Abstract
Species of the genus Wolffia (duckweed) are harvested from natural water bodies in many countries for human consumption. Relative growth rates (RGR) of 25 clones (ecotypes) representing all 11 species of the genus Wolffia were investigated under standardized laboratory conditions in search for potential candidates for production of Wolffia biomass at a biotechnological scale. This is the first report of large-scale screening of physiological properties of Wolffia species. Large differences in RGR of different clones were detected, e.g., in Wolffia globosa. Interestingly, intraspecific differences, i.e., at the level of clones are much higher than differences between species. Rate of photosynthesis (oxygen production in light) and respiration (oxygen consumption in dark) in clones of W. globosa, measured under standardized conditions, are in positive correlation with their respective RGR. Higher rate of photosynthesis seems to be a determining factor for higher RGR. The RGR of the first available axenic clone of the re-discovered species, Wolffia microscopica (clone 2005), depends strongly on the nutrient medium used, in contrast to other investigated species. This clone of W. microscopica has a doubling time of 29.3 h and represents the fastest growing flowering plant known till date.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lemnaceae is a family of monocotyledonous water plants classified into 37 species arranged in five genera (Appenroth et al. 2013; Borisjuk et al. 2015). This family represents the smallest flowering plants (Landolt 1986; Bog et al. 2013). More interesting, however, is the fact that they are gaining increasing importance in terms of their practical applications because of the following reasons: (1) they are the fastest growing angiosperms known till date (Ziegler et al. 2015) producing high amounts of biomass which can be used as feedstock, e.g., for biogas production (Fedler and Duan 2011). (2) Under optimal growth conditions, i.e., light, temperature and nutrient availability, the resulting biomass contains high amounts of valuable proteins that makes it suitable for animal and human nutrition (Bhantumnavin and McGarry 1971; Appenroth et al. 1982; Suppadit et al. 2012; Zetina-Cordoba et al. 2013). (3) When subjected to certain stress conditions, the protein content decreases but the starch content in the duckweed biomass increases to a considerable level. Biomass obtained from such conditions can be used for production of bioethanol via starch degradation and subsequent sugar fermentation (Sree and Appenroth 2014; Cui and Cheng 2015; Sree et al. 2015a, b; Zhao et al. 2015). (4) Duckweeds can grow efficiently on wastewaters. In this process, they take up nutrients from wastewater for their growth and in turn clean it up, thus, eliminating the requirement of external application of fertilizers that can create an additional threat to the environment (Fujita et al. 1999; Verma and Suthar 2014; Cui and Cheng 2015; Zhao et al. 2015).
The natural genetic variability of duckweeds has hardly been tapped for practical applications and there is an urgent need to screen physiological properties at the level of species and clones. This holds true for the rate of biomass production, capacity of protein and starch accumulation in the biomass and so on. Some of the recent physiological investigations demonstrate that such natural variance exists not only exists between different duckweed species but also is intraspecific, i.e., between different clones (ecotypes) of the same species collected from different geographic locations of the world (Kuehdorf et al. 2014; Ziegler et al. 2015).
Wolffia species have been used for many generations as human food in Asian countries like Laos, Cambodia and Thailand (Landolt and Kandeler 1987). The authors have witnessed the use of Wolffia globosa for nutrition of children in Bangladesh. The fact that Wolffia species, unlike duckweeds belonging to other genera, contain oxalate in the soluble form and not as calcium oxalate crystals (that might create problems in digestion), makes them well suited as human food (cf. Landolt and Kandeler 1987). Moreover, the rootless nature of Wolffia species might increase their palatability just by esthetic reasons. Wolffia multiplies mainly by vegetative propagation by the budding of daughter fronds from one single pouch of the mother frond (Sree et al. 2015c). Consequently, clonal offsprings with identical genetic properties are produced. In a previous paper, we investigated rates of vegetative growth of 13 species of duckweeds belonging to all five genera, i.e., Spirodela, Landoltia, Lemna, Wolffiella, and Wolffia (Ziegler et al. 2015). It was shown that the variation in vegetative growth rates exists at the level of clones rather than at the level of species or genera. As biomass production is a key point for any practical application and considering the potential of Wolffia as human food, in the present project we focused on large-scale screening of different species and clones of this genus.
