Abstract
Rosa damascena Mill. is cultivated for its high-value essential oil in different parts of the world. The flower yield and the composition of essential oil of R. damascena are strongly affected by a number of factors. Nevertheless, the interactive effects of foliar application of plant nutrients and kinetin and its time of application on yield and secondary metabolites profile of R. damascena under acidic conditions are still unclear. Thus, a field experiment comprising two different times of spray and five foliar spray treatments was conducted to test the hypothesis that flowering behavior and secondary metabolites profile can be modified through proper nutrient supply at right time. The foliar spray at flower bud appearance stage (S2) significantly (P ≤ 0.05) increased flower yield by about 10.0 % compared with the foliar application at axillary bud development stage (S1) during both years, regardless of plant nutrients. Among the foliar spray treatments, kinetin at 0.20 g L−1 registered about 23–39 % higher flower yield compared with the water spray control; however, remained statistically at par (P ≤ 0.05) with Ca(NO3)2 at 4.06 g L−1. Moreover, the percentage of major fragrance-bearing compounds of essential oil (β-citronellol + nerol, linalool, E-geraniol, and Z-citral) was marginally increased with Ca(NO3)2 compared with kinetin treatment. However, the percentages of major hydrocarbons, nonadecane and heneicosane, were noticeably increased when kinetin was applied at S1. Foliar application of kinetin and Ca(NO3)2 might be done to improve flower yield and essential oil content in R. damascena flowers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rosa damascena Mill. a perennial shrub, belongs to the family of Rosaceae. The species R. damascena, commonly known as Damask rose or oil-bearing rose, is an important industrial crop cultivated for its high-value essential oil in different parts of the world (Pal et al. 2014). Out of 200 species of the genus Rosa, R. damascena is recognized superior for high-value essential oil, which is primarily used in perfumery and cosmetic industries as a base ingredient for its strong fragrance (Lawrence and Reynolds 1991; Rusanov et al. 2009). The antibacterial, anti-infective, and anti-inflammatory (Basim and Basim 2003), and antioxidant (Wei and Shibamoto 2007; Baydar and Baydar 2013) properties of rose oil have also been reported in the literature. Esfandiary et al. (2014) also reported that the extract of R. damascena is a promising treatment for mild memory impairment and Alzheimer’s disease. Damask rose is cultivated for essential oil and medicinal aspects in Turkey, Bulgaria, Morocco, Iran, India, South Russia, South France, China, South Italy, Libya, and Ukraine (Staikov and Kalaijiev 1980; Weiss 1997; Tabaei-Aghdaei et al. 2006). However, Bulgaria and Turkey are the main producers of rose essential oil in the global market, and whole rose industries in Bulgaria and Turkey are based on a single genotype (Rusanov et al. 2005, 2009). Flower yield and composition of essential oil of R. damascena are largely affected by a number of factors, including genetic makeup (Rusanov et al. 2009), growing region (Yousefi et al. 2009), date and time of flower harvest (Baydar and Baydar 2005), time and level of pruning (Pal et al. 2014), method of distillation (Baydar and Baydar 2005), and agronomic practices (Pal and Singh 2013). The trend of using of plant-derived natural products in perfumery, cosmetic, and food industry is gradually increasing. However, the oil content in the flower of R. damascena is very low compared with other essential oil crops. Thus, there is a pressing need to increase the productivity and quality of essential oil of R. damascena to meet the burgeoning demand in the global market. The quality of rose oil is generally assessed by the relative proportion of major components present in the oil.
Among the agronomic practices for essential oil crops, nutrient management is the most important practice, which influences the yield and quality of essential oils. It has been reported that proper application of mineral nutrients improves the performance of plant and also influences the quality and quantity of active substances (Bernath 1986). The essential oils are very diverse chemical compounds, and the biochemical pathways of synthesis of the major components of essential oil have not been fully elucidated so far. However, it has been reported that both macro- and micro-nutrients can increase the essential oil yield and quality of essential oil in some medicinal plants (Zheljazkov et al. 2010, 2011).
Nitrogen (N) is the key nutrient to build many organic compounds like amino acids, proteins, enzymes and nucleic acids (Peng et al. 2007; Nurzynska-Wierdak 2013). Subsequently, amino acids and enzymes play a vital role in the biosynthesis of some major compounds of essential oil (Koeduka et al. 2006). Nurzynska-Wierdak (2013) also reported that the foliar application of N improved essential oil content in some plants and modified the composition of essential oil. Potassium (K), the third most essential nutrient of plant, influences many fundamental metabolic processes, such as turgor-driven movements, osmoregulation, controlling of membrane polarization, protein biosynthesis, and enzyme activation (Clarkson and Hanson 1980; Cherel 2004). The effects of calcium (Ca) and sulfur (S) on essential oil’s yield and composition of essential oil of different medicinal and aromatic plants have also been reported (Dordas 2009; Zheljazkov et al. 2010). Thus, it is clear that plant nutrition is the most important factor for modifying the chemical profile of medicinal and aromatic plants.
In acidic soil, the availability of some metal cations, particularly magnesium (Mg), Ca, and K, is reduced for plant uptake. Subsequently, the nutrient use efficiency is reduced under this situation. The foliar application of plant nutrients is an alternative strategy for coping with this situation. The foliar application ensures quick translocation of nutrients into various plant parts via leaf tissues under various nutrient deficiency conditions (Fageria et al. 2009). However, the effects of foliar application of plant nutrients and kinetin and their time of application on R. damascena have not been studied lucidly under acidic conditions. The objectives of this study were to investigate the impact of foliar application of plant nutrients (N, K, Ca, Cu, and S) and kinetin at different growth stages on flower yield, essential oil content, and composition of essential oil of R. damascena under acidic soil.
Materials and methods
Experimental location, climate and soil characteristics
This investigation was carried out at the experimental farm of CSIR-Institute of Himalayan Bioresource Technology ((32°06′05″N; 76°34′10″E), Palampur, India, during the growing seasons of 2010–2011 and 2011–2012, to study the effect of foliar application of plant nutrients and growth hormone at different growth stages on growth, flower yield, essential oil content, and composition of essential oil of R. damascena. The experimental farm is situated at the altitude of 1393 m from mean sea level. The details of environmental conditions, viz., maximum and minimum temperature, relative humidity, sunshine hours, and rainfall during the crop cycle of the investigation years are presented in Fig. 1. The texture of the experimental soil was silty clay, and the soil was acidic with pH 4.91 (1:2). Available N, phosphorus (P), K, and Ca in the top 20-cm soil layer were 272.86, 87.65, 427.12 and 106.31 kg h−1, respectively.
