Abstract
A long-term experiment was conducted to investigate the alleviative effects of silicon (Si) on cadmium (Cd) toxicity in garlic plants grown in pots. Cd and Si were introduced into soil before sowing. Cd was added at a rate of 20 mg kg−1 soil, and Si was applied at two rates: 50 mg SiO2 kg−1 (Si1) and 500 mg SiO2 kg−1 (Si2). There were totally six treatments consisting of CT (control, no added Cd or Si), Si1, Si2, Cd, Cd + Si1, and Cd + Si2. The results showed that Si addition did not affect the growth of garlic plants under control conditions. Under Cd stress, the plant growth and PSII quantum efficiency were inhibited, and they were significantly improved in the presence of added Si. Added Si at Si1 level did not change the soil pH and Cd availability, while it increased Cd accumulation in both shoot and bulb, and improved Cd tolerance. Si added at Si2 level increased the soil pH and decreased Cd availability, and decreased Cd accumulation in different parts of the plant. Added Si had no effect on the activities of soil catalase, urease or invertase regardless of Cd presence. The results suggest that Si could increase Cd tolerance of garlic plants, and the tolerance increase was attributed to not only decreased Cd availability but also in planta detoxification mechanism. There is no evidence indicating that Si-mediated increase of Cd tolerance is related to improved soil microorganism environment as observed in biotic stress conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Garlic (Allium sativum L.) is an important vegetable and has been cultivated for a long time. Due to its characteristic flavor and medicinal properties, it has become a favorite vegetable for a large population. In recent years, due to anthropogenic activities and industrialization, heavy metal pollution in vegetable production area worldwide is becoming more and more serious. Cadmium (Cd) is among the main elements of heavy metal pollution (Song et al. 2013). It is a very toxic element to human beings when entering the food chain (Aery and Rana 2003; López-Millán et al. 2009). Therefore, decreasing its accumulation in vegetables including garlic is urgent to guarantee food safety.
There are different means to reduce heavy metal accumulation in vegetables as in plants, such as engineering-based soil remediation (e.g., soil dressing), phytoremediation of soil using heavy metal-hyperaccumulating plants (Rascio and Navari-Izzo 2011) and use of metal-excluding cultivars. However, these means may not always be very efficient (Rizwan et al. 2012). In addition, application of exogenous substances may be a feasible pathway to decrease heavy metal accumulation in vegetables.
Silicon (Si) is the second most abundant element in the earth crust, and it has not been considered as an essential element for higher plants (Shi et al. 2014). Despite this, Si has been found to be beneficial for plant growth and development, and be able to increase plant tolerance to various environmental stresses, including heavy metal stress (Wu et al. 2013; Zhu and Gong 2014). Si is the only element that can be massively absorbed and accumulated in plants without toxic symptoms (Ma et al. 2001). Moreover, Si is pollution free and non-corrosive. Therefore, Si fertilizer may be used as a high-quality fertilizer for developing ecological agriculture.
Under heavy metal pollution conditions, many researchers have reported that Si could improve plant growth and alleviate stress-induced adverse effects (Wu et al. 2013). There has been a good amount of the literature reporting that addition of Si can decrease Cd-induced toxicity in plants, but most of the work was conducted in graminaceous plants, which are usually Si accumulators (Liang et al. 2005; Nwugo and Huerta 2011; Liu et al. 2013a; Lukačová et al. 2013; Vaculík et al. 2015). However, there has been relatively less literature reporting on the effect of Si on Cd accumulation and transport in low Si-accumulating plants (Wu et al. 2013). Moreover, in previous reports, different effects of Si on Cd accumulation and transport were also observed. For example, Vatehová et al. (2012) found that Si enhanced the translocation of Cd to the shoot in Brassica spp., whereas Song et al. (2009) reported decreased Cd translocation to the shoot in Brassica chinensis L. Liu et al. (2013b) demonstrated that Si-mediated Cd tolerance in Solanum nigrum L. was due to decreases in Cd uptake by roots, Cd distribution in the leaves, and oxidative damage. Therefore, the effect of Si on Cd accumulation in different species may be different, and the mechanism for Si-mediated Cd tolerance may also be different. In garlic, an important vegetable crop which is facing Cd pollution, the influence of Si application on Cd uptake and tolerance is still unknown, which is worth investigation.
