, Lacerda CF, Silva FB, Silva FLB, Bezerra MA, Gheyi HR (2007) Produtividade do feijão-de-corda e acúmulo de sais no solo em função da fração de lixiviação e da salinidade da água de irrigação. Eng Agríc 27:702–713. doi:10.1590/S0100-69162007000400013
Bradford MM (1976) A rapid and sensitive method for the determination of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochm 72:248–254. doi:10.1016/0003-2697(76)90527-3
Cai X, Niu G, Starman T, Hall C (2014) Response of six garden roses (Rosa x hybrida L.) to salt stress. Sci Hortic 168:27–32. doi:10.1016/j.scienta.2013.12.032
Castro EM, Pereira FJ, Paiva R (2009) Histologia vegetal: estrutura e função dos órgãos vegetativos. Editora UFLA, Lavras
Cavalcanti FR, Oliveira JTA, Martins-Miranda AS, Viégas RA, Silveira JAG (2004) Superoxide dismutase, catalase and peroxidase activities do not confer protection against toxidative damage in salt-stressed cowpea leaves. New Phytol 163:563–571. doi:10.1111/j.1469-8137.2004.01139
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560. doi:10.1093/aob/mcp017
Comissão de Fertilidade do Solo do Estado de Minas Gerais—CFSEMG (1999) Recomendações para o uso de corretivos e fertilizantes em Minas Gerais: 5a aproximação. Imprensa Universitária UFV, Viçosa
Debez A, Rejeb KB, Ghars MA, Gandour M, Megdiche W, Hamed KB, Amor NB, Brown SC, Savouré A, Abdelly C (2013) Ecophysiological and genomic analysis of salt tolerance of Cakile maritima. Environ Exp Bot 92:64–72. doi:10.1016/j.envexpbot.2012
Dias NS, Blanco FF (2010) Efeito dos sais no solo e na planta. In: Gheyi HR, Dias NS, Lacerda CF (eds) Manejo da salinidade na agricultura: estudos básicos e aplicados. INCT-Sal, Fortaleza, pp 129–140
Feijão AR, Silva JCB, Marques EC, Prisco JT, Gomes-Filho E (2011) Efeito da nutrição de nitrato na tolerância de plantas de sorgo à salinidade. Rev Ciênc Agron 42:675–683. doi:10.1590/S1806-66902011000300014
Fernández-García N, Olmos E, García-De La Garma J, López-Berenquer C, Rubio-Asensio JS (2014) Intrinsic water use efficiency controls the adaptation to high salinity in a semi-arid adapted plant, henna (Lawsonia inermis L.). J Plant Physiol 171:64–75. doi:10.1016/j.jplph.2013.11.004
Ferreira DF (2014) Sisvar: a guide for its bootstrap procedures in multiple comparisons. Ciênc Agrotec 38:109–112. doi:10.1590/S1413-70542014000200001
Fraire-Velázquez S, Balderas-Hernández VE (2013) Abiotic stress in plants and metabolic responses. In: Vahdati K, Leslie C (eds) Abiotic stress-plant responses and applications in agriculture. Intech, Rijeka, pp 25–48
Furtini Neto AE, Boldrin KVF, Mattson NS (2015) Nutrition and quality in ornamental plants. Ornam Hort 21:139–150. doi:10.14295/aohl.v21i2.809
Galvan-ampudia CS, Testerink C (2011) Salt stress signals shape the plant root. Curr Opin Plant Biol 14:296–302. doi:10.1016/j.pbi.2011.03.019
Grieve CM, Grattan SR, Maas EV (2012) Plant salt tolerance. In: Wallender WW, Tanji KK (eds) ASCE manual and reports on engineering practice no 71 agricultural salinity assessment and management. ASCE, Reston, pp 405–459
Kraus JE, Arduin M (1997) Manual básico de métodos em morfologia vegetal. EDUR, Rio de Janeiro
Lacerda CF, Cambraia J, Cano MAO, Ruiz HÁ (2001) Plant growth and solute accumulation and distribution in two sorghum genotypes, under NaCl stress. Rev Bras Fisiol Veg 13:270–284. doi:10.1590/S0103-31312001000300003
Maciel MP, Soares TM, Gheyi HR, Rezende EPL, Oliveira GXS (2012) Produção de girassol ornamental com uso de águas salobras em sistema hidropônico NFT. Rev Bras Eng Agric Ambient 16:165–172. doi:10.1590/1807-1929/agriambi.v18n12p1228-1234