The screening strategy in the present investigation is based on the exponential growth rate of the fronds under optimal growth conditions in order to examine their potential for biomass production. It was not intended to imitate any of the ecological conditions concerning temperature, light intensity, photoperiod or abiotic or biotic stress in order to predict the behavior of the clones under out-door conditions. For the first time after several decades, the species endemic to India, Wolffia microscopica, could be integrated into the current investigation after its recent re-discovery (Sree et al. 2015c). During our investigations, we found that the growth of W. microscopica depends largely on the nutrient medium being used. Thus, we compared the growth rates of six selected clones of different Wolffia species in three different growth media. Finally, in order to explain the varying growth rates of different clones of the same species in the genus Wolffia, we selected the clones of W. globosa and tested the hypothesis whether there is any correlation between growth rate and photosynthetic rate.
Materials and methods
Plant material, pre-cultivation and cultivation
All plant material was taken from the collection of the Department of Plant Physiology of the University of Jena. The species identities of the clones were originally defined by the late Elias Landolt, ETH Zurich and confirmed again before the experiments by one of the present authors (KSS). Moreover, 19 out of the 33 clones were characterized before by molecular barcoding (Bog et al. 2013; Borisjuk et al. 2015). The clones investigated are listed in Table 1. The duckweed clones were taken from stock cultures and pre-cultivated under axenic conditions at 25 ± 1 °C and 100 µmol m−2 s−1 photosynthetically active radiation from fluorescence tubes TLD 36W/86 (Philips, Eindhoven, The Netherlands) following the ISO 20079 protocol (Naumann et al. 2007). The plants were pre-cultivated for 4 weeks and nutrient medium was replenished every week. This step of pre-cultivation was necessary to adapt the plants to the different nutrient media, light and temperature conditions, otherwise irreproducible results would be obtained. A modified Schenk-Hildebrandt medium (Schenk and Hildebrandt 1972) was employed with the following composition: CaCl2·2H2O 0.68 mM, KNO3 12.4 mM, MgSO4·7H2O 0.81 mM, (NH4)H2PO4 1.3 mM, MnSO4·H2O 30 µM, H3BO3 40 µM, ZnSO4·7H2O 1.74 µM, KI 3.0 µM, CuSO4·5H2O 0.4 µM, Na2MoO4·2H2O 0.21 µM, CoCl2·6H2O 0.21 µM, FeNaEDTA 27.0 µM, Na2EDTA·2H2O 2.74 µM. The pH of the medium was adjusted to 5.50. For some experiments, modified Hoagland medium with 100 µM EDTA (Venkataraman et al. 1970) or N-medium (Appenroth et al. 1996) were used to test the influence of the nutrient media.
The main phase of cultivation used for determining the growth rates employed the same conditions as described for the pre-cultivation, except that 400 mL glass beakers containing 300 mL autoclaved media were used for cultivation. Ten to 20 fronds were randomly selected from the pre-culture as inoculum for initiating the main (measurement) phase of growth, which lasted for 7 days. The fronds never completely covered the surface of the medium that would lead otherwise to growth limitation.
Growth parameters
The number of fronds (FN) was determined at the onset of the respective experiment (t = 0 day: “t0”) and 7 days later (t = 7 days: “t7”) present in each of the 6 parallel samples. In preliminary investigations counting the number of fronds at 0, 1, 2, 4 and 7 days it was confirmed that all clones in all three media tested followed the law of exponential growth. Therefore, the experiments were simplified using only two data points. Relative growth rates per day (RGR) were calculated using Eq. (1) (Naumann et al. 2007). For measurements involving only two time points, this equation simplifies to Eq. (2) with t7 at the end of the 7-day test period, for each replicate separately.
“RGR” is the increase in parameter value per the unit of time of 1 day.
Doubling time DT (in days) was calculated as:
The yield obtained from the inoculum of 1 frond (or 1 g duckweed biomass) after 7 days of cultivation was termed “relative yield” (RY: analogous to the RGR, but on the basis of the unit of time of 1 week). It was calculated by solving for lnx t7 in Eq. (2) in the following equation:
Since t7 is 7 day and x at t0 is 1 frond or 1 g, x t7 is then equal to elnxt7. The RY on a frond number basis has the dimension of g g−1 7 day−1 or g g−1 week−1.
All quoted data give the mean values obtained from 6 replicate measurements, together with the standard error of the means.
Microelectrode measurements
The oxygen production and oxygen consumption of five different clones of W. globosa were measured using an oxygen microelectrode with a tip diameter of 8–12 μm connected to a microsensor-multimeter OX10 (Unisense, Aarhus, Denmark). The oxygen microelectrode was calibrated using water saturated with air (21 % O2) and 100 % nitrogen (0 % O2). This set up was used with two replicates for each clone of W. globosa weighing approximately 100 mg in fresh weight suspended in 1 ml of the nutrient medium in a sterile GC vial. Each replicate was initially allowed to stabilize over a period of 5 min (steady state) before being plunged into darkness for 150 s to record the rate of oxygen consumption. It followed measurement of the rate of oxygen production for 150 s in the presence of light after a new steady state was reached. The light intensity was set to 100 µmol m−2 s−1 at 25 °C. Results were given as change of relative oxygen concentration (arbitrary units) per g fresh weight of W. globosa per minute.
