Abstract
Drought is a major constraint of agriculture development. The intergeneric somatic hybrids between Brassica napus and Sinapis alba were created by electrofusion to obtain materials with enhanced drought tolerance. The drought tolerance of B. napus cv. Yangyou 6 (Y6) and one offspring line (W146) of the somatic hybrids was evaluated by morphological observation. Physiological parameters were determined in this study. Moreover, the activities of a few antioxidant enzymes and the transcript level of the antioxidant enzyme encoding genes were analyzed by qPCR. W146 and Y6 showed apparent wilting after drought stress for 7 days. However, Y6 wilted more severely than W146. The result of the physiological analysis showed that the relative electronic conductivity and malondialdehyde content of Y6 were higher than that of W146. The relative water content, net photosynthesis rate, proline content, and the activities of superoxide dismutase (SOD) and peroxidase in W146 were higher than that in Y6 after drought stress for 12 days. The DNA and nitrotetrazolium blue chloride staining analysis revealed less accumulation of O2 ·− and H2O2 in W146 than that in Y6 after drought stress. Moreover, the transcript level of some antioxidant enzyme encoding genes, such as Cu/ZnSOD, MnSOD, ascorbate peroxidase, glutathione reductase, and glutathione peroxidase in W146, was higher than that in Y6 under drought stress. Results revealed that line W146 showed more drought stress tolerance than Y6 because line W146 could reduce oxidative damage by efficient antioxidant systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The shortage of water resources has been worsening because of global warming and increasing populations (Hussain and Mumtaz 2014). Drought is becoming one of the most common environmental stresses that limit plant yield and survival (Chaves et al. 2003). In China, arid and semi-arid area accounts for one-third of the total area. Under limited water resource conditions, the selection of crops with high yield and high stress tolerance is important for breeders (Wu et al. 2011).
Brassica napus L. is one of the most important oilseed crops in the world and is a major source of oil and protein. In China, droughts occur frequently and rainfall is uneven, thus affecting the production and yield stability of rapeseed. Droughts account for at least 30 % of the yield loss of rapeseed (Xu et al. 2014). To increase the production and expand the cultivated land of rapeseed, materials with enhanced drought tolerance should be created and the physiological and molecular mechanisms of rapeseed against drought stress need to be revealed. In a previous study, at the rosette stage of oilseed rape development, the relative water content (RWC) decreased when seedlings were under drought stress (Benjamin et al. 2012). The net photosynthesis rate (Pn) significantly decreased (P < 0.05) in B. napus seedlings under drought stress (Yan et al. 2008). Along with increased stress degree, membrane permeability and malondialdehyde (MDA) content increased distinctly in the pot culture of B. napus (Zhao et al. 2010). Moreover, drought stress also preferentially enhanced the activities of superoxide dismutase (SOD) and peroxidase (POD) in ten cultivars of oilseed rape (Abedi and Pakniyat 2010). In addition to the physiological responses of rapeseed under drought stress, the up-regulated expression of a few reactive oxygen species (ROS) genes has a positive relationship with enhanced abiotic stress resistance in plants. The overexpression of ascorbate peroxidase (APX) gene from B. napus enhanced drought tolerance in Arabidopsis thaliana (Chai 2011). Tang and Yang (2008) developed a transgenic potato that expressed tomato Cu/Zn SOD gene, which enhances resistance to salt and oxidative stress.