Weekly mean maximum and minimum temperature (°C), sunshine hours (SS), rainfall (cm) and relative humidity (RH %) during the growing season of 2010–2011 (a) and 2011–2012 (b) at Palampur, India. The starting date of 19th meteorological standard week (MSW) and closing date of 49th MSW are 13th May and 3rd December, respectively
Plant material, crop management and application of treatments
In this study, 3-year-old plants of R. damascena (cv. Jwala) were used, and the planting geometry was 0.75 m × 1.5 m. The plants were pruned during 49th meteorological standard week (MSW) at 75 cm height from the ground level during 2010 and 2011. After pruning, the crop was fertilized with N, P, and K at the rate of 75, 21.85, and 41.50 kg ha−1, respectively, during both the investigating years. The sources of N, P, and K were urea (46 % N), single super phosphate (16 % P2O5), and muriate of potash (60 % K2O), respectively. No irrigation was given during entire crop cycle, since the crop was grown under rain-fed conditions. However, the weeding operation was done as per requirement for better growth and development. The experiment was laid out as two factors factorial arrangement in randomized block design (RBD) with three replications. The first factor was time of foliar spray, and the second factor was foliar spray of different nutrients and kinetin. Ten treatment combinations comprising two different times of foliar spray [axillary bud development stage (S1) and flower bud appearance stage (S2)] and five different foliar spray treatments [water spray control (N1), KNO3 at 5.0 g L−1 (N2), Ca(NO3)2 at 4.06 g L−1 (N3), CuSO4·5H2O at 2.0 g L−1 (N4), and kinetin at 0.20 g L−1(N5)] were tested. The concentration of different nutrients and kinetin were selected based on previous results in different crops (Pal et al. 2013; Farooqi et al. 1993).
Growth and yield data
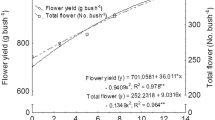
For growth observation, two plants were randomly selected from each replication, and the selected plants were tagged for subsequent observations. After pruning, total number of shoots (No. bush−1) was recorded. The reproductive data like number of flowers (No. shoot−1), flower weight (g flower−1), and flower yield (g shoot−1) were recorded on day-to-day basis from the first day of the harvesting to completion of the flowering. The average values of the entire harvesting period are presented. The dynamics of flowering pattern are also presented (Fig. 2). However, the diameter of flower (cm) and number of petal per flower were recorded at the time of pick flowering stage. Harvesting was done by manual picking of the flowers early in the morning (6:00–9:00 am) to prevent the loss of volatile compounds of essential oil.
The dynamic of the flowering pattern (a–d) and flower yield (e–f) under different treatments. The length of flowering period was 35 during both the cropping seasons. S1 and S2 are the time of foliar spray at axillary bud development stage and flower bud appearance stage, respectively, whereas F1, F2, F3, F4, and F5 are denoting the spraying of water, KNO3 at 5.0 g L−1, Ca(NO3)2 at 4.06 g L−1, CuSO4·5H2O at 2.0 g L−1, and kinetin at 0.20 g L−1, respectively
Chlorophyll (Chl) determination
The leaves were collected from each experimental unit at the time of pick flowering stage. After removal of main veins from the collected leaf samples, 200 mg fresh leaf sample was separated from each sample. Chlorophyll was extracted in the solution of 80 % acetone (v/v) (Arnon 1949). Finally, the absorbance of the extracts at 645 and 663 nm was recorded with a spectrophotometer (model T 90+ UV/vis, PG Instrument Ltd.). The total Chl (mg g−1 tissue) was estimated based on the absorbance values as per standard equations suggested by Arnon (1949).
Determination of NPK in leaf
For the determination of N, P, and K content in leaves, the leaves were collected from each experimental unit at the end of both cropping seasons. The third leaf from the top was used to eliminate the effect of leaf positioning. Dry leaf samples were powered with a laboratory grinder having a sieve spacing of 0.7 mm. The samples were digested with a mixture of concentrated H2SO4 and selenium (Se) as per the procedure suggested by Sahrawat et al. (2002). Then, micro-Kjeldahl method was used for estimation of total N, whereas a spectrophotometer (model T 90+ UV/vis, PG Instrument Ltd.) and a flame photometer (model BWB XP, BWB technologies UK Ltd., UK) were used for estimation of total P and K, respectively, as per the procedures suggested by Prasad et al. (2006).
Extraction of essential oil
The freshly harvested (at the morning) flowers of R. damascena were used for extraction of essential oil. The oil was extracted through hydro-distillation using a Clevenger-type apparatus. The flower-to-water ratio was 1:3 (w/v), and the distillation process was carried out for 4 h with slow heating (Pal et al. 2014). The oil content (w/w) in flower was expressed as percentage on a fresh weight basis of flower, and then the oil yield was expressed in kg per hectare (kg ha−1). The oil samples were collected in glass vial and dehydrated by anhydrous Na2 SO4 (Merck). Then, the oil samples were stored in a dark place at 4 °C until analyzed by gas chromatography (GC) and gas chromatography–mass spectrometry (GC–MS) (Baydar and Baydar 2005).
GC analysis and quantification
The GC analyses of rose oil were carried out by a Shimadzu GC-2010 gas chromatograph (Shimadzu, Tokyo, Japan) equipped with flame ionization detector (FID) and a DB-5 capillary column (30 m × 0.25 mm, fused silica, and film thickness 0.25 µm). The oven temperature was programmed from 70 (4 min) to 220 °C with an increasing rate of 4 °C min−1 and held for 5 min at 220 °C, while injector and detector temperatures were set at 240 and 250 °C, respectively. The carrier gas was nitrogen with a velocity of 1.05 mL min−1. The individual compounds of essential oil were quantified in percentage based on peak area percentage of the chromatogram.
