Abstract
Cyclotides are small plant disulfide-rich and cyclic proteins with a diverse range of biological activities. Cyclotide-like genes show key sequence features of cyclotides and are present in the Poaceae. In this study the cDNA of the nine cyclotide-like genes were cloned and sequenced using 3′RACE from Zea mays. The gene expression of two of these genes (Zmcyc1 and Zmcyc5) were analyzed by real-time PCR in response to biotic (Fusarium graminearum, Ustilago maydis and Rhopalosiphum maydis) and abiotic (mechanical wounding, water deficit and salinity) stresses, as well as in response to salicylic acid and methyl jasmonate elicitors to mimic biotic stresses. All isolated genes showed significant similarity to other cyclotide-like genes and were classified in two separate clusters. Both Zmcyc1 and Zmcyc5 were expressed in all studied tissues with the highest expression in leaves and lowest expression in roots. Wounding, methyl jasmonate and salicylic acid significantly induced the expression of Zmcyc1 and Zmcyc5 genes, but the higher expression was observed for Zmcyc1 as compared with Zmcyc5. Expression levels of these two genes were also induced in inoculated leaves with F. graminearum, U. maydis and also in response to insect infestation. In addition, the 1000-base-pairs (bp) upstream of the promoter of Zmcyc1 and Zmcyc5 genes were identified and analyzed using the PlantCARE database and consequently a large number of similar biotic and abiotic cis-regulatory elements were identified for these two genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclotides are a family of bioactive small proteins from plants that contain a cyclized backbone and a knotted array of three disulfide bonds (Craik et al. 1999). They normally consist of 28–37 amino acids and are the largest family of circular proteins. The discovery of cyclotides tracs back to the time when native medicine was used in Africa in 1970. Oldenlandia affinis as a medicinal plant has long been used in parts of Africa by women to assist childbirth. In the early 1970s, the kalata B1 peptide was identified as the first cyclotide that is responsible for the uterotonic activity (Gran 1973). Later several other plant-derived peptides were identified with a similar circular backbone and cysteine content to kalata B1 (Daly et al. 1999).
It has been shown that cyclotides accomplish various biological activities including uterotonic activity (Gran 1973; Koehbach et al. 2013), neurotensin inhibition (Witherup et al. 1994), anti-HIV (Gustafson et al. 1994), haemolytic (Barry et al. 2003), anti-bacterial (Ovesen et al. 2011), insecticidal (Jennings et al. 2001; Gruber et al. 2007), and cytotoxic activities (Lindholm et al. 2002). Cycloviolacin VYI—a cyclotide from Viola yedoensis is active against influenza A H1N1 virus (Liu et al. 2014). Most of the studies have focused on animal or human antifungal and anti-bacterial activities while less works have been carried out on cyclotides effects on plant pathogens. Generally, it has been shown that cyclotides play a role in plants against biotic/abiotic stresses. Cyclotides from Iranian V. odorata showed antimicrobial activity against the plant pathogenic bacteria Xanthomonas oryzae, Ralstonia solanacearum, Ralstonia cicil, and Bacillus sp. (Zarrabi et al. 2013).
Cyclotides show tissue specific expression patterns and are induced by both biotic and abiotic stresses. Variation of cyclotides in different tissues have been reported (Trabi and Craik, 2004; Seydel and Dornenburg 2006; Mylne et al. 2010; Poth et al. 2012). Semi-quantitative analysis of 20 cyclotides from Viola uliginosa in different tissues and in suspension cultures showed that they are differentially expressed in wild type plant tissues and suspension cultures (Slazak et al. 2015). Expression analysis of cyclotide genes from V. baoshanensis showed that some cyclotide genes are induced by cadmium stress and wounding (Zhang et al. 2015). This wide range of expression response of cyclotides in plant cells, is probably associated with the role of these genes in host defense against both biotic and abiotic stresses.
Most cyclotides have been isolated from the Rubiaceae and Violaceae families (Craik and Conibear 2011). However, recently cyclotides have been found in the Cucurbitaceae (Hernandez et al. 2000), Solanaceae (Poth et al. 2012), Fabaceae (Poth et al. 2011) and Poaceae (Mulvenna et al. 2006). In addition to regular cyclic cyclotides, a few acyclic variants of cyclotides have been reported. Acyclic cyclotides contain the similar cysteine arrangement and show high sequence similarity with cyclotides, but they cannot be cyclized (Ireland et al. 2006; Nguyen et al. 2011, 2012). Acyclic cyclotides have been referred as “uncyclotides”. or “acyclotides” (Nguyen et al. 2011; Poth et al. 2012). Recently, nine novel linear cyclotides has been reported from Panicum laxum belonging to Poaceae and it has been shown that they possess a cystine knot arrangement similar to cyclotides (Nguyen et al. 2013).