During the seedling growth stage, drought stress not only affects the growth of leaves and roots but also suppresses the bolting and flowering of rapeseed, thus causing yield reduction. Many metabolic processes may change under drought, including water transportation, photosynthesis, osmotic regulation, and enzyme activities (Kishor et al. 2005; Martínez et al. 2007; Chaves et al. 2009; Mittler et al. 2011). When plants are exposed to drought stress conditions, the ROS-scavenging/producing homeostasis is broken and the excessive accumulation of ROS may cause damage to plant cells, which can lead to cell death (Cruz de Carvalho 2008). Plants have evolved efficient enzymatic and non-enzymatic antioxidant systems to scavenge excess ROS (Apel and Hirt 2004). Superoxide radicals are dismutated by SOD, which is the first barrier to withstand ROS (Hasanuzzaman et al. 2012). The excess H2O2 participates in the ascorbate–glutathione (AsA–GSH) cycle, in which APX utilizes AsA to restore H2O2 to H2O and monodehydroascorbate (MDHA) (Foyer and Noctor 2011). MDHA is instable in plant cells. A part of MDHA is restored to AsA by monodehydroascorbate reductase (MDHAR), whereas other parts are oxidized to dehydroascorbate (DHA) (Lisenbee et al. 2005). DHA can be reduced by GSH to generate AsA under the function of dehydroascorbate reductase (DHAR) (Saruhan et al. 2009). Simultaneously, the generated GSSG is catalyzed to GSH by glutathione reductase (GR) (Sharma and Dubey 2005; Queval et al. 2007; Munné Bosch et al. 2013).
In our previous study, we created intergeneric somatic hybrids between B. napus and Sinapis alba via electrofusion (Wang et al. 2005). The hybrids were then subsequently backcrossed with B. napus cv. Yangyou 6 (Y6) (Li et al. 2012). S. alba (white mustard) has many desirable agronomic traits, including tolerance to drought (Brown et al. 1997). We compared one offspring line (W146) with Y6 to evaluate their performance under drought stress. The physiological and biochemical responses to drought stress in line W146 and Y6 were investigated by determining multiple physiological indexes. Furthermore, the function of antioxidant enzymes in enhancing rapeseed drought tolerance was examined by quantitative analysis of the expression of ROS-related enzyme genes, such as MnSOD, Cu/ZnSOD, APX, GPX, MDHAR, DHAR, GR, and Ferritin (Fer). This study will help establishing reliable methods for screening rape materials with improved drought tolerance for genetic breeding. Our results revealed that the offspring line W146 might obtain drought tolerance traits from S. alba and provide enhanced drought tolerance to rapeseed.
Materials and methods
Plant materials and treatment
Line W146 from the intergeneric somatic hybrid between Brassica napus and Sinapis alba was backcrossed with Y6 for multiple generations and B. napus cv. Y6 were used in this study (Li et al. 2012). Healthy and plump seeds were sown into the seedling-raising plate filled with nutrient soil and vermiculite and cultivated in a growth chamber at 25 °C with a 16-h light/8-h dark photoperiod. After germination, seedlings were transplanted into pots (16 cm × 12 cm). The rape seedlings at the four-leaf stage were subjected to drought treatment. For drought stress, water was withheld from plants. After the plants were exposed to drought stress for 0, 7, and 12 days, leaves from six seedlings were collected for each of three replications and were stored at −80 °C for further use.
Relative water content
Leaf samples were used for RWC assay according to the method described previously (Barrs and Weatherley 1962). The fresh weight (W 1), turgid weight (W 2), and dry weight (W 3) of leaves were measured, and the RWC was calculated as follows:
Relative electronic conductivity and lipid peroxidation
Electronic conductivity was measured using an EL30 conductivity meter (Shanghai Mettler-Toledo, China) according to the method described preciously (Yang et al. 1996). Fresh leaves (0.5 g) were immersed in 25 ml ddH2O at room temperature for 4 h. The tubes were then shaken at 280 rpm for 2 h at 28 °C, and the initial electronic conductivity of the solution (C1) was measured. The solution was boiled for 30 min, and the electronic conductivity (C2) was re-measured. Relative electronic conductivity (REC) was calculated as C1/C2 × 100 %. The MDA content was measured using thiobarbituric acid according to the method described previously (Hodges et al. 1999).
Net photosynthesis rate measurement
Pn was measured with a portable photosynthetic apparatus (Li-COR 6400, USA). Five seedlings randomly selected from the Line W146 and Y6 were used for determination. All measurements were conducted under natural conditions at 8:30–12:00 a.m. on sunny days.