GC–MS analysis and compound identification
GC–MS analyses of rose oil were performed by a Shimadzu QP2010 GC–MS system (Shimadzu, Tokyo, Japan) attached with an AOC-5000 auto injector and a DB-5 (SGE International, Ringwood, Australia) fused silica capillary column (30 m × 0.25 mm i.d., and film thickness 0.25 μm). The working conditions were as follows: the temperature was programmed from 70 (4 min) to 220 °C (5 min) with a ramp of 4 °C min−1; injector and interface temperatures were at 240 and 250 °C, respectively. Helium was used as a carrier gas with a flow rate of 1.1 mL min−1, and ionization voltage was 70 eV.
The compounds were identified based on the retention indices (RI) of all volatile components, which were calculated with reference to homologous series of n-alkanes (C8–C24). Then the constituents of essential oil were identified based on the comparison of RI and mass spectra with National Institute of Standards and Technology-mass spectral (NIST-MS) database (Stein 2005).
Statistical analysis
All of the data obtained from R. damascena during both the years were subjected to analysis of variance (ANOVA) to estimate the variance components of main (time of foliar spray and foliar spray of different nutrients and hormone) effects and their reciprocal interactions effect using Statistica 7 software (Stat Soft Inc., Tulsa, OK, USA). The data about compositions of essential oil are presented as mean ± standard error (SE). When the ANOVA F test showed significance at P = 0.05, the least significant difference (LSD) value was used to assess the difference among treatment means. Principal component analysis (PCA) was used to evaluate the nature of influences of treatment combinations on chemical profile of essential oil as a bi-plot. Factor loading values of the respective variables (different component of essential oil) indicate the correlations with the principal component (PC).
Results
Growth data
The data presented in the Table 1 revealed that the main yield attribute of R. damascena, number of flowers (No. shoot−1), was significantly (P ≤ 0.05) affected by the foliar application of different plant nutrients and kinetin at different growth stages during both the years. Irrespective of plant nutrition, the foliar application at the flower bud appearance stage registered significantly (P ≤ 0.05) higher number of flowers (7.23 and 6.55 no. shoot−1) compared with the foliar application at the axillary bud development stage during both the years. Among the foliar sprays, the application of kinetin produced significantly (P ≤ 0.05) higher number of flowers (7.35 and 6.84 No. shoot−1) compared with the water spray control, but remained statistically at par with Ca(NO3)2 and KNO3 during both cropping seasons. The lowest number of flowers (6.40 and 5.27 no. shoot−1) was found with water spray control. The interaction effect between time of foliar spray and plant nutrients on the number of flowers of R. damascena was insignificant (P ≥ 0.05).
On the other hand, diameter of flower (cm) and petal (no. flower−1) were not markedly influenced either by time of foliar spray or by different concentrations of plant nutrients (Table 1); nevertheless, maximum diameter of flower (3.30 and 3.38 cm) was recorded with the application of kinetin during both the cropping seasons. The maximum number of petals (32.00 and 32.50 no. flower−1) was recorded with Ca(NO3)2 and kinetin during 2010–2011 and 2011–2012, respectively. Weight of flower, the important yield component of R. damascena, was not significantly (P ≥ 0.05) influenced by the time of foliar spray during both the years. However, irrespective of time of the foliar spray, kinetin registered significantly (P ≤ 0.05) higher average flower weight (3.29 g flower−1) compared with the water spray control and CuSO4·5H2O during 2010–2011 (Table 1). The application of Ca(NO3)2 also recorded significantly (P ≤ 0.05) higher average flower weight (3.27 g flower−1) compared with water spray control.
The periodic number of flowers production (no. bush−1) and flower yield (g bush−1) is presented in Fig. 2. The trend of flower production (No. bush−1) and flower yield (g bush−1) during ontogeny was not changed due to the foliar application of plant nutrients and plant hormone at different growth stages during both the years, and the peak flowering stage was attained during 16–20 days of flower harvest under all the treatments (Fig. 2a–h). Irrespective of stage of the foliar spray, the application of kinetin continuously produced higher number of flowers (Fig. 2b, d) and flower yield (Fig. 2f, h) onward 16–20 days of harvest during 2010–2011 and 2011–2012.
The analyzed data (Table 1) revealed that the overall effects of time of foliar spray and foliar application of different plant nutrients and kinetin on the flower yield (g shoot−1) of R. damascena were significant (P ≤ 0.05) during both the years. Regardless of plant nutrients, the foliar application at the flower bud appearance stage significantly (P ≤ 0.05) increased flower yield by about 9.8 and 9.6 % compared with foliar application at the axillary bud development stage during 2010–2011 and 2011–2012, respectively. Among the plant nutrients and growth hormone, the foliar application of kinetin, irrespective of time of application, recorded significantly (P ≤ 0.05) higher flower yield (24.28 and 20.66 g shoot−1) compared with the water spray control, CuSO4·5H2O and KNO3 during 2010–2011 and 2011–2012 (Table 1). The application of kinetin produced about 23–39, 15–22, and 11–15 % higher flower yield compared with water spray control, CuSO4·5H2O, and KNO3, respectively. However, the foliar application of Ca(NO3)2 produced statistically at par (P ≤ 0.05) flower yield (23.90 and 19.53 g shoot−1) compared with the application of kinetin during both the years. In this study, the interaction effect between time of foliar spray and different concentrations of plant nutrients and plant hormone on the flower yield was insignificant (P ≥ 0.05).
The percentage of blind shoots was not significantly (P ≥ 0.05) affected by the time of foliar application of plant nutrients and kinetin; however, least percentage (4.09 %) of blind shoots was recorded with Ca(NO3)2 followed by KNO3 during both the seasons (Table 1).
Oil content (%) and oil yield (kg ha−1)
Results showed that the essential oil content (%) in flowers of R. damascena was not significantly (P ≥ 0.05) influenced by the time of foliar spray and different concentrations of plant nutrients and plant hormone (Fig. 3a, b). However, the foliar application of different plant nutrients and hormone at the flower bud appearance stage registered marginally higher oil content (0.038 and 0.04 %) compared with the foliar application at the axillary bud development stage during both the years. Regardless of time of foliar spray, the maximum oil content (0.042 %) was recorded with the foliar application of Ca(NO3)2 during 2011–2012; nevertheless, during 2010–2011, the equal amount of oil content (0.04 %) was recorded with KNO3, Ca(NO3)2, and kinetin (Fig. 3b). The water spray control registered lowest oil content (0.032 and 0.036 %) during both the cropping seasons. However, irrespective of time of the foliar spray, the yield of essential oil (kg ha−1) was significantly (P ≤ 0.05) influenced by the foliar application of different concentrations of plant nutrients and plant hormone (Fig. 3d). The maximum oil yield (1.925 and 1.588 kg ha−1) was recorded with the foliar application of kinetin and Ca(NO3)2 during 2010–2011 and 2011–2012 cropping season, respectively.