Statistics
The mean values of the RGR and DT of each clone were calculated from two sets of three parallel samples each from two independent experiments (n = 6). They are presented in Table 1 and Fig. 1 together with the standard errors of the means. In Fig. S1 each “error bar” for a particular column indicates the span of the lowest and the highest mean values for the individual clones associated with that column.
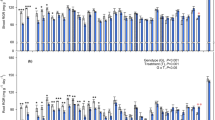
Relative yield (RY) of all the 25 clones of Wolffia investigated in the present project (black bars) compiled together with 8 of those investigated previously (white bars; Ziegler et al. 2015). Schenk-Hildebrandt medium was used in all cultivations. More details about these 33 clones are available in Table 1
Results and discussion
In the present project, RGRs of 25 clones spanning all 11 species of the genus Wolffia were determined. The previously published RGRs of 8 additional clones belonging to three of the Wolffia species (Ziegler et al. 2015) were integrated in the present data in order to make the data set more comprehensive. Thus, in the present paper growth rates of 33 clones of different Wolffia species investigated under identical, standardized growth conditions were analyzed. The RGR and the DT of all clones were summarized in Table 1, and RY is depicted in Fig. 1.
In Schenk-Hildebrandt nutrient medium, the lowest growth rate was recorded in W. globosa, clone 9196, with an RGR of 0.155 day−1 and a DT of 4.47 days (Table 1; Fig. 1). In terms of RY, this clone produced 2.96 g biomass from a starting material of 1 g in 1 week. Interestingly, under the same cultivation conditions, the highest growth rate was also recorded in the species W. globosa, clone 9498, with an RGR of 0.559 day−1 and a DT of 29.8 h. In addition, in terms of RY, this clone produces 50 g biomass per week from a starting material of 1 g. Evidently, there is a huge variation in the growth rates of these two clones. These results were obtained under standardized laboratory conditions in Schenk-Hildebrandt nutrient medium. The performance of the clones will, of course, be different under natural conditions. However, cultivation under standardized, optimal conditions gives information about the growth capacity of the investigated clones and makes it possible to perform a comparative analysis of the clones under the same defined conditions. As discussed above, both the slowest and the fastest growing clones belong to the species W. globosa demonstrating a very high intraspecific variation. Intraspecific variations of vegetative growth can also be seen in W. angusta, W. arrhiza, W. brasiliensis and W. columbiana (Fig. 1; Table 1). The intraspecific variations in the growth rates between some of the clones of a species are larger than the interspecific variation of the averages of growth rates of all the clones of a species (Fig. S1, supplementary material). It can be concluded that the rate of vegetative propagation of a clone is not completely defined by the species it belongs to. In general, clones of a duckweed species are isolated from locally adapted populations. Therefore, it can be assumed that the adaptation of plants to local ecological conditions must be a crucial factor in determining their physiological properties, e.g., rate of vegetative growth. This is in accordance with our previous results for representative clones belonging to different species and genera of duckweed (Ziegler et al. 2015), however, this assumption can now be firmly based on the present, more detailed analysis of the data comprising of 33 clones, all belonging to the genus Wolffia.
According to Venkataraman et al. (1970), the growth rate of one of the clones of W. microscopica was reported to be 0.78 day−1, equivalent to a DT of 21 h and RY of 235 g g−1 week−1. This growth rate is much higher than any of the ones in the present data set. Unfortunately, this clone was lost in all stock collections and no representative of the species, W. microscopica, was available (Bog et al. 2013; Lam et al. 2014) until its recent re-discovery (Sree et al. 2015c). We were now able to include the very first data about the re-discovered W. microscopica, clone 2005 (Table 1). However, the RGR of this clone measured in Schenk-Hildebrandt medium was not especially high in comparison with other Wolffia clones (Fig. 1).