Proline content determination
Proline (Pro) content was determined according to the protocol described preciously by Bates et al. (1973). Leaves (0.5 g) were used to extract the Pro homogenized in 3 % sulfosalicylic acid, and the supernatant was mixed with equal volume of glacial acetic acid and acidic ninhydrin for the reaction. Following heating under 100 °C for 30 min, a volume of 5 ml methylbenzene was added to the mixture. The absorbance of the supernatant was measured at 520 nm using a UV–vis spectrometer (Perkinelmer lambda 25, USA).
Enzyme activity assays
Leaves (0.5 g) were homogenized in 5 ml 0.05 M Na phosphate buffer (pH 7.8) including 1 % (w/v) polyvinyl pyrrolidone (PVP). A supernatant was used for enzyme assays. Low temperature operations at 4 °C were required. All spectral analysis was conducted on a UV–vis spectrometer (Perkinelmer lambda 25, USA).
SOD activity was measured according to the method described preciously (Giannopolitis and Ries 1977). The mixture contained 0.05 M Na phosphate buffer (pH 7.8), 130 mM methionine, 750 μM nitrotetrazolium blue chloride (NBT), 100 μM EDTA-Na2, 20 μM riboflavin, and 0.1 ml enzyme extract. The absorbance was monitored at 560 nm after the mixture was under illumination for 30 min.
POD activity was measured according to the method proposed by Rao et al. (1996). The reaction mixture contained 0.05 M Na phosphate buffer (pH 7.8), 1 % (v/v) guaiacol, and 10 mM H2O2. After the mixture was pre-incubated at 25 °C for 10 min, 0.2 ml crude enzyme extract was added to initiate the reaction. The absorbance changes were recorded for 2 min at 470 nm. An absorbance change of 0.01 unit min−1 was defined as 1 unit (U) of POD activity.
DAB and NBT staining
The in situ formations of H2O2 and O2 ·− in the leaves were visually detected by the staining of diaminobenzidine (DAB) and NBT, respectively (Tian et al. 2013). To detect hydrogen peroxide accumulation, the detached leaves were immersed in 0.1 mg/ml DAB in 50 mM Tris acetate buffer, pH 5.0, at 25 °C for 24 h in the dark. To detect superoxide anion accumulation, the detached leaves were immersed in a 0.5-mg/ml NBT solution in 10 mM potassium phosphate buffer, pH 7.8, at 25 °C for 6 h in the dark. After staining, the leaves were boiled in 95 % (v/v) ethanol for 15 min and were stored in 40 % (v/v) glycerol. Images were captured with a Canon camera. Each experiment was conducted using at least five individual plants.
qRT-PCR of antioxidant enzyme gene expression
The expression level of eight antioxidant enzyme genes was examined in Y6 and Line W146. First, total RNA was isolated from rapeseed leaves according to the manufacturer’s protocol (RNAiso plus, TAKARA, China). The total RNA was subsequently used to synthesize cDNA with HiScipt reverse transcriptase according to the manufacturer’s instructions (Vazyme, China).
Primers used for qPCR were designed using Primer Express 3.0, and the primer sequences are listed in Table S1. PCR reactions were performed on a fluorescence quantitative instrument (Agilent Mx3005P, USA). The 10-μL reactions contained 5 μL SYBR Green (Roche, USA), 1 ng cDNA sample, and 0.2 μM gene-specific primers. Three technological replicates were performed per cDNA sample. Relative expression levels were calculated by the 2−ΔΔT method (Pfaffl 2001). Bnactin (NCBI AF111812) was used as an internal standard to normalize the cDNA template content.
Statistical analysis
The statistical significance of difference in indexes was tested by DPS (Zhejiang University, China). Differences between the means among rapeseed lines and treatments were compared by Duncan’s multiple range tests at 0.05 levels.
Results
Phenotype changes of rapeseed leaves under drought stress
Under normal conditions, the performance of Y6 was similar to that of W146. When the seedlings were exposed to drought stress, a significant morphological change was observed at the 7-day stress (Fig. 1). The leaves exhibited apparent withering at 7 days (leaf color changed from green to yellow). At 12 days, the leaves of both line W146 and Y6 withered seriously and began to fall off. However, Y6 wilted more seriously than line W146, which appeared to be nearly dead (Fig. 1).