It is usually reported that pH rise due to Si application contributes to reduced Cd availability (Liang et al. 2005). In maize, Liang et al. (2005) observed that when Si addition was added at a low rate that did not change Cd availability in growth medium, the Cd accumulations in both shoot and root were increased. In view of the different effects of Si on Cd accumulation and transport reported in different plants (Song et al. 2009; Vatehová et al. 2012), the influence of Si (added at a low rate that does not change Cd availability) on Cd uptake in garlic plants needs to be investigated. Changes of soil enzyme activities can reflect the health status of soil (Wang et al. 2013), which can influence the growth of plants. Up to date, few reports are available on the possible effect of Si on soil enzyme activities. Wang et al. (2013) reported that in soil-grown tomato which was infected by Ralstonia solanacearum, application of Si could increase the activities of soil urease and acid phosphatase, suggesting that Si-mediated changes of soil enzyme activities were involved in the resistance response of tomato against R. solanacearum. However, under other environmental stresses including Cd pollution, whether Si affects the activities of soil enzymes (such as catalase, urease and invertase) remains unknown. In addition, previous studies on the role of Si in enhancing Cd tolerance have mostly been conducted based on short-term treatment, in which the interactions between Si and Cd may have been underestimated in the long term (Zhang et al. 2008), as in the field.
In this work, a long-term pot experiment was conducted to investigate the effect of Si applied at two contrasting rates on plant growth, photosynthesis, Cd accumulation and soil enzyme activities under Cd stress conditions, the aims being to explore the effect of Si on Cd tolerance in garlic plants, as well as the feasibility of Si application in controlling Cd pollution in vegetable production.
Materials and methods
Plant materials
The experiments were conducted at the experimental site of College of Horticulture (34°17′N, 108°04′E), Northwest A&F University. In winter, the temperature is as low as about −10 °C, while in spring, it can reach low 30 s. The properties of the soil used in the experiment were as follows: 27.0 g kg−1 of organic matter, 1.38 g kg−1 of total N, 0.96 g kg−1 of total P and 14.3 g kg−1 K (Wang et al. 2014). The soil was air dried, passed through a 2-mm sieve before introduction of heavy metal and Si. On 31 October 2012, Cd or Si was introduced into the soil. Cd was added at a rate of 20 mg Cd kg−1 (as CdCl2). Si was added at 50 mg SiO2 kg−1 (Si1) or 500 mg SiO2 kg−1 (Si2, as Na2SiO3·9H2O). There were totally six treatments, including CT (control), Si1, Si2, Cd, Cd + Si1, and Cd + Si2. The garlic bulbs (‘Gailiangsuan’) were sown in plastic pots (upper diameter 18 cm, lower diameter 10 cm, height 13 cm) filled with the above soils on 7 November 2012. After seedlings emerged, the garlic plants were regularly watered. The plants were harvested on 12 May 2013 for analysis.
Si concentration determination
The powder of dried tissues (0.1 g) was weighed and put in a 15-mL plastic tube, and 3 mL of 50 % (w/v) NaOH was added. Then the tubes were sterilized at 121 °C for 20 min, after which the solution was made up to 10 mL with the NaOH solution. Si in the solutions was determined according to the method of Dai et al. (2005) with slight modifications. To 0.5 mL of the supernatant was added 2.5 mL of 20 % (v/v) acetic acid and 1 mL of ammonium molybdate tetrahydrate (50 g L−1, pH 7.0). Five minutes later, 0.5 mL of 20 % (w/v) tartaric acid, 0.1 mL of reducing reagent and 0.4 mL of 20 % (v/v) acetic acid were added. The reducing reagent was a mixture of solution A (2 g Na2SO3 and 0.4 g 1-amino-2-naphthol-4-sulfonic acid in 25 mL of distilled water) and solution B (25 g NaHSO3 in 200 mL of distilled water). After 30 min, the supernatant absorbance was measured at 650 nm.