Melo GM, Cunha PC, Pereira JAF, Willadino L, Ulisses C (2011) Alterações anatômicas em folhas e raízes de Jatropha curcas L. cultivadas sob estresse salino. Rev Ciênc Agron 42:670–674. doi:10.1590/S1806-66902011000300013
Miller GL (1959) Determination of reducing sugar by DNS method. Anal Chem 31:426–428
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250. doi:10.1046/j.0016-8025.2001.00808
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. doi:10.1146/annurev.arplant.59.032607.092911
Navarro A, Elia A, Conversa G, Campi P, Mastrorilli M (2012) Potted mycorrhizal carnation plants and saline stress: growth, quality and nutritional plant responses. Sci Hortic 140:131–139. doi:10.1016/j.scienta.2012.03.016
Nawaz K, Hussain K, Majeed A, Khan F, Afghan S, Ali K (2010) Fatality of salt stress to plants: morphological, physiological and biochemical aspects. Afr J Biotechnol 9:5475–5480. doi:10.5897/AJB10.100
Paiva PDO, Almeida EFA (2012) Produção de flores de corte. Editora UFLA, Lavras
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349. doi:10.1016/j.ecoenv.2004.06.010
Prisco T, Gomes Filho E (2010) Fisiologia e bioquímica do estresse salino em plantas. In: Gheyi HR, Dias NS, Lacerda CF (eds) Manejo da salinidade na agricultura: Estudos básicos e aplicados. INCT-Sal, Fortaleza, pp 147–163
Rasool S, Hameed A, Azooz MM, Rehman M, Siddigi TO, Ahmad P (2013) Salt stress: causes, types and responses of plants. In: Ahmad P, Azooz MM, Prasad MNV (eds) Ecophysiology and responses of plants under salt stress. Springer, New York, pp 1–24
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. Soil Sci 78:154. doi:10.1097/00010694-195408000-00012
Rivero RM, Mestre TC, Mittler R, Rubio F, Garcia-Sanchez F, Martinez V (2014) The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ 37:1059–1073. doi:10.1111/pce.12199
Shabala S, Munns R (2012) Salinity stress: physiological constraints and adaptative mechanisms. In: Shabala S (ed) Plant stress physiology. Cabi, New York, pp 59–93
Silva EC, Nogueira RJMC, Araújo FP, Melo NF, Azevedo Neto AD (2008) Physiological responses to salt stress in young umbu plants. Environ Exp Bot 63:147–157. doi:10.1016/j.envexpbot.2007.11.010
Tarchoune I, Degl’innocenti E, Kaddour R, Guidi L, Lachaâl M, Navari-Izzo F, Ouerghi Z (2012) Effects of NaCl or Na2SO4 salinity on plant growth, ion content and photosynthetic activity in Ocimum basilicum L. Acta Physiol Plant 34:607–615. doi:10.1007/s11738-011-0861-2
Travassos KD, Soares FAL, Gheyi HR, Dias NS, Nobre RG (2011) Crescimento e produção de flores de girassol irrigado com água salobra. Rev Bras Agric Irrigada 5:123–133. doi:10.7127/rbai.v5n200036
Veatch-Blohm ME, Morningstar L (2011) Calla lily growth and development under saline irrigation. HortScience 46:222–227
Veatch-Blohm ME, Malinowski M, Keefer D (2012) Leaf water status, osmotic adjustment and carbon assimilation in colored calla lilies in response to saline irrigation. Sci Hortic 144:65–73. doi:10.1016/j.scienta.2012.06.036
Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57:508–514. doi:10.1042/bj0570508
Yiotis C, Psaras GK (2011) Dianthus caryophyllus stems and Zantedeschia aethiopica petioles/pedicels show anatomical features indicating efficient photosynthesis. Flora 206:360–364. doi:10.1016/j.flora.2010.07.004
Acknowledgements
The authors would like to thank Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B Zheng.
Rights and permissions
About this article
Cite this article
Figueiredo, J.R.M., Paiva, P.D.d.O., dos Reis, M.V. et al. Development changes in calla lily plants due to salt stress. Acta Physiol Plant 39, 147 (2017). https://doi.org/10.1007/s11738-017-2446-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2446-1