Physiological response of line W146 and Y6 under drought stress
The leaf RWC and Pn decreased under drought stress (Fig. 2a, b). The decrease of RWC and Pn was more dramatic in Y6 than in line W146 after drought stress for 12 days. After 12 days of stress treatment, the RWC in Y6 leaves (33.2 %) was significantly less than that in line W146 leaves (44.8 %). Leaf RWC is a criterion for the evaluation of drought tolerance and plant water status (Zhou et al. 2012). This result demonstrated that line W146 may be more drought tolerant than Y6.
The effect of drought treatment during experimental period (0, 7 and 12 days) on physiological parameters. a Relative water content (RWC) in leaves of Y6 and W146 before and after drought stress. b Net photosynthetic rate (Pn) of Y6 and W146 before and after drought stress. c Relative electrical conductivity in leaves of Y6 and W146 before and after drought stress. d MDA content in leaves of Y6 and W146 before and after drought stress. e SOD activity of Y6 and W146 before and after drought stress. f POD activity of Y6 and W146 before and after drought stress. g Proline content in leaves of Y6 and W146 before and after drought stress. Data represent mean ± SE (n = 3). Different letters on bars indicate significant difference (P < 0.05) according to Duncan’s Test
Limited water caused decreased membrane stability in both lines, W146 and Y6. Under normal conditions, the REC and MDA contents in Y6 and line W146 showed no obvious differences. After 12 days of drought stress, the REC and MDA contents in Y6 were significantly higher than that in line W146 (Fig. 2c, d). These results illustrated that the membrane damage in Y6 was more severe than that in line W146 under drought stress conditions.
Free Pro accumulates rapidly with increased drought stress degree; hence, it can be used as an evaluation index for plant drought tolerance (Gomes et al. 2010). Before stress, the Pro content in Y6 was equivalent to that in line W146. After stress, the Pro contents of both lines, W146 and Y6, significantly increased. Under drought stress, the Pro content in line W146 was significantly higher than that in Y6 (Fig. 2g). After 12 days of drought stress, the Pro contents in Y6 and line W146 were 671.2 and 729.0 μg/gFW, which up-regulated 9.4 and 11.4-fold than those under normal conditions, respectively.
Oxidative stress damage and antioxidant enzyme activity
The third leaf counting downwards from the top of the plantlets was used to detect the levels of ROS accumulation and the activities of antioxidant enzyme in rapeseed. We determined the O2 ·− and H2O2 contents by NBT staining and DAB staining, respectively. As shown in Fig. 3, no significant differences were detected between line W146 and Y6 under normal conditions. With the development of drought stress, the accumulation of O2 ·− and H2O2 dramatically increased. However, a light color was witnessed in the leaves of line W146 compared with that in Y6 after 7 and 12 days of drought stress. These data demonstrated that line W146 had higher ROS-scavenging capacity than Y6 under drought conditions.
We further examined the activities of antioxidant enzyme, including SOD and POD in leaves of line W146 and Y6. Under normal conditions, the activity of SOD in Y6 was significantly higher than that in line W146 (Fig. 2e). However, no significant difference was found in POD activity between W146 and Y6 (Fig. 2f). After 12 days of drought stress, significantly higher antioxidant enzyme activities were detected in line W146 than those in Y6 (Fig. 2e, f).
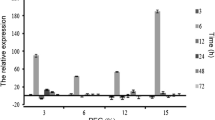
Moreover, we examined some genes that encode antioxidant enzymes, including MnSOD, Cu/ZnSOD, APX, GPX, MDHAR, DHAR, GR, and Fer. As shown in Fig. 4, the transcript abundance of the detected genes showed different change patterns under drought stress. The expression of Cu/ZnSOD was remarkably up-regulated by drought, which was up-regulated approximately 40-fold in line W146 after 7 days of drought stress. MnSOD, APX, GPX, DHAR, and GR were also up-regulated under drought. The expression of MDHAR and Fer showed no significant difference between drought stress and normal conditions in W146. The expression levels of up-regulated genes, except DHAR, were higher in line W146 after drought for 12 days than in Y6. These results suggest that the up-regulated expression genes were likely to be involved in enhancing the activities of antioxidant enzymes in line W146 and subsequently increased drought tolerance.