Measurement of chlorophyll fluorescence
Chlorophyll fluorescence parameters were assayed on the second fully expanded leaves from the top at 9:00–11:30 in the morning on 10 May 2013 with an FMS-2 Hansatech pulse-modulated fluorometer (Hansatech Instruments Ltd, UK). After 20–30 min of dark adaptation, the leaf minimal chlorophyll fluorescence (F 0) was determined. The maximal chlorophyll fluorescence (F m) was recorded during a subsequent saturating light pulse (8000 μmol m−2 s−1 for 0.8 s). After continuously being illuminated with actinic light at an intensity of 300 μmol m−2 s−1, the steady-state fluorescence (F s) was recorded. A second saturating light pulse (8000 μmol m−2 s−1 for 0.8 s) was imposed to measure the maximum fluorescence in the light-adapted state (F m′). The maximum PSII photochemical efficiency F v/F m = (F m − F 0)/F m, basal quantum yield F v/F 0 and PSII quantum efficiency ΦPSII = (F m′ − F s)/F m′ were calculated.
Determination of Cd concentrations in plants
On the harvest day, the garlic roots and bulb were quickly washed with distilled water and the plant was divided into roots, bulb and shoot. The tissues were then dried at 70 °C for 3 days. Dry plant materials were ground to powder, weighed, and digested in concentrated nitric acid. The Cd concentrations in digested solutions were determined by atomic adsorption spectrophotometer (Hitachi Z-2000, Japan).
The translocation factor (TF) of Cd was calculated based on the study by Shi et al. (2010):
Soil pH and Cd concentration
On the day of plant harvest, the soil samples were collected, air dried, ground and passed through a 2-mm sieve for analysis. The pH of the soils was measured with a glass electrode (soil:water 1:1).
The extraction of Cd was followed by the procedures of Hou and Yin (2007). Five gram of soil sample was extracted in 12.5 mL of 0.5 M MgCl2 for 3 h, after which the mixture was filtered and the supernatant was used to measure the exchangeable Cd. To the deposit was added 12.5 mL of solution containing 0.1 M NaOH and 0.1 M Na4P2O7, and the mixture was left overnight, followed by 2 h of shaking. The mixture was filtered and the supernatant was used for determination of humic acid-bound Cd. The deposit was extracted in 12.5 mL of 1 M HCl for 1 h and the mixture was filtered. The supernatant obtained from this step was used for determination of carbonate- or Fe/Mn oxide-bound Cd. To the deposit was added 12.5 mL of 30 % H2O2. The mixture was heated at 80 °C for 4 h, cooled down to room temperature and shaken for 30 min. The mixture was filtered and the supernatant was used for determination of organic Cd. The deposit was extracted in 12.5 mL of concentrated HNO3 and HCl (v/v, 1:3) in boiling water bath for 1.5 h. The mixture was filtered and the supernatant was used for determination of residual Cd. Cd was analyzed by atomic adsorption spectrophotometer (Hitachi Z-2000, Japan).
Soil enzyme activities
The soil catalase activity was determined according to Yang et al. (2011). Briefly, to 1 g soil was added 20 mL of water and 2.5 mL of 0.3 % H2O2. The mixture was shaken for 20 min, after which 0.5 mL of saturated KAl(SO4)2 was added and the mixture was immediately filtered. The supernatant was added with 2.5 mL of 1.5 M H2SO4 and the solution absorbance was measured at 240 nm. Controls without addition of soil or H2O2 were included. The soil catalase activity was expressed as the amount of H2O2 consumed per gram of soil within 20 min.
The soil urease activity was assayed using indophenol blue colorimetric method (He et al. 2002) with slight modifications. Briefly, 1 g soil and 0.5 mL of methylbenzene was added in a 15-mL tube. After 15 min, 2.5 mL of 10 % (w/v) urea and 5 mL of citrate buffer (pH 6.7) were added. The mixture was incubated at 37 °C for 24 h, after which it was filtered. To 1 mL of the filtrate was added 7.6 mL of water, 0.8 mL of 1.35 M sodium phenoxide and 0.6 mL of sodium hypochlorite. After 20 min, the absorbance was recorded at 578 nm. The urease activity was expressed as the amount of NH3–N formed.
The soil invertase activity was determined using 3,5-dinitrosalicylic acid method (Li et al. 2005) with slight modifications. Briefly, to 1 g soil was added 7.5 mL of 8 % (w/v) sucrose, 2.5 mL of 70 mM phosphate buffer (pH 5.5) and 0.125 mL of methylbenzene. The mixture was incubated at 37 °C for 24 h, after which it was filtered. To 0.2 mL of the filtrate was added 0.6 mL of 5 g L−1 3,5-dinitrosalicylic acid, followed by being incubated in boiling water for 5 min. The solution was let to cool down and then 9.2 mL of water was added. The absorbance was recorded at 508 nm. The invertase activity was expressed as the amount of glucose released within 24 h at 37 °C.