Also, some cyclotide-like genes have been identified from data mining of nucleotide databases in several plants of the Poaceae (Mulvenna et al. 2006). Searching the databases using cyclotides as queries has resulted in the finding of 32 putative cyclotide-like genes, including Zea mays (11), Triticum aestivum (6), Setaria italica (5), Agrostis stolonifera (3), Pennisetum glaucum (1), Sorghum bicolor (1), Schedonorus arundinaceus (2), Hordeum vulgare (1), Saccharum officinarum (1), and Oryza sativa (1), respectively (Mulvenna et al. 2006; Nguyen et al. 2013). Cyclotide-like genes show significant similarity with other cyclotide genes. The amino acid sequence similarities include six-Cys residues, an absolutely conserved Glu residue in loop 1—a hydroxyl-bearing (Thr or Ser) residue immediately after the Glu residue in loop 1 and the last residue in loop 3 (Mulvenna et al. 2006). Deduced protein sequence of Poaceae cyclotide-like genes are classified into two broad classes. The first class shows significant sequence identity in upstream of the first Cys residue and generally has short tails after the final Cys residue. In the second class the upstream region of the Cys residue is less conserved and the tail after the last Cys residue is longer. In addition, numbers of amino acids between the first and second Cys residues are variable. These show that the Poaceae family contains a wide range of cyclotide-like genes, which have hindered their structural and functional identification.
In the present work, nine cyclotide-like genes were isolated from maize using 3′RACE-PCR. Also, the gene expression of two cyclotide-like genes including Zmcyc1 and Zmcyc5 were analyzed using real-time PCR (RT-PCR). Results showed that cyclotide-like genes expression were dynamic and induced in response to fungal disease, insect attack and signaling molecule treatments which probably reflects their direct or indirect roles in plant defense systems.
Materials and methods
Plant material
Maize (Zea mays L. cv. SC. 704) plants were grown in 10-L pots with a 1:1:1 mix of peat: vermiculite: perlite with fertilizers in a green house with a daily cycle of 14-h light (70–80 W/m2) at 25 °C and 10-h dark.
Stress treatment
To investigate the effect of wounding on gene expression, the leaf lamina was cut with a razor blade and harvested at different time points after wounding. Control samples were collected from healthy leaves as well. For other treatments, leaves were sprayed with 1 mM salicylic acid (SA), 0.1 mM methyl jasmonate (JA) and water as control. For drought treatment, irrigation was withheld for 96 h and three of the youngest leaves were collected from both irrigated and non-irrigated plants for gene expression analysis (Andersen et al. 2002; Boyer and McLaughlin 2007). For salinity treatments, 3-week-old plants were watered with EC = 8 deci Siemens/m (5.12 g/L NaCl) and samples were collected 48 h after treatment. The leaf samples were collected, frozen using liquid nitrogen, and were used for RNA extraction and subsequent analysis.
Fusarium graminearum inoculation
Three-week-old seedlings were subjected to pathogen inoculation using the root-dip method (Elmer and Anagnostakis 1991; Katan et al. 1994). Inoculates for root-dip assays were formulated as liquid cultures of 2% (w/v) diet fiber at 20–28 °C with shaking and subsequently filtered through two layers of cheesecloth. Suspensions containing microconidia were prepared at the density of 106 cfu/ml using a hemocytometer. Roots of 3-week-old seedlings were removed from trays and carefully washed free of soil, then dipped for 3–5 min in spore suspensions for treatment(s) and in dH2O for control plant prior to planting. Sampling was conducted at 0, 3, 6, 9 and 12 days after treatment.
Ustilago maydis inoculation
Teliospores of smut fungi were collected from a corn farm and mixed, powdered and sterilized in 5% sodium hypochlorite solution for 10 min on a shaker, washed twice with dH2O, and then cultured on PDA medium containing 10 g/L dextrose and subsequently filtered through two layers of cheesecloth, and sporidia were collected in dH2O (Thakur et al. 1989; Zamani et al. 2011). A mixture of sporidia with a final concentration of 106 cell/ml was injected into 3-week plant leaf hypodermal tissue. Sampling was conducted at 0, 3, 6, 9, and 12 days after treatment.
Aphid infection
Rhopalosiphum maydis were collected from different sections of maize plants in field. Three weeks after planting, each plant was infested with adult insects. Aphids were transferred to experimental plants with a fine paint brush (Gao et al. 2008). Samples were collected at 0, 3 and 6 days after infection. For the all treatments, leaves of 5 plants per treatment bulked as one replicate and at the end 3 replicates were used in each treatment for gene expression analysis.
RNA extraction
Total RNA was extracted from roots, stems, male and female flowers, coleoptile, coleorhiza, control leaves, and in the leaves of plants subjected to different stresses using TRIzol reagent (Invitrogen), following the manufacturer’s instructions. The quality of RNA was checked by visualizing the ethidium bromide (EtBr)-stained ribosomal RNA in 1% agarose gels. The ratio of 260/280 and 230/260 nm were determined by Eppendorf BioPhotometer Plus.
cDNA synthesis
For cDNA synthesis, 5 μg of total extracted RNA were treated with RNase-free DNase (Promega) according to the manufacture’s instruction to ensure no DNA contamination exist. First strand cDNA was synthesized in a 20 μl reaction system (Fermentas) containing1 μl oligo dT, 1 μl dNTP (50 mM), 8 μl total RNA (1 μg) at 65 °C for 5 min, then 2-min incubation on ice followed by addition of 0.5 μl M-MLV reverse transcriptase (Fermentas), 2 μl reaction buffer and 7.5 μl distilled water and subsequently placed at 42 °C for 60 min.