The effect of foliar spray of plant nutrient and phytohormones on the oil content (%) and oil yield (kg ha−1). The oil yield was estimated based on oil content recorded after hydro-distillation using a Clevenger-type apparatus. Vertical bars indicate mean standard error (±) at P = 0.05, and the vertical bars with the same letter are statistically at par (P ≥ 0.05) in the respective years
Correlation matrix
The correlation matrix among the flower yield, yield attributes, oil content, and oil yield of R. damascena showed that the flower yield (g shoot−1) was significantly (P ≤ 0.05) and positively correlated with the diameter of flower and weight of flower (g flower−1) with correlation coefficients of 0.65 and 0.75, respectively (Table 2). Strong correlation (r = 0.97, P ≤ 0.01) was also found between number of flowers (no. shoot−1) and flower yield (g shoot−1). On the other hand, negative correlation (r = − 0.42) was found between blind shoot (%) and flower yield (g shoot−1). A significant (P ≤ 0.05) and positive correlation (r = 0.65) was found between flower weight (g flower−1) and petal (no. flower−1). The oil content in flower was positively correlated with the number of flowers (r = 0.82, P ≤ 0.01), diameter of flower (r = 0.75, P ≤ 0.05), and flower yield (r = 0.76, P ≤ 0.05). In this experiment, yield of essential oil was positively and significantly correlated with the number of flowers per shoot (r = 0.74, P ≤ 0.05), flower weight (r = 0.77, P ≤ 0.01), flower yield (r = 0.82, P ≤ 0.01), and oil content in the flowers (r = 0.73, P ≤ 0.05).
Total Chl and NPK concentration in leaf
The total Chl content (mg g−1 fresh leaf) in the leaves of R. damascena was significantly (P ≤ 0.05) influenced by the time of foliar spray during 2011–2012, and the maximum total Chl content (2.92 mg g−1 fresh leaf) was recorded with foliar spray at the flower bud appearance stage (Table 3). On the other hand, the effects of different plant nutrients and kinetin on total Chl content in leaves of R. damascena were insignificant (P ≥ 0.05) during both the years. However, the application of kinetin registered about 13.06 and 8.18 % higher Chl content compared with the water spray control during 2010–2011 and 2011–2012, respectively.
The effect of time of foliar spray on total N content in the leaves of R. damascena was insignificant (P ≥ 0.05); however, the foliar spray at the flower bud appearance stage resulted in a marginal increase in N concentration in the leaves during both the years (Table 3). Among the nutritional and hormonal sprays, irrespective of time of spray, the application of Ca(NO3)2 registered significantly (P ≤ 0.05) higher N content (25.9 mg g−1 dry leaf) in the leaves compared with CuSO4·5H2O during 2010–2011 (Table 3). The trend of P accumulation in the leaves was not clear; nevertheless, the lowest P content (1.8 and 1.1 mg g−1 dry leaf) was recorded with kinetin during both the cropping seasons. The K content (mg g−1 dry leaf) in the leaves was not significantly (P ≥ 0.05) affected either by the time of foliar spray or by different plant nutrients and kinetin during both the years. However, irrespective of time of foliar spray, the maximum K content (6.5 and 10.2 mg g−1 dry leaf) in the leaves was recorded with the foliar application of KNO3. The interaction effects between time of foliar spray and different concentrations of plant nutrients and plant hormone on total Chl, N, P, and K content in the leaves were insignificant (P ≥ 0.05) during 2010–2011 and 2011–2012 (Table 3).
Compositions of oil
The compositions of essential oil of R. damascena were noticeably influenced by the time of foliar spray and different concentrations of plant nutrients and kinetin. We have identified and quantified a total of 32 compounds, which contribute about 77–92 % of total volume of the essential oil of R. damascena; nevertheless, only important components are presented in Table 4. In this study, β-citronellol and nerol, the most abundant compounds, were collectively quantified for all treatments. The highest quantity of β-citronellol + nerol (31.42 and 33.5 %) was recorded with the foliar application at the flower bud appearance stage during both the cropping seasons. On the other hand, nonadecane, the most represented hydrocarbon, content (13.32 and 17.3 %) was low with the foliar application at the flower bud appearance stage during 2010–2011 and 2011–2012 (Table 4).
In our experiment, the concentration of major compounds of rose essential oil such as β-citronellol + nerol, linalool, β-myrcene, E-geraniol, germacrene D, nonadecane, eicosane, and heneicosane was also influenced by different nutritional treatments and plant hormone (Table 4). Flowers obtained from the water spray control treatment produced substantially highest concentration (38.05 and 38.85 %) of β-citronellol + nerol during both the years. The least quantity (22.95 %) of β-citronellol + nerol was recorded with the foliar application of kinetin during 2010–2011, irrespective of the time of foliar spray. Moreover, the foliar application of kinetin also registered lowest percentage (13.6 and 14.5 %) of E-geraniol during both the cropping seasons, while the maximum quantity (19.7 %) of E-geraniol was found with the foliar application of Ca(NO3)2 during 2010–2011. In this study, nonadecane was the most represented hydrocarbon, and accounted for approximately 11.05–21.9 % of the total volume of the rose essential oil (Table 4). Irrespective of time of foliar spray, the highest levels of nonadecane (20.65 and 21.90 %) and heneicosane (8.70 and 8.20 %) were recorded with the foliar application of kinetin during both the cropping seasons, whereas the foliar spray did not markedly influence the concentration of eicosane during both the years (Table 4).