Discussion
Drought is one of the major factors that cause rapeseed growth inhibition and yield loss. Screening out drought-tolerant rapeseed materials will improve crop yield under drought stress. To establish effective screening methods, we need a better understanding of the morphological, physiological, and molecular basis of drought tolerance in rapeseed. A number of redox metabolism and associated signaling have been studied on plants response to abiotic stress (Miller et al. 2008; Pyngrope et al. 2013). The capability of ROS scavenging may be associated with drought tolerance of plants (Tsugane et al. 1999). Pavlović et al. (2014) found that the activities of antioxidant enzymes (catalase, guaiacol peroxidase, and APX) were higher in drought conditions than those of the control; this finding suggests that antioxidant enzymes have important functions in B. rapa response to drought. Tohidi-Moghadam et al. (2009) demonstrated that canola with higher levels of antioxidants showed higher resistance to drought stress condition.
In this study, drought severely affected rapeseed growth and morphologically caused leaf wilting. Wilting is the passive movement of plant leaves to prevent excess water consumption under drought stress (Fang and Xiong 2015). The ability of plant tissues to store water is also a mechanism for drought tolerance (Malinowski and Belesky 2000). Our results suggested that line W146 was able to maintain more water in leaves than Y6 at severe drought stress.
As previously reported, drought inhibits photosynthesis rate in various plant species (Han and Kakubari 1996; Reddy et al. 2004; Hou et al. 2006). Under drought stress, photosynthesis is inhibited because of the stomata closure and the decrease in leaf internal CO2 concentration (Zhou et al. 2014). Furthermore, with the development of drought stress, chlorophyll and photosynthetic pigments will be damaged; these pigments are important for plants to harvest light and to produce reducing powers resulting in the inhibition of photosynthesis (Farooq et al. 2009). Our results showed the Pn values of both Y6 and line W146 were inhibited under drought stress, while line W146 had higher Pn than Y6 under drought conditions.
Drought stress damaged cell membrane integrity and caused increased electrolyte leakage (Premachandra et al. 1990). The ion leakage was higher in Y6 than that in line W146 under drought stress. This result revealed more severe membrane damage in Y6. Drought stress is often accompanied by oxidative stress. The enhanced accumulation of H2O2 results in the membrane lipid peroxidation and solute leakage (Dionisio Sese and Tobita 1998). MDA is an important index for lipid peroxidation degree (Smirnoff 1993). In this study, the MDA content in line W146 was significantly higher than that in Y6 under drought stress. This finding indicated that continuous drought treatment caused more severe membrane lipid peroxidation in Y6 than in line W146 (Türkan et al. 2005; Wang 2006).
The relationship between Pro accumulation and drought tolerance has been reported in many plants (Simon-Sarkadi et al. 2006; Shukla et al. 2012). The increase of Pro in plants can be considered an indicator of the degree of plant suffering from the stress (Zhao et al. 2008). Pro acts as a cytoplastic protective agent for enzymes and cell structure and can be used to adjust the redox potential and reduce cell acidity (Fang and Xiong 2015). In the current study, the Pro content was significantly increased under drought stress and the accumulation of Pro in line W146 was significantly higher than that in Y6. These results suggested that line W146 had stronger drought tolerance than Y6.