Isolation of partial sequences by 3′RACE
The sequence of putative cyclotide-like genes of Zea mays including CF060985, CF014141, CK369406, BM379838, CF630454, CF013901, CN070702, BI674581, BM080572, and CK368015 were aligned using CLUSTALW. A forward primer SF1, 5′ AGAGAGAGAGGAAAGCTAGC was designed based on conserved regions of maize sequences (Table 1). First strand cDNA for 3′ RACE was synthesized through reverse transcription using anchor primer (5′ GACCACGCGTATCGATGTCGACTTTTTTTTTTTTTTTTV 3′) and First strand cDNA synthesis kit (Thermo-Fisher Scientific former. Fermentas, Schwerte, Germany). The first cycle of PCR was done in a total volume of 25 μl including 8 μl H2O, 12 μl Master Mix (CinnaGen), 2 μl First strand cDNA template, 1.5 μl SF1, and 1.5 μl PCR anchor primer, GACCACGCGTATCGATGTCGAC. PCR temperature program was 1 cycle of 5 min at 94 °C, 35 cycles of 30 s at 94 °C, 30 s at 53 °C, 40 s at 72 °C, 1 cycle of 20 min at 72 °C. The 1% agarose gel was used to separate PCR product.
Molecular cloning and DNA sequencing
The expected PCR fragments were purified from the gels using a Nucleic Acid Extraction kit (Vivantis) and were ligated into the TA vector using the TA cloning kit (Fermentas). Recombinant plasmids were transformed into competent cells of Escherichia coli DH5a strain. Positive white clones were checked by colony PCR, and their plasmids were extracted. Sequencing of cloned fragments were done by a commercial sequencing service (Bioneer Inc. Bioneer Corporation).
Bioinformatics analysis
The sequence of putative cyclotide-like genes were blasted using BLAST program at National Center for Biotechnology Information Server (http://www.ncbi.nlm.nih.gov/) to their homology. Deduced protein sequence of cyclotide-like genes were aligned using Clustal-Omega. In addition to our new cyclotide-like genes, other cyclotide-like gene sequences were obtained from the GenBank database for phylogenetic analysis (Table 2). The phylogenetic tree of cyclotide-like gene was constructed using MEGA4.0.2 software based on the method of Neighbor-Joining (NJ). Theoretical isoelectric point and mass values for the protein was predicted using ExPASyProtParam tool (http://us.expasy.org/tools/protparam.html). To sequence translate to protein, cyclotide sequence was translated by online software ExPASy in each 6 ORF and then the obtained sequences were analyzed for Cys position and true cyclotide structure. For promoter sequence analysis, first the maize chromosomes were downloaded from ensemblgenome.org (ftp.ensemblgenome.org/pub/plants/release-23/fasta/zea_mays/dna/) and then aligned with cyclotide sequence by offline software stand-aloneBLAST version 2.2.26. After finding the gene position on chromosome, a 1000 bp sequence in upstream of gene was analyzed using PLACE (http://www.dna.affrc.go.jp) and PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
qRT-PCR analysis
Real-time PCR runs was done using Applied BioSystem thermal cycler instrument. For quantification of real-time PCR product, the threshold cycle (Ct) of the cyclotide-like genes were normalized with Actin1 as reference gene. The relative gene expression for each selected genes was quantified for both the control and treatment plants. For real-time PCR expression analysis three independent biological replicates and three technical replicates of each biological replicate were used. The PCR efficiency of each primer pair for target genes and reference genes was evaluated on five log serial dilutions. Power SYBR Green PCR Master Mix (Applied Biosystems) was used for qRT-PCR. Melting curve analysis was conducted by increasing temperature from 60 to 95 °C (0.5 °C per 10 s) along with gel electrophoresis of the final product to check the specific amplification. To confirm the absence of genomic DNA negative control reactions were done using RNA as a template. For relative expression fold changes of each gene, its Ct value was normalized to the Ct value of the reference gene and was calculated relative to a calibrator using the ΔCt method as follows: ΔCt = Ct(target gene) − Ct (Actin 1) and the RGE as: RGE = POWER(2−ΔCt) (Livak and Schmittgen 2001).
Statistical analysis
The expression data was analyzed using SAS version 9.1 (SAS Institute, Inc, Cary, NC, USA). One-way analysis of variance (ANOVA) with LSD post hoc tests was used to reveal significant differences among treatments.