Principal component analysis (PCA)
Multivariate analysis was performed by the means of principal component analysis (PCA) using the set of 17 major compounds of essential oil of R. damascena. The results from PCA revealed that the first two components, PC1 and PC2, jointly explained 75.31 % of the total variation (Fig. 4). The PCA bi-plot indicated that there was no distinct clustering among the treatment combinations. However, the treatment S1N5 (foliar spray of kinetin at the axillary bud development stage) was separated by the PC1 from the rest of the treatments and placed in the negative end of the PC1. On the other hand, the treatments S1N1 (foliar spray of water spray control at the axillary bud development stage), S2N1 (foliar spray of water spray control at the flower bud appearance stage), and S2N2 (foliar spray of KNO3 at flower bud appearance stage) were placed in the positive end of PC1; nevertheless, S1N1 is separated from S2N1 and S2N2 by the PC2 (Fig. 4).
Principal component analysis of the major compounds of rose essential oil influenced by plant nutrients and kinetin. Principal component 1 and 2 (PC1 and PC2) jointly explained 75.31 % of the initial variability of the data. Loading scores of compounds (variable) with PC1 and PC2 are presented in inset at upper left corner, whereas eigenvalues are presented in lower left corner of the factor plane. The case distributions (treatment combinations) are also presented in the PCA bi-plots. S1 and S2 are the time of foliar spray at axillary bud development stage and flower bud appearance stage, respectively, whereas F1, F2, F3, F4, and F5 are denoting the spraying of water, KNO3 at 5.0 g L−1, Ca(NO3)2 at 4.06 g L−1, CuSO4·5H2O at 2.0 g L−1, and kinetin at 0.20 g L−1, respectively. Var 1–17 are β-myrcene, linalool, α-terpineol, β-citronellol + nerol, z-citral, E-geraniol, E-citral, geranyl acetate, E-caryophyllene, α-guaiene, α-humulene, germacrene D, pentadecane, E, E-α-farnesene, nonadecane, eicosane, and heneicosane, respectively
The relationships among the variables in the space of the first two components (PC1 and PC2) and factor loadings with PC1 and PC2 are also presented in Fig. 4. In this study, PC1 has positive coefficients with thirteen variables [β-myrcene (Var 1), linalool (Var 2), α-terpineol (Var 3), β-citronellol + nerol (Var 4), z-citral (Var 5), E-geraniol (Var 6), E- citral (Var 7), geranyl acetate (Var 8), E-caryophyllene (Var 9), α-guaiene (Var 10), α-humulene (Var 11), germacrene D (Var 12), and E, E-α-farnesene (Var 14)] and negative coefficients with four variables [pentadecane (Var 13), nonadecane (Var 15), eicosane (Var 16), and heneicosane (Var 17)]. However, the loading values of linalool (0.81), α-terpineol (0.87), β-citronellol + nerol (0.89), z-citral (0.91), and E-geraniol (0.92) with PC1 were quite high (Fig. 4). Figure 4 also reveals that nonadecane, eicosane, and heneicosane have almost similar negative heavy loadings (−0.97, −0.94, and −0.96) with PC1. Thus, it is clear that these three variables are highly correlated with each other, and influenced by different treatments in a similar way.
Discussions
The results of this study showed that the number of flowers (No. shoot−1) and flower yield (g shoot−1) of R. damascena were significantly (P ≤ 0.05) influenced by the foliar application of different plant nutrients and kinetin under acidic soil (Table 1). The foliar application at the flower bud appearance stage produced 9.21–10.08 % higher number of flowers compared with the foliar application at axillary bud development. Though the diameter of flower (cm) and weight (g flower−1) were not significantly (P ≥ 0.05) affected by the time of spray, the foliar application at flower bud appearance stage registered 9.6–9.8 % higher flower yield, regardless of different plant nutrients. This result may be due to the fact that the time of exogenous nutrient supply at flower bud appearance stage coincides with the critical nutrient requirement stage of R. damascena. In most of the plants, the rate of nitrogen and potassium uptakes is initially slow but reaches its peak during flowering stage. It has been reported that the maximum N uptake rates for oilseeds during branching and early bud formation, and total N accumulation have been recorded to peak during the flowering stage (Jones et al. 2011). Moreover, spraying at the flower bud appearance stage increased flower yield of R. damascena owing to effective absorbance of nutrients and enhancement of physiological activities. The foliar application of plant nutrients ensures instant translocation of nutrients to various plant parts through leaf tissues under various nutrient deficiencies (Fageria et al. 2009). Thus, the foliar fertilization is the most effective tool under the situations of low soil nutrients bioavailability during the reproductive growth stage of plants (Fageria et al. 2009). Sarkar and Pal (2006) also reported that foliar spray of nitrate salts during 50 % flowering stage increased growth and yield of green gram [Vigna radiata (L.) Wilczek] compared with the control.
On the other hand, irrespective of time of foliar spray, the maximum flower yield was recorded with the foliar application of kinetin followed by foliar spray of Ca(NO3)2. This result may be due to the fact that the exogenous application of kinetin increases the endogenous cytokinins level, which acts as a floral stimulus. Lynas (1981) reported that cytokinins increased the number of flowers in beans. It has also been reported that cytokinins play an important role in floral stimulus in Sinapis alba L. (Lejeune et al., 1988). On the other hand, Ca(NO3)2 supplied Ca2+ and NO3 − −N, which regulated many cellular functions (Ward and Shroeder 1994) and enhanced enzyme activities (Bush 1995). Furthermore, calcium protects the plant under oxidative stress conditions through enhancing the antioxidant enzyme activity (Gong et al. 1998; Jiang and Huang 2001). Bernier et al. (1988) also reported that flower development is dependent on nutrient supply, which influences phytohormone balance and source sink relationship through the transport of photoassimilate. In this study, total Chl concentration in the leaves was also higher with kinetin- and Ca(NO3)2-treated plants. This was also a possible cause to increase the maximum number of flowers and flower yield because higher Chl concentration may hasten photosynthetic activities and others physiological processes related to plant growth and development. It has been reported that kinetin application controls the Chl breakdown in leaves (Fletcher 1969; Richmond and Lang 1957; Thimann 1985).