Statistical analysis
The data were analyzed by one-way or two-way ANOVA (analysis of variance) using SPSS software (SPSS Statistics 22).
Results
Plant Si concentrations and growth
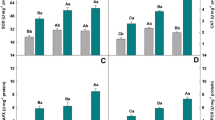
Under non-stress conditions, Si addition did not change the Si concentration in the below-ground part of the plant (Fig. 1a). The Si concentration in the below-ground part was significantly decreased under Cd presence, and it was increased by added Si at Si2 level. In the shoot, the Si concentration was not affected by Si or Cd treatments (Fig. 1b).
Effect of silicon application on the silicon concentrations in the below-ground part and shoot of garlic plants grown in soil supplemented with Cd. Data are mean ± SD (n = 6). Two-way ANOVA was performed to see the effects of Cd and Si, and their interactions. *P < 0.05; ***P < 0.001; NS not significant at P < 0.05. Bars with the same letters are not significantly different at P < 0.05
Addition of Si did not significantly affect the dry weights of shoot and below-ground part of plants under non-stress conditions (Fig. 2). Cd addition decreased the garlic dry weights, which were improved in the presence of added Si (Fig. 2). By and large, there were no significant differences in the shoot or below-ground dry weights between Cd + Si1 and Cd + Si2 treatments. Similar phenomena were also observed on the growth of garlic bulb in the presence of added Cd and/or Si (Fig. 3).
Effect of silicon on the dry matter yield of garlic grown in soil supplemented with Cd. Data are mean ± SD (n = 7). Two-way ANOVA was performed to see the effects of Cd and Si, and their interactions. *P < 0.05; **P < 0.01; NS not significant at P < 0.05. Bars with the same letters are not significantly different at P < 0.05
Chlorophyll fluorescence
In the control conditions, Si addition did not change the maximum PSII photochemical efficiency (F v/F m, Fig. 4b) or PSII quantum efficiency (ΦPSII, Fig. 4c), and it only increased the basal quantum yield (F v/F 0, Fig. 4a) when Si was added at Si1 level. Cd presence decreased all these fluorescence parameters, and Si addition avoided the decreases (Fig. 4).
Effect of silicon on chlorophyll fluorescence parameters in garlic plants under Cd stress. Basal quantum yield = F v/F 0; maximum PSII photochemical efficiency F v/F m = (F m − F 0)/F m; PSII quantum efficiency ΦPSII = (F m′ − F s )/F m′. Data are mean ± SD (n = 8). Two-way ANOVA was performed to see the effects of Cd and Si, and their interactions. **P < 0.01; ***P < 0.001; NS not significant at P < 0.05. Bars with the same letters are not significantly different at P < 0.05
Cd accumulation in plants
Under Cd stress, application of Si at Si1 level significantly increased the Cd level in the shoot (Fig. 5a) and bulb (Fig. 5c), but it did not affect the level in the root (Fig. 5b); whereas Si application at Si2 level decreased the Cd levels in the shoot, root and bulb (Fig. 5a, b, c).
In the absence of added Si, the translocation factors of Cd were 0.340 ± 0.018 and 0.178 ± 0.006 from the root to both shoot and bulb, respectively. Si addition at Si1 level did not significantly change the translocation factors. However, at Si2 level, the translocation factors of Cd from the root to both shoot and bulb were significantly increased to 0.522 ± 0.101 and 0.305 ± 0.080, respectively.
Soil pH and percentage of different forms of Cd in soil
Si addition at Si1 level did not change the soil pH when compared to the corresponding controls (CT and Cd treatments, respectively). However, a higher Si level at Si2 significantly increased the soil pH (from around 7.6 to over 8.1).
In Cd-incorporated soil, addition of Si at Si1 level did not change the availability of Cd (Table 1). However, application of Si at Si2 level significantly decreased the percentage of exchangeable Cd while increased the percentages of organic bound and residual Cd (Table 1).
Soil enzyme activities
The soil catalase activity was significantly decreased in the presence of added Cd, but it was not changed by added Si (Fig. 6a). The activities of urease and invertase were not changed in the presence of added Cd or Si (Fig. 6b, c).