Results
Isolation and sequence analysis of Zmcyc genes
Based on the alignment of 10 expressed sequence tags (ESTs) of Z. mays, a band of the predicted size (~500 bp) was observed after PCR amplification using forward and oligo dT anchor PCR primers. The cDNA synthesized from total RNA extracted from whole plant tissues was used as a template in PCR . The expected fragment was excised from agarose gel and cloned into the plasmid vector (TA cloning, Fermentas). Positive clones were picked and used for screening. Twenty unique clones were chosen for DNA sequencing, of which 9 clones contained the primer sites. BLAST analysis against GenBank database revealed that 9 sequences were highly similar to cyclotide-like genes with e-value of 5e−25 to 1e−155. Conceptual translations of the above 9 sequences revealed that they code for two classes of cyclotide-like proteins named as Zmcyc1 to Zmcyc9 (Fig. 1). These sequences were classified into two groups. The first group had a continuous open reading frame (ORF) and the characteristics of six cysteine motifs and was significantly similar to Poaceae cyclotide-like genes in database. Zmcyc1, Zmcyc2, and Zmcyc3 had an identical amino acid sequence in spite of different nucleotide sequences. All of the group I cyclotide-like genes, Zmcyc1 and Zmcyc5 encode an endoplasmic reticulum signal peptide with 34 amino acids and a putative precursor protein with 54 amino acids that includes a C-terminal domain with 26 amino acids that in turn contains six Cys residues at positions 62, 66,71, 78, 80, and 85 similar to known cyclotides. All the isolated sequences contain a short tail with three residues after the C-terminal Cys residue (Fig. 2).
Putative cyclotide-like genes identified in Z. mays. Zmcyc1-Zmcyc5 shows the conserved structure of the genes in Poaceae species. Each sequence contains a signal sequence (horizontal boxes), a precursor region, the six-Cys domain, and a short C-terminal tail region. The numbering of the loops connecting each Cys residue is indicated at top
The second group includes four sequences which showed less conservation of the six-Cys domain, and also displayed an expansion in the number of amino acids between the first and second or other Cys residues. The lengths of these sequences also varied from 60 to 140 amino acids.
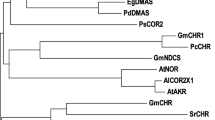
Phylogenetic profiling of gramineous cyclotide-like genes was carried out using MEGA software. The phylogenetic tree showed that gramineous cyclotide genes were separated into two distinct clusters, consistent with the amino acid sequence similarity analysis as shown in Fig. 3. All of the isolated cyclotides-like genes in this study along with nine other putative cyclotide-like genes from maize were placed in one cluster. In another cluster, cyclotide-like genes from other gramineous plants were located along with four linear cyclotides from P. laxum. However, linear cyclotides were found in a separate sub cluster.
Dendrogram of cyclotide-like peptide sequence in Poaceae family. Accession number for each sequence are listed on the right side of the plant name: more details are listed in Table 2. This phylogram is plotted by Criterion neighbor-joining method
Database assisted sequence analysis
Based on genome draft of maize the 1000 bp upstream of both Zmcyc1 and Zmcyc5 genes were isolated and analyzed using PlantCARE. Comparison of the two promoter sequences revealed the presence of different cis-elements at different positions. The promoter sequences of both genes were AT rich with 60.5% and 56.34% AT for Zmcyc1 and Zmcyc5, respectively. The results of some of the identified general transcription and potential regulatory cis-elements for both Zmcyc1 and Zmcyc5 have been summarized in Tables 3 and 4.
Most of cis-elements found in both genes promoters were similar, but some different elements were also found. For both genes TATA box sequence elements required for the critical and precise transcription initiation. However, in Zmcyc5 INRNTPSADB sequence that is called initiator and works in TATA less promoter instead of TATA box were also detected. The CAAT box sequences which are responsible for the tissue specific promoter activities were also found in both gene promoters. In both promoters calcium responsive cis-element ABRERATCAL and ACGTATERD sequence elements were identified. Conserved sequence of CBFHV was found in both promoters that is a low temperature response element. Abiotic stress related elements including LTRECOREATCOR15, MYB1AT, MYB2CONSENSUSAT, and MYBCORE were also detected in both promoters. Some important wounding and disease induced regulatory elements like, WBOXATNPR1, WBOXHVISO1, WBOXNTCHN48, WBOXNTERF3, and WRKY71OS were also found in the promoter region of both genes.
Expression of the Zmcyc1 and Zmcyc5 in different tissues and development stage
Quantitative real-time PCR showed significant differences in gene expression of Zmcyc1 and Zmcyc5 between different tissues and the relative gene expression of Zmcyc1 was significantly higher than that of Zmcyc5. Despite different levels in gene expression of two genes the expression patterns in different tissues were similar. For both genes the highest expression was observed in leaves and stems, respectively. The gene expression of Zmcyc1 and Zmcyc5 in leaves were approximately eight- and three-fold higher than that observed in roots and flowers, respectively (Fig. 4a, b). The lowest gene expression was observed in roots.
Expression of two cyclotide-like genes Zmcyc1 and Zmcyc5 at mRNA level a, b in different tissues (root, stem, leaf, male and female flower) and c, d in different leaf stages over 70-day period at the time of 7 day (cleoptile), 21 day (trifoliate), 30 day (the first stem node), 50 day (appearance of male flowers), and 70 day (appearance of female flowers). Error bars are standard deviations from three biological triplicates (P < 0.05)
Results of gene expression during leaf development showed a dynamic pattern of both genes as that the lowest level of expression for both genes were in 30-day-old leaves, but the highest level of expression for Zmcyc1 was at the time of emergence of female flowers (70-day-old leaves) and for Zmcyc5 at the time of emergence of male flowers (50-day-old leaves) (Fig. 4c, d). The relative expression at these stages was about 70- and 40-fold higher than that of younger leaves (30-day-old leaves).