Though the application of Ca(NO3)2 at 4.06 g L−1 and KNO3 at 5.0 g L−1 supplied equal amount of N (650 ppm), a slightly higher flower yield was observed with Ca(NO3)2. This result may be due to the effect of counter ion of Ca2+. In this experiment, the application of CuSO4·5H2O at 2.0 g L−1 did not significantly (P ≥ 0.05) influence on the flower yield of R. damascena. Although Cu is an essential nutrient for normal plant growth and development, it is also potentially toxic at higher concentration. It has also been reported that Cu in plants leads to oxidative stress inducing changes in the activity and content of some components of the biosynthetic pathways (De Vos et al. 1992; Foyer et al. 1994; Luna et al. 1994; Drazkiewicz et al. 2003).
In this experiment, the average oil content in the fresh flower varied from 0.032 to 0.042 % depending upon the time of foliar spray, nutrient treatment, and year (Fig. 3). This variation of essential oil content (%) in the fresh flower due to time of foliar spray and different plant nutrients was not significant (P ≥ 0.05) during both the cropping seasons. However, the foliar spray at flower bud appearance stage recorded marginally higher oil content (%) during both the years (Fig. 3a). This result may be due to the fact that the foliar application of plant nutrition at flower bud appearance stage increases the bioavailability of essential plant nutrients, which influences physiological and enzymatic activities for essential oil synthesis. Fageria et al. (2009) also reported that foliar fertilization is the most useful under the situations of low nutrient bioavailability in soil during the reproductive growth stage of plants. Although the essential oil content in flower did not show significant (P ≥ 0.05) differences among the treatments, higher value (0.042 %) was found with the Ca(NO3)2 treatment during 2011–2012 cropping season (Fig. 3b). The result may be due to the fact that the foliar application of NO3 − with Ca2+ enhances photosynthetic pigments and N content in the leaves; this is confirmed from the present investigation (Table 3). The beneficial effects of Ca fertilization on yield of essential oil and the composition of essential oil have been reported by several workers (Suh and Park 2000; Dordas 2009).
On the other hand, the application of kinetin and Ca(NO3)2 significantly (P ≤ 0.05) increased oil yield (kg ha−1) by about 56 and 33 %, regardless of the time of foliar spray, compared with the water spray control treatment during 2010–2011 and 2011–2012, respectively. These results may be due to significant (P ≤ 0.05) increase in fresh flower yield coupled with higher oil content in flower. It has been reported that the foliar application of Ca(NO3)2 increases the yield of essential oil of Artemisia dracunculus (Heidari et al. 2014) and Origanum vulgari (Dordas 2009). Zheljazkov et al. (2010) also reported that N application increased the oil yield of Mentha canadensis. In contrast, kinetin controls the breakdown of Chl in leaves (Richmond and Lang 1957; Fletcher 1969; Thimann 1985), minimizes Chl losses (Mukherjee and Kumar 2007), and suppresses senescence enzymes (Thimann 1980). However, the effects of kinetin and Ca(NO3)2 on the oil yield (kg ha−1) were statistically (P ≥ 0.05) at par during both the cropping seasons.
The foliar application of different plant nutrients and kinetin at flower bud appearance stage increased total Chl content in leaves during both the cropping season. This result might be due to efficient absorbance of plant nutrients and kinetin through fully developed canopies. On the other hand, among the plant nutrients and kinetin, the maximum Chl content in leaves was recorded with kinetin. These results may be due to the fact that the kinetin controls the breakdown of Chl in leaves (Fletcher 1969; Richmond and Lang 1957; Thimann 1985). The results are in conformity with the findings of Venkatarayappa et al. (1984) in Phaseolus vulgaris. Foliar application of NO3 − with either Ca2+ or K+ also appreciably enhanced the concentration of photosynthetic pigments in this study because N is an essential component of green pigments of plants (Lawlor 2002). Moreover, the role of counter ions (Ca2+ and K+) on formation of photosynthetic pigment has been reported by many researchers (Tanaka and Tsuji 1980; Misra et al. 2001). In our experiment, regardless of time of spray, foliar application of different plant nutrients, kinetin did not alter the concentration of N, P, and K in the leaves of R. damascena in 2011–2012 cropping season. This result might be due to the dilution effect of nutrient content (Pal et al. 2015). Nevertheless, during 2010–2011, application of CuSO .4 5H2O registered significantly (P ≤ 0.05) lower N content compared with the Ca(NO3)2. This result may be due to the accumulation of toxic levels of Cu in shoot tissues, which may have decreased Chl and N content in leaves (Table 3). The concentration of K in leaves was also increased marginally with the foliar application of KNO3 compared to rest of the treatments. The results are in conformity with the findings of Davies et al. (2009) and Heidari et al. (2014).
Foliar application of plant nutrients and phytohormones changes in percentage of major components of essential oils have been reported in some medicinal and aromatic plant species such as oregano (Dordas 2009), French tarragon (Heidari et al. 2014), and lemongrass (Zheljazkov et al. 2011). The relative percentage of major compounds is one of the important parameters to determine the quality of rose oil. In this experiment, the foliar application of KNO3, Ca(NO3)2, CuSO4·5H2O, and kinetin under acidic condition modified the percentage of major compounds of rose oil. The foliar application of Ca(NO3)2 registered higher percentage of β-citronellol + nerol, linalool, z-citral, and E-geraniol compared with the kinetin. On the other hand, substantially higher amounts of hydrocarbons such as nonadecane (20.65–21.90 %), eicosane (1.6–2.05 %), and heneicosane (8.2–8.7 %) were recorded with the kinetin treatment compared with rest of the treatments. However, the PCA showed that higher amounts nonadecane, eicosane, and heneicosane were recorded with the kinetin applied at axillary bud development stage. A high content of hydrocarbons is not desirable for high-quality rose oil. This result suggested that changes in compositions seem to be a direct consequence of enzyme activities involved in different metabolic pathways. Koeduka et al. (2006) also reported that amino acid and enzymes play a key role in the biosynthesis of several compounds, which are constituents of essential oil. In this study, the percentage of major compounds was varied over the year under all the treatments. Thus, results indicated the presence of other factors, which are also responsible to modify the composition of essential oil of R. damascena.