Effect of silicon on activities of catalase, urease and invertase in soil supplemented with Cd. Data are mean ± SD (n = 3). Two-way ANOVA was performed to see the effects of Cd and Si, and their interactions. *P < 0.05; NS not significant at P < 0.05. Bars with the same letters are not significantly different at P < 0.05
Discussion
Cd is a highly toxic element and its pollution in agricultural land threatens the human health through the food chain. Previous studies showed that Si could inhibit Cd accumulation in cereal crops (Liang et al. 2005; Shi et al. 2005; da Cunha and do Nascimento 2009; Wu et al. 2013), suggesting a possible application value of Si fertilizer in controlling Cd pollution in vegetables. Garlic is an important vegetable crop. In this study, we observed that Si addition did not change the Si concentrations in the shoot or root in the control conditions (Fig. 1). The result indicates that garlic is a low Si accumulator. Up to date, little information is available on the possible effect of Si on Cd accumulation in garlic plants. Moreover, there have been very few long-term soil-based studies on the effect of Si on Cd toxicity in plants. In this study, it was observed that Si application avoided the decrease in the biomass of garlic plant under Cd stress (Figs. 2, 3). These results clearly indicate that exogenous Si could increase Cd tolerance of garlic plants. However, we observed that the effect of Si on Cd accumulation in garlic was related to the application rate of Si. A low level of Si application enhanced the Cd accumulations in both shoot and bulb of garlic plants, while application of high Si level significantly decreased the Cd accumulation in both edible parts (Fig. 5). These results suggest a potential value of Si application in controlling Cd pollution in garlic plants. However, a suitable application rate that can increase soil pH and decrease Cd availability needs to be determined in every case.
The increase in Cd tolerance of plants could be due to decreased Cd uptake, transport or improved in planta tolerance. In this study, compared with Cd treatment alone, when Si was applied at a high rate (Si2), Cd accumulations in garlic root, shoot and bulb were all significantly decreased (Fig. 5), while the Cd translocations from the root to both shoot and bulb were increased, suggesting that Si-mediated decrease in Cd uptake (rather than translocation) contributed to the increased Cd tolerance. At the application rate of Si2, the soil pH was significantly increased and the proportion of available Cd was decreased (Table 1). Therefore, Si-mediated decreases in the Cd concentrations of shoot, root and bulb at Si2 level were attributed to the reduced phytoavailability of Cd. Similar results have also been observed in maize at a high rate of Si application (Liang et al. 2005). In addition, it should be noted that unlike the soils used in previous studies that were usually acidic (Liang et al. 2005; Rizwan et al. 2012), the soil used in this study was alkaline. Due to the high soil pH, the proportion of exchangeable Cd level in the soil was much lower (Table 1) than that reported in a previous study (Liang et al. 2005). Even so, addition of Si at a high level (Si2) could still further decrease the Cd availability (Table 1), and thus decrease Cd uptake and accumulation in garlic plants (Fig. 5). Therefore, a decrease in Cd phytoavailability due to pH rise mediated by Si is a chemical mechanism for Si-mediated tolerance of plants to heavy metals.
However, at a lower application rate of Si (Si1), where the soil pH and Cd availability were not changed (Table 1), the Cd tolerance of garlic was still increased, as can be seen from the improved plant growth (Figs. 2, 3) and PSII photochemical efficiency (Fig. 4). Si application at Si1 level did not decrease, but even increase Cd accumulation in the shoot and bulb of garlic plants (Fig. 5a, c). Moreover, since the growth of both shoot and below-ground part of Cd-treated garlic was improved by Si, the total Cd uptake was further increased by Si application. For example, the total Cd accumulation in the shoot was increased 2.9 times in Si1-applied plants (2.424 μg) compared with plants treated with Cd alone (0.850 μg). The effects of Si on Cd uptake and accumulation have also been mixed in the literature (Shi et al. 2005; Vaculík et al. 2009) and may be related to plant species. Shi et al. (2005) observed that Si addition decreased root-to-shoot Cd transport and its accumulation in the shoot of rice. In maize, Vaculík et al. (2009) found that added Si increased the Cd concentrations in both roots and shoots. Liang et al. (2005) observed that Si inclusion into Cd-incorporated soil at a rate of 50 mg Si kg−1 soil significantly increased the Cd concentration in maize root while decreased the Cd concentration in the shoot, suggesting a decrease in root-to-shoot Cd transport, which is different from the result observed in this study. In rice, a Si-accumulating plant, it has been found that Si deposition in the root forms a physical barrier, which reduces Cd transport to the shoot (Shi et al. 2005). In garlic, a non-silicon-accumulating plant as observed in this work (Fig. 1), since no decrease in the root-to-shoot or root-to-bulb Cd transport was observed in Si-applied plants, it is unlikely that the formation of Si deposition-induced physical barrier in the root occurred. In addition, the differences of Si effects on shoot Cd accumulation might also be related to the Cd and Si concentrations applied, as observed in maize (Lukačová et al. 2013). More work is needed to investigate whether the effect of Si on shoot Cd accumulation differs at different Cd and Si concentrations when the phytoavailability of Cd is not altered.