Wounding, aphid attack
Results showed that the expression of Zmcyc1 was elevated under mechanical wounding and within 24 h reached highest level (about ten-fold higher than the control). Afterwards, Zm cyc1 expression was reduced gradually, but after 72 h the gene expression level was still seven-fold higher than control plants (Fig. 5a). For Zmcyc5 after 24 h the gene expression level was dropped quickly down to the level of control plants. Results showed that the gene expression patterns for both genes were similar and the gene expression reached the highest level 6 days after insect feeding (Fig. 5b, c).
Expression of two cyclotide-like genes Zmcyc1 and Zmcyc5 at mRNA level under wonding on leaf a intact leaf in control plant at the time of 0, 24. 48 and 72 h and b insect attack of corn aphids inside control plant at the time of day 1, day 3 and 6. c Corn leaf aphid infestation on corn leaf in 6-day period. Error bars are standard deviations from three biological triplicates (P < 0.05)
Salicylic acid and methyl jasmonate treatments
Results showed that during the first 24 h after salicylic acid treatment the expression level of Zmcyc1 significantly increased, then gradually decreased at 48 h and remained constant until 72 h (Fig. 6a). However, the expression level of Zmcyc5 was reduced after 48 h and reached the control plants level.
The expression of Zmcyc1 increased significantly 24 h after methyl jasmonate treatment, remained constant until 48 h and increased sharply 72 h after treatment (Fig. 6b). Zmcyc5 transcript levels gradually increased and reached the highest level 48 h after treatment and decreased at 72 h. The expression levels of Zmcyc1 were higher than Zmcyc5 after methyl jasmonate treatment.
Ustilago mydis and Fusarium graminearum
Gene expression analysis of Zmcyc1 and Zmcyc5 under infection by F. graminearum showed that 3 days after inoculation, the expression levels of both genes increased significantly,
but at day 4 decreased gradually until day 12 then reached the level of control plants (Fig. 7a, d).
Expression of two cyclotide-like genes Zmcyc1 and Zmcyc5 at mRNA level under treatment with fungal a F. graminarium agent of ear rot and b U. maydis agent of smut after 1, 3, 6, 9 and 12 days. c U. maydis infection in 12-day period, symptoms appeared after 3 days and gall after 9 days. d F. graminearium infection in 12-day period, symptoms appeared after 3 days. Error bars are standard deviations from three biological triplicates (P < 0.05)
The gene expression level for cyclotide-like genes under infection by Ustilago maydis sporidium increased significantly in comparison with control plant and the gene expression coordinately increased with the progression of the disease and symptoms. Three days after infection, the gene expression of both genes increased and remained at that level until day 6 (Fig. 7b, c). The highest gene expression was observed at day 9, when gall symptoms were observed on leaves. 12 days after infection, the level of expression of both genes was decreased to the level of Day 6.
Drought and salinity
The expression levels of Zmcyc1 and Zmcyc5 were significantly increased in plants subjected to drought treatment. In treated plants, the expressions of Zmcyc1 and Zmcyc5 were almost 6 and 8 times higher than control plants at 96 h after latest irrigation, respectively (Fig. 8a). Results showed that the expression of Zmcyc1 and for Zmcyc5 at 48 h after irrigation with salt water (EC = 8) increased 5.2 fold as compared with non-irrigated condition (Fig. 8b).
Expression of two cyclotide-like genes Zmcyc1 and Zmcyc5 at mRNA level under salinity and drought condition. a Zmcyc1 under control and salinity, b Zmcyc5 under control and salinity, c Zmcyc1 under control and drought, d Zmcyc5 under control and drought. For drought treatment, irrigation was withheld for 96 h and three of the youngest leaves were collected for both irrigated and non-irrigated plants for expression analysis. For salinity treatments 3-week-old plants were watered with EC = 8 dSiemens/m (5.12 g NaCl per liter) and samples were collected at 48 h after treatment. Error bars are standard deviations from three biological triplicates (P < 0.05)
Discussion
In this study, nine putative cyclotide-like genes from maize were cloned and sequenced using 3′RACE. The obtained cyclotide-like genes were classified in two groups. Five of them contained six cysteine residues and showed high similarity to the computationally identified cyclotide-like genes in gramineous plants. In Violaceae and Rubiaceae, cyclotides belong to a relatively large family. For example, more than 50 cyclotides have been identified in Viola hederacea (Trabi and Craik 2004). However, less putative cyclotide-like genes have been identified in gramineous plants. Most gramineous plants have less than ten and within them the majority have one or two cyclotide genes (Mulvenna et al. 2006). There are no comprehensive studies on cyclotide-like genes in gramineous plant and most of the studies are based on similarity searches in EST databases. In addition, a recent study showed that cyclotide-like genes in gramineous plant show variation in their structure and types. Nine novel linear cyclotides have been reported from the Panicum laxum of the Poaceae family (Nguyen et al. 2013). Linear cyclotides have been reported in two species including A. stolonifera and S. italica, from the Poaceae by searching nucleotide databases. Linear cyclotides possesses a cysteine knot arrangement similar to cyclotides, hence it is possible to identify more cyclic or acyclic cyclotide genes from gramineous plants in the future.