Conclusion
The foliar application of different plant nutrients and kinetin modulates the flowering behavior, flower and essential oil yield, and profiling of secondary metabolites of R. damascena under acid stress conditions. The foliar spray at S2 stage increased flower yield by about 10.0 % compared with foliar application at S1 stage. The application of kinetin and Ca(NO3)2 produced about 23–39 % and 20.83–34.48 % higher flower yield compared with the water spray control, respectively. Substantial variations in composition of essential oil were also observed due to plant nutrients and its time of application. The foliar application of Ca(NO3)2 at 4.06 g L−1 and kinetin at 0.20 g L−1 at flower bud appearance stage may be adopted to increase the oil yield with maintaining the desired quality. However, further studies are required to understand the role of other factors, particularly environmental factors on the chemical compositions of rose essential oil. Besides, standardization of the Ca(NO3)2 and kinetin doses is also needed.
Author contribution statement
Probir Kumar Pal designed and conducted the experiments, statistical analysis, and wrote the manuscript. Mitali Mahajan conducted the experiments, biochemical analysis, collected and analyzed data, and the literature search. The chemical compositions of essential oil have been identified by Vijai K. Agnihotri.
Abbreviations
- Ca(NO3)2 :
-
Calcium nitrate
- Chl:
-
Chlorophyll
- CuSO4·5H2O:
-
Copper sulfate
- GC:
-
Gas chromatography
- GC–MS:
-
Gas chromatography–mass spectrometry
- MSW:
-
Meteorological standard week
- KNO3 :
-
Potassium nitrate
- PCA:
-
Principal component analysis
References
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Basim E, Basim H (2003) Antibacterial activity of Rosa damascena essential oil. Fitoterapia 74(4):394–396
Baydar H, Baydar NG (2005) The effects of harvest date, fermentation duration and Tween 20 treatment on essential oil content and composition of industrial oil rose (Rosa damascena Mill.). Ind Crops Prod 21:251–255
Baydar NG, Baydar H (2013) Phenolic compounds, antiradical activity and antioxidant capacity of oil bearing rose (Rosa damascena Mill.) extracts. Ind Crops Prod 41:375–380
Bernath J (1986) Production ecology of secondary plant products. In: Craker LE, Simon JE (eds) Herb, spice and medicinal plants recent advances in botany horticulture and pharmacology. Oryx Press, Phoenix, pp 185–234
Bernier G, Lejeune P, Jacqmard A, Kinet JM (1988) Cytokinins in flower initiation. In: Pharis RP, Rood SB (eds) Plant growth substances. Springer, Berlin, pp 486–489
Bush DS (1995) Calcium regulation in plant cells and its role in signaling. Ann Rev Plant Physiol Plant Mol Biol 46:95–122
Cherel L (2004) Regulation of K+ channel activities in plants: from physiological to molecular aspects. J Exp Bot 55:337–351
Clarkson DT, Hanson JB (1980) The mineral nutrition of higher plants. Annu Rev Plant Physiol. 31:239–298
Davies MJ, Atkinson CJ, Burns C, Woolley JG, Hipps NA, Arroo RR, Dungey N, Robinson T, Brown P, Flockart I, Hill C, Smith L, Bentley S (2009) Enhancement of artemisinin concentration and yield in response to optimization of nitrogen and potassium supply to Artemisia annua. Ann Bot 104:315–323
De Vos CH, Vonk MJ, Voojis R, Schat H (1992) Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol 98:853–858
Dordas C (2009) Foliar application of calcium and magnesium improves growth, yield, and essential oil yield of oregano (Origanum vulgare ssp. hirtum). Ind Crops Prod 29:599–608
Drazkiewicz M, Skórzynska-Polit E, Krupa Z (2003) Response of the ascorbate-glutathione cycle to excess copper in Arabidopsis thaliana (L.). Plant Sci 164:195–202
Esfandiary E, Karimipour M, Mardani M, Alaei H, Ghannadian M, Kazemi M, Mohammadnejad D, Hosseini N, Esmaeili A (2014) Novel effects of Rosa damascena extract on memory and neurogenesis in a rat model of Alzheimer’s disease. J Neurosci Res 92:517–530
Fageria NK, Filho MPB, Moreira A, Guimares CM (2009) Foliar fertilization of crop plants. J Plant Nutr 32(6):1044–1064
Farooqi AH, Sharma S, Naqvi AA, Khan A (1993) The effect of Kinetin on flower and oil production in Rosa damascena. J Essent Oil Res 5:305–309
Fletcher RA (1969) Retardation of leaf senescence by benzyladenine in intact bean plants. Planta 89(1):1–8
Foyer CH, Lelandias M, Kunert KJ (1994) Photooxidative stress in plants. Physiol Plant 92:696–717
Gong M, Van der Liut AH, Knight MR, Trewaves AJ (1998) Heat shock induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiol 116:429–437
Heidari S, Azizi M, Soltani F, Hadian J (2014) Foliar application of Ca(NO3)2 and KNO3 affects growth, essential oil content, and oil composition of French tarragon. Ind Crops Prod 62:526–532
Jiang Y, Huang B (2001) Effects of calcium on antioxidant activities and water relations associated with heat tolerance in two cool-season grasses. J Exp Bot 52:341–349
Jones C, Olson-Rutz K, Dinkins CP (2011) Nutrient uptake timing by crops: to assist with fertilizing decisions. EB0191 revised June 2011, MSU extension 1–8
Koeduka T, Fridman E, Gang DR, Vassao DG, Jackson BL, Kish CM, Orlova I, Spassova SM, Lewis NG, Noel JP, Baiq TJ, Dudareva N, Pichersky E (2006) Eugenol and isoeugenol, characteristic aromatic constituents of spices, are biosynthesized via reduction of a coniferyl alcohol ester. Pro Natl Acad Sci USA 103(26):10128–10133
Lawlor DW (2002) Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. J Exp Bot 53:773–787
Lawrence BL, Reynolds RJ (1991) Progress in essential oils. Perfum Flavor 16:44–77
Lejeune P, Kinet J-M, Bernier G (1988) Cytokinin fluxes during floral induction in the long day plant Sinapis alba L. Plant Physiol 86(4):1095–1098
Luna CM, Gonzalez CA, Trippi VS (1994) Oxidative damage caused by excess of copper in oat leaves. Plant Cell Physiol 35(1):11–15
Lynas C (1981) Cytokinins, photoperiod and flower bud development in Phaseolus vulgaris. Cambridge, p 184