In this study, Si-mediated increase in Cd tolerance of garlic plants at Si1 level may suggest some effects of in planta mechanism. Cd-induced oxidative damage could be alleviated by enzymatic and non-enzymatic antioxidants in plants (Gonçalves et al. 2007). It has been widely reported that Si application can ameliorate oxidative stress status through enhancing antioxidant defense capacities in plants (Wu et al. 2013). However, up to date, it is still difficult to prove whether the effect of Si on antioxidant defense is direct or just secondary. Further research is needed to investigate the in planta mechanism for Si-mediated Cd tolerance in garlic plants.
Compartmentation of heavy metal in the apoplast is an important strategy for the plant survival (Ye et al. 2012). Under Mn stress, it has been observed that added Si decreased the Mn concentration in the symplast by locating more Mn to the cell wall (Rogalla and Römheld 2002; Dragisic Maksimovic et al. 2012), suggesting a stronger binding of Mn to the cell walls. Under Cd stress, Vaculík et al. (2012) also observed that Si enhanced the binding of Cd to the apoplast in the shoot of maize. Liu et al. (2013a) found Cd–Si co-complexation in the cell wall matrix in rice suspension cells. Ma et al. (2015) further confirmed that the formation of [Si-hemicellulose matrix]Cd complexation in cell walls contributed to the inhibition of Cd uptake in rice. Recently, Wu et al. (2015) also found that Si modified the organic acid levels and cell wall components in tomato and cucumber under Cd stress. These studies suggest that comparting more Cd in the cell wall is an important mechanism for Si-mediated Cd tolerance in plants. Whether this is the case in garlic plants is unclear. Future work regarding the Si effect on the distribution of Cd between the symplast and the apoplast is needed.
Soil enzyme activities can be used to evaluate soil health status (Wang et al. 2013). Wang et al. (2013) found that Si enhanced R. solanacearum resistance in tomato by modifying soil microbial activity and microbial community structure. However, to our knowledge, under abiotic stresses such as heavy metal stress, the effect of Si on soil enzyme activities has not been investigated before. In this study, we investigated the effect of Si on the activities of soil catalase, urease and invertase under Cd presence (Fig. 6). Catalase can catalyze the decomposition of hydrogen peroxide (Cheng et al. 2013). Urease catalyzes the hydrolysis of urea into NH3 as well as CO2 (Mackie et al. 2013). To some extent, the urease activity reflects the supply of inorganic nitrogen of soil. Soil invertase catalyzes the hydrolysis of sucrose into glucose and fructose (Gu et al. 2009). Therefore, the invertase plays an important role in the decomposition and utilization of organic matter. In this study, no effects of Si on the activities of soil catalase, urease and invertase were observed (Fig. 6). These results do not suggest any Si-mediated change in the soil microorganism environment.
The results of this study suggest that Si could increase the Cd tolerance of garlic plants. On one hand, the increase of tolerance is attributed to decreased Cd availability; on the other hand, the increase of Cd tolerance may also be attributed to in planta detoxification mechanism which still remains to be investigated. There is no evidence indicating that Si-mediated increase of Cd tolerance is related to improved soil microorganism environment.
Author contribution statement
Yichao Wang and Yanhong Hu conducted the experiment, collected and analyzed the data, and wrote the draft. Yaoke Duan and Ru Feng helped with the experiment. Haijun Gong planned the experiment and revised the manuscript.