When plants confront drought, their first option is to close the stomata, which will decrease the CO2 assimilation in leaves and spare more electrons to form ROS, such as O2 ·− (Farooq et al. 2009). The excess ROS causes oxidative stress, which leads to membrane lipid peroxidation, ion leakage, metabolism perturbations, and severe injury or plant death (Gill and Tuteja 2010). However, plants have a sophisticated mechanism to scavenge excess ROS and to maintain ROS under dynamic equilibrium. Several major antioxidant enzymes, including SOD, POD, APX, and GPX, are involved in this process. The activities of SOD and POD enzymes were higher in line W146 than in Y6. In previous studies, drought preconditioning resulted in higher expression of Cu/Zn SOD gene, which may be one of the critical reasons in acquiring drought tolerance in white clover (Li et al. 2013). Transgenic rice overexpressing Cu/ZnSOD gene from Avicennia marina showed better drought tolerance in comparison to untransformed plants (Prashanth et al. 2008). TaMnSOD transgenic cotton obtained increased drought tolerance through the regulation of superoxide scavenging as well as developed root and leaf system (Zhang et al. 2014). Miao et al. (2006) found that the overexpression of ATGPX3 in Arabidopsis caused decreased sensitivity to drought stress and that ATGPX3 might play crucial role in H2O2 homeostasis. We found the transcript level of Cu/ZnSOD, MnSOD, and GPX genes was significantly up-regulated in W146. This result revealed that line W146 might possess strong drought tolerance by up-regulating the expression of the ROS-scavenging genes and by enhancing the activities of the antioxidant enzymes.
In conclusion, our data suggested that line W146 showed more drought tolerance than Y6. On the basis of physiological parameters (RWC, REC, Pn, MDA, Pro content, SOD, and POD activity), line W146 had higher RWC, Pn, Pro content, SOD, and POD activity, but lower REC and MDA contents than Y6 under drought stress. These physiological parameters may be effective for evaluating drought tolerance in rapeseed. Moreover, the gene expression of a few antioxidant enzymes revealed that the antioxidant system in line W146 functioned more effectively to reduce ROS accumulation under drought conditions. The increased drought tolerance of line W146 may be partly attributed to the accumulation of free Pro, enhanced antioxidant system, lower ROS level, more water content, and higher photosynthesis. These results will be helpful for selecting drought-tolerant rapeseed materials, introducing the trait of drought tolerance from S. alba to B. napus, and understanding the mechanism of enhanced drought tolerance in intergeneric somatic hybrids.
Author contribution statement
Y.J. F. and Y.P.W. conceived and designed the experiments; L.J.X., L.Q.Y., and N.L.S performed the experiments; Y.J.F. analyzed the data; J.L. contributed reagents/materials/analysis tools; and L.J.X., and Y.P.W. wrote the paper.
Abbreviations
- APX:
-
Ascorbate peroxidase
- AsA:
-
Ascorbate
- AsA–GSH:
-
Ascorbate–glutathione
- DAB:
-
Diaminobenzidine
- DHA:
-
Dehydroascorbate
- DHAR:
-
Dehydroascorbate reductase
- Fer:
-
Ferritin
- GPX:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GSSG:
-
Glutathione oxidized
- MDA:
-
Malondialdehyde
- MDHA:
-
Monodehydroascorbate
- MDHAR:
-
Monodehydroascorbate reductase
- NBT:
-
Nitrotetrazolium blue chloride
- Pn:
-
Net photosynthesis rate
- POD:
-
Peroxidase
- Pro:
-
Proline
- PVP:
-
Polyvinyl pyrrolidone
- REC:
-
Relative electronic conductivity
- ROS:
-
Reactive oxygen species
- RWC:
-
Relative water content
- SOD:
-
Superoxide dismutase
References
Abedi T, Pakniyat H (2010) Antioxidant enzyme changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.). Czech J Genet Plant Breed 46:27–34
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Barrs H, Weatherley P (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413–428
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Benjamin A, Franoise LC, Marie-Franoise N et al (2012) Nitrogen availability impacts oilseed rape (Brassica napus L.) plant water status and proline production efficiency under water-limited conditions. Planta 236:659–676
Brown J, Brown AP, Davis JB et al (1997) Intergeneric hybridization between Sinapis alba and Brassica napus. Euphytica 93:163–168