Misra AN, Srivastava A, Strasser RJ (2001) Utilization of fast chlorophyll a fluorescence technique in assessing the salt/ion sensitivity of mung bean and Brassica seedlings. J Plant Physiol 158(9):1173–1181
Mukherjee D, Kumar R (2007) Kinetin regulates plant growth and biochemical changes during maturation and senescence of leaves, flowers, and pods of Cajanus cajan L. Biol Plant 51(1):80–85
Nurzyńska-Wierdak R (2013) Does mineral fertilization modify essential oil content and chemical composition in medicinal plants? Acta Sci Pol Hortorum Cultus 12(5):3–16
Pal PK, Singh RD (2013) Understanding crop-ecology and agronomy of Rosa damascena Mill. for higher productivity. Aust J Crop Sci 7(2):196–205
Pal PK, Prasad R, Pathania V (2013) Effect of decapitation and nutrient applications on shoot branching, yield, and accumulation of secondary metabolites in leaves of Stevia rebaudiana Bertoni. J Plant Phys 170:1526–1535
Pal PK, Agnihotri VK, Gopichand Singh RD (2014) Impact of level and timing of pruning on flower yield and secondary metabolites profile of Rosa damascena under western Himalayan region. Ind Crops Prod 52:219–227
Pal PK, Kumar R, Guleria V, Mahajan M, Prasad R, Pathania V, Gill BS, Singh D, Gopichand Singh B, Singh RD, Ahuja PS (2015) Crop-ecology and nutritional variability influence growth and secondary metabolites of Stevia rebaudiana Bertoni. BMC Plant Biol 15:67
Peng M, Hannam C, Gu H, Bi YM, Rothstein SJ (2007) A mutation in NLA, which encodes a RING-type ubiquitin ligase, disrupts the adaptability of Arabidopsis to nitrogen limitation. Plant J 50(2):320–337
Prasad R, Shivay YS, Kumar D, Sharma SN (2006) Learning by doing exercise in soil fertility- practical manual for soil fertility. Division of Agronomy, IARI, New Delhi (p 68)
Richmond AE, Lang A (1957) Effect of kinetin on protein content and survival of detached Xanthium leaves. Science 125:650–651
Rusanov K, Kovacheva N, Vosman B, Zhang L, Rajapakse S, Atanassov A, Atanassov I (2005) Microsatellite analysis of Rosa damascena Mill. accessions reveals genetic similarity between genotypes used for rose oil production and old Damask rose varieties. Theor Appl Genet 111:804–809
Rusanov K, Kovacheva N, Stefanova K, Atanassov A, Atanassov I (2009) Rosa damascene—genetic resources and capacity building for molecular breeding. Biotechnol Biotechnol Equip 23(4):1436–1439
Sahrawat KL, Ravi Kumar G, Rao JK (2002) Evaluation of triacid and dry ashing procedures for determining potassium, calcium, magnesium, iron, zinc, manganese and copper in plant materials. Commun Soil Sci Plant Anal 33:95–102
Sarkar RK, Pal PK (2006) Effect of pre-sowing seed treatment and foliar spray nitrate salts on growth and yield of greengram (Vigna radiata). Indian J Agric Sci 76(1):62–65
Staikov V, Kalaijiev I (1980) Study of oil roses (Rosa damascena Mill.) originated from India, Morocco, Iran and Bulgaria (in Bulgarian). Plant Sci 17(4):58–68
Stein SE (2005) Mass spectral and database software, version 3.02. National Institute of Standards and Technology (NIST), Gaithersburg
Suh EJ, Park KW (2000) Effect of calcium ion in nutrient solution on the content and composition of essential oil of sweet basil in hydroponics. J Korean Soc Hortic Sci 41:598–601
Tabaei-Aghdaei SR, Hosseini Monfared H, Fahimi H, Ebrahimzade H, Jebelly M, Naghavi MR, Babaei A (2006) Genetic variation analysis of different populations of Rosa damascena in NW. Iran using RAPD markers. Iran J Bot 12(2):121–127
Tanaka A, Tsuji H (1980) Effects of calcium on chlorophyll synthesis and stability in the early phase of greening in cucumber cotyledons. Plant Physiol 65(6):1211–1215
Thimann KV (1980) The senescence of leaves. In: Thimann KV (ed) Senescence in plants. CRC Press, Boca Raton, pp 85–115
Thimann KV (1985) The interaction of hormonal and environmental factors in leaf senescence. Biol Plant 27:83–91
Venkatarayappa T, Fletcher RA, Thompson JE (1984) Retardation and reversal of senescence in bean leaves by benzyladenine and decapitation. Plant Cell Physiol 25(3):407–418
Ward JM, Schroeder JI (1994) Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell 6(5):669–683
Wei A, Shibamoto T (2007) Antioxidant activities and volatile constituents of various essential oils. J Agric Food Chem 55(5):1737–1742
Weiss EA (1997) Essential oil crops. CAB International, Wallingford
Yousefi B, Tabaei-Aghdaei SR, Darvish F, Assareh MH (2009) Flower yield performance and stability of various Rosa damascena Mill. landraces under different ecological conditions. Sci Hortic 121(3):333–339
Zheljazkov VD, Cantrell CL, Astatkie T, Ebelhar MW (2010) Peppermint productivity and oil composition as a function of nitrogen, growth stage, and harvest time. Agron J 102:124–128
Zheljazkov VD, Cantrell CL, Astatkie T, Cannon JB (2011) Lemongrass productivity, oil content, and composition as a function of nitrogen, sulphur, and harvest time. Agron J 103(3):805–812
Acknowledgments
The authors are thankful to Dr. P.S. Ahuja, former Director of IHBT, Palampur for his constant encouragement for this work. The authors are also thankful to Dr. Gopichand for providing the necessary support for field experiment. We thank Mr. Ramdeen Prasad and Mrs Vijaylata Pathaniya for technical support. The authors acknowledge the Council of Scientific and Industrial Research (CSIR), Government of India, for financial support. This research work has been undertaken under the CSIR network project—BSC-0110 and MLP-0010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Li.
Rights and permissions
About this article
Cite this article
Pal, P.K., Mahajan, M. & Agnihotri, V.K. Foliar application of plant nutrients and kinetin modifies growth and essential oil profile in Rosa damascena under acidic conditions. Acta Physiol Plant 38, 176 (2016). https://doi.org/10.1007/s11738-016-2187-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2187-6