References
Aery NC, Rana DK (2003) Growth and cadmium uptake in barley under cadmium stress. J Environ Biol 24:117–123
Cheng F, Peng X, Zhao P, Yuan J, Zhong C, Cheng Y, Cui C, Zhang S (2013) Soil microbial biomass, basal respiration and enzyme activity of main forest types in the Qinling Mountains. PLoS One 8(6):e67353
da Cunha KPV, do Nascimento CWA (2009) Silicon effects on metal tolerance and structural changes in maize (Zea mays L.) grown on a cadmium and zinc enriched soil. Water Air Soil Poll 197:323–330
Dai W, Zhang K, Duan B, Sun C, Zheng K, Cai R, Zhuang J (2005) Rapid determination of silicon content in rice. Rice Sci 12:145–147 (in Chinese with English abstract)
Dragisic Maksimovic J, Mojovic M, Maksimovic V, Romheld V, Nikolic M (2012) Silicon ameliorates manganese toxicity in cucumber by decreasing hydroxyl radical accumulation in the leaf apoplast. J Exp Bot 63:2411–2420
Gonçalves JF, Becker AG, Cargnelutti D, Tabaldi LA, Pereira LB, Battisti V, Spanevello RM, Morsch VM, Nicoloso FT, Schetinger MRC (2007) Cadmium toxicity causes oxidative stress and induces response of the antioxidant system in cucumber seedlings. Braz J Plant Physiol 19:223–232
Gu Y, Wang P, Kong CH (2009) Urease, invertase, dehydrogenase and polyphenoloxidase activities in paddy soil influenced by allelopathic rice variety. Eur J Soil Biol 45:436–441
He W-X, Zhu M-E, Zhang Y-P (2002) Crop effect on the relationship between soil urease activity and mercury. J Northwest Sci Tech Univ Agric For (Nat Sci Ed) 30(2):68–72 (in Chinese with English abstract)
Hou M, Yin H-A (2007) Transformation of Hg forms in a soil-vegetable system. Soils 39:561–566 (in Chinese with English abstract)
Li D-P, Wu Z-J, Chen L-J, Zhu P, Ren J (2005) Dynamics of invertase activity of black soil treated by a long-term located fertilization and its influence. Chin J Eco Agric 13(2):102–105 (in Chinese with English abstract)
Liang Y, Wong JW, Wei L (2005) Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 58:475–483
Liu J, Ma J, He C, Li X, Zhang W, Xu F, Lin Y, Wang L (2013a) Inhibition of cadmium ion uptake in rice (Oryza sativa) cells by a wall-bound form of silicon. New Phytol 200:691–699
Liu J, Zhang H, Zhang Y, Chai T (2013b) Silicon attenuates cadmium toxicity in Solanum nigrum L. by reducing cadmium uptake and oxidative stress. Plant Physiol Biochem 68:1–7
López-Millán AF, Sagardoy R, Solanas M, Abadía A, Abadía J (2009) Cadmium toxicity in tomato (Lycopersicon esculentum L.) plants grown in hydroponics. Environ Exp Bot 65:376–385
Lukačová Z, Švubová R, Kohanová J, Lux A (2013) Silicon mitigates the Cd toxicity in maize in relation to cadmium translocation, cell distribution, antioxidant enzymes stimulation and enhanced endodermal apoplasmic barrier development. Plant Growth Regul 70:89–103
Ma JF, Miyake Y, Takahashi E (2001) Silicon as a beneficial element for crop plants. In: Datonoff L, Snyder G, Korndorfer G (eds) Silicon in agriculture. Elsevier Science Publishing, New York, pp 17–39
Ma J, Cai H, He C, Zhang W, Wang L (2015) A hemicellulose-bound form of silicon inhibits cadmium ion uptake in rice (Oryza sativa) cells. New Phytol 206:1063–1074
Mackie KA, Müller T, Zikeli S, Kandeler E (2013) Long-term copper application in an organic vineyard modifies spatial distribution of soil micro-organisms. Soil Biol Biochem 65:245–253
Nwugo CC, Huerta AJ (2011) The effect of silicon on the leaf proteome of rice (Oryza sativa L.) plants under cadmium-stress. J Proteome Res 10:518–528
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180:169–181
Rizwan M, Meunier JD, Miche H, Keller C (2012) Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J Hazard Mater 209–210:326–334