Four of the cloned putative cyclotide-like genes from maize were completely different in the number of cysteine residues, their positions and also in the length of the putative peptides. They showed significant similarity to cyclotide-like genes and clustered in a phylogenetic tree with cyclotide-like genes from maize and wheat. Current functions of these genes still remains poorly understood. They may come from a new class of cyclotide-like genes that have not yet been studied. It is also possible that they are a new class of small peptides similar to cyclotide genes or maybe pseudogenes.
In the present work, the gene expression patterns of two cyclotide-like genes including Zmcyc1 and Zmcyc5 were studied under different conditions and treatments, both biotic and abiotic. Both Zmcyc1 and Zmcyc5 genes were expressed in all mature plant tissues, but a higher expression levels were observed in leaves and stems. The highest expression of both genes was detected in mature leaves. In Viola hederacea different cyclotide genes are expressed in different tissues, e.g., a root specific cyclotide has been identified (Trabi and Craik 2004). Gene expression analysis of a cyclotide-like gene Bcl 1 in barley has shown that Bcl 1 gene expression in the coleoptile and in the first leaf is higher than other tissues (Mulvenna et al. 2006). Rcl is a cyclotide-like gene in rice expressed in all tissues except flowers and its highest expression is observed in roots (Mulvenna et al. 2006). The expression of Barley Bcl1 in the coleoptile over 7 days shows that its expression gradually increased until day 5. Zmcyc1 and Zmcyc5 genes showed a similar expression pattern in different tissues and, in different developmental stages of leaves. Analysis of cis-elements in Zmcyc1 and Zmcyc5 promoters showed that they contain similar cis-elements, consistent with their similar expression patterns.
Methyl jasmonate and salicylic acid act as global signals for defense genes. Cross-talk between methyl jasmonate and salicylic acid pathways appears to be very common and important in the regulation of defense gene expression. There is no report on the expression of cyclotide-like genes in response to signaling molecules such as JA, ethylene and SA. Many reports show that SA and JA regulate many pathogenesis-related (PR) genes. To our knowledge there are no reports on expression analysis of cyclotides following signaling molecule treatments. Cyclotides show antimicrobial activities against a wide range of pathogens, hence they might be responding to signaling molecules such as SA and JA.
There are many reports of antifungal activity of cyclotides (Jennings et al. 2001; Tam et al. 1999). It has been reported that infection of maize plants with the smut fungus Ustilago maydis upregulates the expression of many genes including a cyclotide-like named Umi11 that is significantly induced, especially in gall development stage (Basse 2005). In agreement with the latter study, both Zmcyc1 and Zmcyc5 expression significantly increased in 9 days after inoculation which coincided with the time of gall symptoms emergence. Similar results were observed for both genes after F. graminearum infection in maize. Also integrated analysis of proteomics and transcriptomics data showed that the resistance to Fusarium Head Blight (FHB) is mediated by regular and coordinated expression of signal molecules, e.g., salicylic acid, jasmonic acid, ethylene, and ROS inside secondary metabolites (Ding et al. 2011). It has been reported that PR proteins have a role in resistance to FHB in wheat (Gottwald et al. 2012; Li and Yen 2008; Xiao et al. 2013). Consistent with this, it is possible that Zmcyc1 and Zmcyc5 encoding PR proteins could also play defense roles in the maize defense systems against fungi.
To examine whether Zmcyc1 and Zmcyc5 are dynamically regulated by abiotic stresses, maize plants subjected to drought and salinity and their expression were examined over 48 h and results showed that their expression increased after treatments. Already few reports suggest that cyclotides are induced by abiotic factors. In Oldenlandia affinis, a cyclotide kalata B5 is only detected during winter (Plan et al. 2010). Also, cyclotide peptide profile between two plants of the same species of Viola hederacea is different at diverse locations (Trabi and Craik 2004). Furthermore, the levels of some cyclotides are significantly different in Swedish violets during the warm month of July relative to April and September (Trabi and Craik 2004). In addition, six cyclotides from Viola baoshanensis show cadmium-dependent up-regulation (Zhang et al. 2009). These authors have speculated that cyclotide proteins in V. baoshanensis possibly play a role as a reactive oxygen species scavenger. Therefore, the up-regulation of these cyclotide-like genes in abiotic stress may be related to a similar function.
Taking together, cyclotide like genes in this study showed various response to a range of biotic and abiotic stresses in maize. Therefore, it is possible that they spatially and developmentally regulated and their expression is triggered by different stimuli. Further analysis and experimentation would need to be performed to determine the detail function of these genes in each stress and whether or not they provide enhanced plant defense.
Author contribution statement
BB conceived and designed the experiments; HS performed the experiments; HS, and MM analyzed the data; and BB and HS wrote the paper.