Chai L (2011) Ascorbate peroxidase gene from Brassica napus enhances salt and drought tolerances in Arabidopsis thaliana. Afr J Biotechnol 10:18085–18091
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought from genes to the whole plant. Funct Plant Biol 30:239–264
Chaves M, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot-London 103:551–560
Cruz de Carvalho MH (2008) Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal Behav 3:156–165
Dionisio Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Fang Y, Xiong L (2015) General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci 72:673–689
Farooq M, Wahid A, Kobayashi N et al (2009) Plant drought stress: effects, mechanisms and management. J Sustain Agr 29:153–188
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant Physiol 59:309–314
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gomes FP, Oliva MA, Mielke MS et al (2010) Osmotic adjustment, proline accumulation and cell membrane stability in leaves of Cocos nucifera submitted to drought stress. Sci Hortic 126:379–384
Han Q, Kakubari Y (1996) Drought-dependent responses of photosynthesis, transpiration and water use efficiency of Japanese cypress and Japanese red pine seedlings. J For Res 1:73–78
Hasanuzzaman M, Hossain MA, Da Silva JAT et al (2012) Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. Crop stress and its management: perspectives and strategies. Springer, Berlin, pp 261–315
Hodges DM, DeLong JM, Forney CF et al (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hou X, Duan C, Liu G et al (2006) Photosynthesis characters of tree peony in response to soil water content. Acta Agr Boreali-Sin 21:91–94
Hussain M, Mumtaz S (2014) Climate change and managing water crisis: Pakistan’s perspective. Rev Environ Health 29:71–77
Kishor PK, Sangam S, Amrutha R et al (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438
Li A, Jiang J, Zhang Y et al (2012) Molecular and cytological characterization of introgression lines with yellow seed derived from somatic hybrids between Brassica napus and Sinapis alba. Mol Breed 29:209–219
Li Z, Shi P, Peng Y (2013) Improved drought tolerance through drought preconditioning associated with changes in antioxidant enzyme activities, gene expression and osmoregulatory solutes accumulation in white clover (Trifolium repens L.). Plant Omics 6:481–489
Lisenbee CS, Lingard MJ, Trelease RN (2005) Arabidopsis peroxisomes possess functionally redundant membrane and matrix isoforms of monodehydroascorbate reductase. Plant J 43:900–914
Malinowski DP, Belesky DP (2000) Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40:923–940
Martínez JP, Silva H, Ledent JF et al (2007) Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.). Eur J Agron 26:30–38
Miao Y, Lv D, Wang P et al (2006) An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18:2749–2766
Miller G, Shulaev V, Mittler R (2008) Reactive oxygen signaling and abiotic stress. Physiol Plant 133:481–489
Mittler R, Vanderauwera S, Suzuki N et al (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Munné Bosch S, Queval G, Foyer CH (2013) The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiol 161:5–19
Pavlović I, Ludwig-Müller J, Salopek-Sondi B (2014) Hormonal profile and antioxidant defense system of Brassica rapa plants during drought and recovery period. Plant Biol 6:21–26
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Prashanth SR, Sadhasivam V, Parida A (2008) Over expression of cytosolic copper/zinc superoxide dismutase from a mangrove plant Avicennia marina in indica Rice var. Pusa Basmati-1 confers abiotic stress tolerance. Transgenic Res 17:281–291
Premachandra G, Saneoka H, Ogata S (1990) Cell membrane stability, an indicator of drought tolerance, as affected by applied nitrogen in soyabean. J Agr Sci 115:63–66
Pyngrope S, Bhoomika K, Dubey R (2013) Reactive oxygen species, ascorbate-glutathione pool, and enzymes of their metabolism in drought-sensitive and tolerant indica rice (Oryza sativa L.) seedlings subjected to progressing levels of water deficit. Protoplasma 250:585–600
Queval G, Issakidis Bourguet E, Hoeberichts FA et al (2007) Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J 52:640–657