Rogalla H, Römheld V (2002) Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell Environ 25:549–555
Shi XH, Zhang CC, Wang H, Zhang FS (2005) Effect of Si on the distribution of Cd in rice seedlings. Plant Soil 272:53–60
Shi GR, Cai QS, Liu CF, Wu L (2010) Silicon alleviates cadmium toxicity in peanut plants in relation to cadmium distribution and stimulation of antioxidative enzymes. Plant Growth Regul 61:45–52
Shi Y, Zhang Y, Yao HJ, Wu JW, Sun H, Gong HJ (2014) Silicon improves seed germination and alleviates oxidative stress of bud seedlings in tomato under water deficit stress. Plant Physiol Biochem 78:27–36
Song A, Li Z, Zhang J, Xue G, Fan F, Liang Y (2009) Silicon-enhanced resistance to cadmium toxicity in Brassica chinensis L. is attributed to Si-suppressed cadmium uptake and transport and Si-enhanced antioxidant defense capacity. J Hazard Mater 172:74–83
Song W, Chen B-M, Liu L (2013) Soil heavy metal pollution of cultivated land in China. Res Soil Water Conserv 20:293–298 (in Chinese with English abstract)
Vaculík M, Lux A, Luxová M, Tanimoto E, Lichtscheidl I (2009) Silicon mitigates cadmium inhibitory effects in young maize plants. Environ Exp Bot 67:52–58
Vaculík M, Landberg T, Greger M, Luxová M, Stoláriková M, Lux A (2012) Silicon modifies root anatomy, and uptake and subcellular distribution of cadmium in young maize plants. Ann Bot 110:433–443
Vaculík M, Pavlovič A, Lux A (2015) Silicon alleviates cadmium toxicity by enhanced photosynthetic rate and modified bundle sheath’s cell chloroplasts ultrastructure in mainze. Ecotoxicol Environ Safety 120:66–73
Vatehová Z, Kollárová K, Zelko I, Richterová-Kučerová D, Bujdoš M, Lišková D (2012) Interaction of silicon and cadmium in Brassica juncea and Brassica napus. Biologia 67:498–504
Wang L, Cai K, Chen Y, Wang G (2013) Silicon-mediated tomato resistance against Ralstonia solanacearum is associated with modification of soil microbial community structure and activity. Biol Trace Elem Res 152:275–283
Wang M, Wu C, Cheng Z, Meng H, Zhang M, Zhang H (2014) Soil chemical property changes in eggplant/garlic relay intercropping systems under continuous cropping. PLoS One 9:e111040
Wu J-W, Shi Y, Zhu Y-X, Wang Y-C, Gong H-J (2013) Mechanisms of enhanced heavy metal tolerance in plants by silicon: a review. Pedosphere 23:815–825
Wu J, Guo J, Hu Y, Gong H (2015) Distinct physiological responses of tomato and cucumber plants in silicon-mediated alleviation of cadmium stress. Front Plant Sci 6:453
Yang L-F, Zeng Q, Li H-B, Yan J-J (2011) Measurement of catalase activity in soil by ultraviolet spectrophotometry. Chin J Soil Sci 42(1):207–210 (in Chinese with English Abstract)
Ye J, Yan C, Liu J, Lu H, Liu T, Song Z (2012) Effects of silicon on the distribution of cadmium compartmentation in root tips of Kandelia obovata (S., L.) Yong. Environ Pollut 162:369–373
Zhang C, Wang L, Nie Q, Zhang W, Zhang F (2008) Long-term effects of exogenous silicon on cadmium translocation and toxicity in rice (Oryza sativa L.). Environ Exp Bot 62:300–307
Zhu Y, Gong H (2014) Beneficial effects of silicon on salt and drought tolerance in plants. Agron Sustain Dev 34:455–472
Acknowledgments
This study is supported by the National Natural Science Foundation of China (31272152, 31471866) and Program for New Century Excellent Talents in University of China (NCET-11-0441).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G Klobus.
Y. Wang and Y. Hu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, Y., Hu, Y., Duan, Y. et al. Silicon reduces long-term cadmium toxicities in potted garlic plants. Acta Physiol Plant 38, 211 (2016). https://doi.org/10.1007/s11738-016-2231-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2231-6