References
Andersen MN, Asch F, Wu Y, Jensen CR, Næsted H, Mogensen VO, Koch KE (2002) Soluble invertase expression is an early target of drought stress during the critical, abortion-sensitive phase of young ovary development in maize. Plant Physiol 130:591–604
Barry DG, Daly NL, Clark RJ, Sando L, Craik DJ (2003) Linearization of a naturally occurring circular protein maintains structure but eliminates hemolytic activity. Biochemistry 42:6688–6695
Basse CW (2005) Dissecting defense-related and developmental transcriptional responses of maize during Ustilago maydis infection and subsequent tumor formation. Plant Physiol 138:1774–1784
Boyer JS, McLaughlin JE (2007) Functional reversion to identify controlling genes in multigenic responses: analysis of floral abortion. J Exp Bot 58:267–277
Craik DJ, Conibear AC (2011) The chemistry of cyclotides. J Org Chem 76:4805–4817
Craik DJ, Daly NL, Bond T, Waine C (1999) Plant cyclotides: a unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J Mol Biol 294:1327–1336
Daly NL, Love S, Alewood PF, Craik DJ (1999) Chemical synthesis and folding pathways of large cyclic polypeptides: studies of the cystine knot polypeptide kalata B1. Biochemistry 38:10606–10614
Ding L, Xu H, Yi H, Yang L, Kong Z, Zhang L, Xue S, Jia H, Ma Z (2011) Resistance to hemi-biotrophic Fusarium graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PLoS One 6:e19008
Elmer WH, Anagnostakis S (1991) Vegetative compatibility groups of Fusarium proliferation from Asparagus and comparisons of virulence, growth rates, and colonization of Asparagus residues among groups. Phytopathology 81:852–857
Gao L-L, Klingler JP, Anderson JP, Edwards OR, Singh KB (2008) Characterization of pea aphid resistance in Medicago truncatula. Plant Physiol 146:996–1009
Gottwald S, Samans B, Luck S, Friedt W (2012) Jasmonate and ethylene dependent defence gene expression and suppression of fungal virulence factors: two essential mechanisms of Fusarium head blight resistance in wheat. BMC Genom 13:369
Gran L (1973) On the effect of a polypeptide isolated from “Kalata-Kalata”(Oldenlandia affinis DC) on the oestrogen dominated uterus. Acta Pharmacol Tox 33:400–408
Gustafson KR, Sowder RC, Henderson LE, Parsons IC, Kashman Y, Cardellina JH, McMahon JB, Buckheit RW Jr, Pannell LK, Boyd MR (1994) Circulins A and B. Novel human immunodeficiency virus (HIV)-inhibitory macrocyclic peptides from the tropical tree Chassalia parvifolia. J Am Chem Soc 116:9337–9338
Gruber CW, Cemazar M, Clark RJ, Horibe T, Renda RF, Anderson MA, Craik DJ (2007) A novel plant protein-disulfide isomerase involved in the oxidative folding of cystine knot defense proteins. J Biol Chem 282:20435–20446
Hernandez J-F, Gagnon J, Chiche L, Nguyen TM, Andrieu J-P, Heitz A, Trinh Hong T, Pham TTC, Le Nguyen D (2000) Squash trypsin inhibitors from Momordica cochinchinensis exhibit an atypical macrocyclic structure. Biochemistry 39:5722–5730
Ireland DC, Colgrave ML, Nguyencong P, Daly NL, Craik DJ (2006) Discovery and characterization of a linear cyclotide from Viola odorata: implications for the processing of circular proteins. J Mol Biol 357:1522–1535
Jennings C, West J, Waine C, Craik D, Anderson M (2001) Biosynthesis and insecticidal properties of plant cyclotides: the cyclic knotted proteins from Oldenlandia affinis. Proc Nat Acad Sci USA 98:10614–10619
Katan T, Katan J, Gordon T, Pozniak D (1994) Physiologic races and vegetative compatibility groups of Fusarium oxysporum f. sp. melonis in Israel. Phytopathol New York Baltimore Then St Paul- 84:153
Koehbach J, O’Brien M, Muttenthaler M, Miazzo M, Akcan M, Elliott AG, Daly NL, Harvey PJ, Arrowsmith S, Gunasekera S, Smith TJ (2013) Oxytocic plant cyclotides as templates for peptide G protein-coupled receptor ligand design. Proc Natl Acad Sci USA 110:21183–21188
Li G, Yen Y (2008) Jasmonate and ethylene signaling pathway may mediate Fusarium head blight resistance in wheat. Crop Sci 48:1888–1896
Lindholm P, Göransson U, Johansson S, Claeson P, Gullbo J, Larsson R, Bohlin L, Backlund A (2002) Cyclotides: a novel type of cytotoxic agents. Mol Cancer Ther 1:365–369
Liu M, Yang Y, Zhang S, Tang L, Wang H, Chen C, Shen Z, Cheng K, Kong J, Wang W (2014) A cyclotide against influenza A H1N1 virus from Viola yedoensis. Yao Xue Xue Bao 49:905–912
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Mylne JS, Wang CK, van der Weerden NL, Craik DJ (2010) Cyclotides are a component of the innate defense of Oldenlandia affinis. Peptide Sci 94:635–646