Rao MV, Paliyath G, Ormrod DP (1996) Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol 110:125–136
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202
Saruhan N, Terzi R, Saglam A et al (2009) The relationship between leaf rolling and ascorbate-glutathione cycle enzymes in apoplastic and symplastic areas of Ctenanthe setosa subjected to drought stress. Biol Res 42:315–326
Sharma P, Dubey RS (2005) Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul 46:209–221
Shukla N, Awasthi R, Rawat L et al (2012) Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol Biochem 54:78–88
Simon-Sarkadi L, Kocsy G, Várhegyi Á et al (2006) Stress-induced changes in the free amino acid composition in transgenic soybean plants having increased proline content. Biol Plant 50:793–796
Smirnoff N (1993) Tansley review no. 52. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Tang L, Yang XL (2008) Improving potato plants oxidative stress and salt tolerance by gene transfer both of Cu/Zn superoxide dismutase and ascorbate peroxidase. Chin Biotechnol 28:25–31
Tian F, Gong J, Zhang J et al (2013) Enhanced stability of thylakoid membrane proteins and antioxidant competence contribute to drought stress resistance in the tasg1 wheat stay-green mutant. J Exp Bot 64:1509–1520
Tohidi-Moghadam H, Shirani Rad A, Nour Mohammadi G et al (2009) Effect of super absorbent application on antioxidant enzyme activities in Canola (Brassica napus L.) cultivars under water stress conditions. Am J Agric Biol Sci 4:215–223
Tsugane K, Niwa Y, Ohba YK et al (1999) A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell 11:1195–1206
Türkan İ, Bor M, Özdemir F et al (2005) Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci 168:223–231
Wang Q (2006) Effects of drought stress on protective enzymes activities and membrane lipid peroxidation in leaves of soybean seedlings. J Agro-Environ Sci 25:918–921
Wang Y, Soontag K, Rudloff E et al (2005) Intergeneric somatic hybridization between Brassica napus L. and Sinapis alba L. J Integr Plant Biol 47:84–91
Wu N, Guan Y, Shi Y (2011) Effect of water stress on physiological traits and yield in rice backcross lines after anthesis. Energy Proced 5:255–260
Xu L, Lin Z, Tao Q et al (2014) Multiple nuclear factor Y transcription factors respond to abiotic stress in Brassica napus L. PLoS One 9:e111354
Yan L, Zhao X, Xia X et al (2008) Effects of oligochitosan on photosynthetic parameter of Brassica napus seedlings under drought stress. Acta Agron Sin 34:326–329
Yang GP, Rhodes D, Joly RJ (1996) Effects of high temperature on membrane stability and chlorophyll fluorescence in glycinebetaine-deficient and glycinebetaine-containing maize lines. Funct Plant Biol 23:437–443
Zhang D, Yang H, Li X et al (2014) Overexpression of Tamarix albiflonum TaMnSOD increases drought tolerance in transgenic cotton. Mol Breed 34:1–11
Zhao Z, Cai Y, Fu M et al (2008) Response of the soils of different land use types to drought: eco-physiological characteristics of plants grown on the soils by pot experiment. Ecol Eng 34:215–222
Zhao L, Wang W, Song Y (2010) Changes of photosynthesis and membrane damage of Brassica napus L. under soil water stress. J Henan Agr Sci 8:33–35
Zhou S, Hu W, Deng X et al (2012) Overexpression of the wheat aquaporin gene, TaAQP7, enhances drought tolerance in transgenic tobacco. PLoS One 7:e52439
Zhou S, Sun X, Yin S et al (2014) The role of the F-box gene TaFBA1 from wheat (Triticum aestivum L.) in drought tolerance. Plant Physiol Biochem 84:213–223
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grants 31330057, 31501335), the Jiangsu Province Science Foundation (Grants BK20150441, 15KJB180019), China Postdoctoral Science Foundation (2015M570482), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and Yangzhou University for Excellent Talent Support Program.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by W Zhou.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xia, L., Yang, L., Sun, N. et al. Physiological and antioxidant enzyme gene expression analysis reveals the improved tolerance to drought stress of the somatic hybrid offspring of Brassica napus and Sinapis alba at vegetative stage. Acta Physiol Plant 38, 88 (2016). https://doi.org/10.1007/s11738-016-2111-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2111-0