Mulvenna JP, Mylne JS, Bharathi R, Burton RA, Shirley NJ, Fincher GB, Anderson MA, Craik DJ (2006) Discovery of cyclotide-like protein sequences in graminaceous crop plants: ancestral precursors of circular proteins? Plant Cell 18:2134–2144
Nguyen GKT, Zhang S, Wang W, Wong CTT, Nguyen NTK, Tam JP (2011) Discovery of a linear cyclotide from the bracelet subfamily and its disulfide mapping by top-down mass spectrometry. J Biol Chem 286:44833–44844
Nguyen GKT, Lim WH, Nguyen PQT, Tam JP (2012) Novel cyclotides and uncyclotides with highly shortened precursors from Chassalia chartacea and effects of methionine oxidation on bioactivities. J Biol Chem 287:17598–17607
Nguyen GKT, Lian Y, Pang EWH, Nguyen PQT, Tran TD, Tam JP (2013) Discovery of linear cyclotides in monocot plant Panicum laxum of Poaceae family provides new insights into evolution and distribution of cyclotides in plants. J Biol Chem 288:3370–3380
Ovesen RG, Nielsen J, Bruun Hansen HC (2011) Biomedicine in the environment: Sorption of the cyclotide kalata B2 to montmorillonite, goethite, and humic acid. Environ Toxicol Chem 30:1785–1792
Plan MR, Rosengren KJ, Sando L, Daly NL, Craik DJ (2010) Structural and biochemical characteristics of the cyclotide kalata B5 from Oldenlandia affinis. Peptide Sci 94:647–658
Poth AG, Colgrave ML, Lyons RE, Daly NL, Craik DJ (2011) Discovery of an unusual biosynthetic origin for circular proteins in legumes. Proc Nat Acad Sci USA 108:10127–10132
Poth AG, Mylne JS, Grassl J, Lyons RE, Millar AH, Colgrave ML, Craik DJ (2012) Cyclotides associate with leaf vasculature and are the products of a novel precursor in Petunia (Solanaceae). J Biol Chem 287:27033–27046
Seydel P, Dornenburg H (2006) Establishment of in vitro plants, cell and tissue cultures from Oldenlandia affinis for the production of cyclic peptides. Plant Cell Tissue Org 85:247–255
Slazak B, Sliwinska E, Saługa M, Ronikier M, Bujak J, Słomka A, Goransson U, Kuta E (2015) Micropropagation of Viola uliginosa (Violaceae) for endangered species conservation and for somaclonal variation-enhanced cyclotide biosynthesis. Plant Cell Tissue Org 120:179–190
Tam JP, Lu Y-A, Yang J-L, Chiu K-W (1999) An unusual structural motif of antimicrobial peptides containing end-to-end macrocycle and cystine-knot disulfides. Proc Nat Acad Sci USA 96:8913–8918
Thakur R, Leonard K, Pataky J (1989) Smut gall development in adult corn plants inoculated with Ustilago maydis. Plant Dis 73:921–925
Trabi M, Craik DJ (2004) Tissue-specific expression of head-to-tail cyclized miniproteins in Violaceae and structure determination of the root cyclotide Viola hederacea root cyclotide1. Plant Cell 16:2204–2216
Witherup KM, Bogusky MJ, Anderson PS, Ramjit H, Ransom RW, Wood T, Sardana M (1994) Cyclopsychotride A, a biologically active, 31-residue cyclic peptide isolated from Psychotria longipes. J Nat Prod 57:1619–1625
Xiao J, Jin X, Jia X, Wang H, Cao A, Zhao W, Pei H, Xue Z, He L, Chen Q (2013) Transcriptome-based discovery of pathways and genes related to resistance against Fusarium head blight in wheat landrace Wangshuibai. BMC Genomics 14:197
Zamani M, Rahjoo V, Parchamian M (2011) Evaluation of the reaction of early maturing maize genotypes to common smut using artificial inoculation. Zbornik Matice Srpske Za Prirodne Nauke 121:71–77
Zarrabi M, Dalirfardouei R, Sepehrizade Z, Kermanshahi R (2013) Comparison of the antimicrobial effects of semipurified cyclotides from Iranian Viola odorata against some of plant and human pathogenic bacteria. J Appl Microbiol 115:367–375
Zhang J, Liao B, Craik DJ, Li J-T, Hu M, Shu W-S (2009) Identification of two suites of cyclotide precursor genes from metallophyte Viola baoshanensis: cDNA sequence variation, alternative RNA splicing and potential cyclotide diversity. Gene 431:23–32
Zhang J, Li J, Huang Z, Yang B, Zhang X, Li D, Craik DJ, Baker AJ, Shu W, Liao B (2015) Transcriptomic screening for cyclotides and other cysteine-rich proteins in the metallophyte Viola baoshanensis. J Plant Physiol 178:17–26
Acknowledgements
This project was supported by the University of Kurdistan, Faculty of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Q. Wang.
Rights and permissions
About this article
Cite this article
Salehi, H., Bahramnejad, B. & Majdi, M. Induction of two cyclotide-like genes Zmcyc1 and Zmcyc5 by abiotic and biotic stresses in Zea mays . Acta Physiol Plant 39, 131 (2017). https://doi.org/10.1007/s11738-017-2425-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2425-6