In an effort to understand the large intraspecific differences in growth rates of duckweeds, we hypothesized that RGR correlates positively with photosynthetic rate of the given clone. Clones belonging to W. globosa were selected for testing this hypothesis because they displayed large intraspecific differences in growth rates. As shown in Fig. 2a, there is a positive correlation between the logarithms of the oxygen evolution (OE) and growth rates of the clones following the equation:
This relationship has a coefficient of determination of R 2 = 0.796 and the statistical test indicated high significance (F = 31.159, P < 0.001). It can be concluded that the clones with high apparent photosynthetic rates also possess high growth rates. The high rates of photosynthesis might make high growth rates possible. This inference does not exclude the influence of other factors on a complex physiological process like growth but demonstrates that it is a factor of significant impact.
Correlation of the relative growth rates (RGR) of five clones of Wolffia globosa with rates of oxygen formation (a) and consumption (b), respectively. Oxygen formation and consumption were measured in relative units (see “Materials and methods” section). The clone numbers are indicated parallel to the two data points that represent the replicate measurements of the oxygen formation or consumption correlating to the RGR of that particular clone
We tested also the respiration in darkness in all five clones by measuring the consumption of oxygen (OC) in darkness (Fig. 2b). The following equation describes the relationship between the logarithm of the rate of oxygen consumption and the growth rates with an R 2 = 0.771; with the statistical test indicating high significance (F = 19.776, P = 0.003):
From the results, it can be concluded that high growth rates are associated with high respiratory activity or, in a broader sense, may indicate high metabolic activity. It should be finally added that the logarithm of gross photosynthetic oxygen production (net photosynthesis) and the growth rate also correlated significantly with an R 2 = 0.777 (data not shown). Already many years ago, Zelitch (1982) stressed that measuring photosynthesis of single leaves of a plant cannot result in satisfactory results to comprehend the rate of plant growth and postulated the requirement of measuring photosynthesis and respiration of whole plants, which was easily possible using duckweed system in the present study. Poorter et al. (1990) demonstrated a significant relationship between photosynthesis and dry mass of the leaves in many wild species. This is similar to the present results with different clones of W. globosa showing a significant correlation between photosynthesis and growth rate.
In general, for the measurement of growth rates of duckweeds, modified Schenk-Hildebrandt medium is used as in Ziegler et al. (2015) and also in the present work because a preliminary experiment with a set of selected duckweed clones belonging to all five different genera including Wolffia showed a good performance in this medium in terms of their growth and appearance (data not shown). Apart from this, we wanted to replicate the method of Venkataraman et al. (1970) for the measurement of growth rate especially of W. microscopica in order to be able to compare their published results with those of the present ones. Therefore, we decided to measure the RGR of W. microscopica in three different nutrient media (modified Schenk-Hildebrandt medium, modified Hoagland medium, and N medium; cf. “Materials and methods” section). For comparison the clones of other Wolffia species, i.e. W. globosa, W. arrhiza, and W. angusta which showed fast and slow growth in modified Schenk-Hildebrandt medium (Fig. 1) were also tested in modified Hoagland medium and N medium (Fig. 3). Interestingly, there was no significant difference in the growth rates of Wolffia clones in different nutrient media except for that of W. microscopica clone 2005. This clone showed huge differences in its growth rates when cultivated in different nutrient media, the slowest being in modified Schenk-Hildebrandt medium and the highest in N medium (Fig. 2). The reason for this exceptional behavior of W. microscopica is not clear. Of course, there exist differences in the composition of the three nutrient media used but it is very intriguing why only W. microscopica reacts so sensitively to different growth media. In N medium, the RGR of W. microscopica clone 2005 was 0.568 ± 0.006 day−1 (DT = 29.3 h, RY = 51.2 g g−1 week−1). This is the fastest growth rate out of all the investigated clones, significantly higher than that of W. globosa clone 9498 grown in the N medium or in modified Schenk-Hildebrandt medium. This means that this clone is presently the fastest growing Angiosperm.
Relative growth rates of six clones of Wolffia in three different nutrient media. For the composition of the nutrient media refer to the “Materials and methods” section. The investigated clones belong to the following species: W. globosa (9196, 9498), W. arrhiza (8871, 9567), W. angusta (8878), W. microscopica (2005). The values are represented by means together with the standard errors of the means
Although the fastest growing in the present data set, W. microscopica clone 2005 showed much lower RGR than that of the lost clone which was investigated by Venkataraman et al. (1970). The huge difference between the growth rates of the two clones of W. microscopica is not very surprising; such differences were also detected in W. globosa, e.g., in the present study. These results provide an additional proof that the growth rate is essentially defined at the level of clones rather than at the species level.
Author contribution statement
K. Sowjanya Sree and Klaus-J. Appenroth designed and carried out the experiments and evaluated the data. The measurements of the oxygen formation and consumption were carried out by Sailendharan Sudakaran. The manuscript was written in cooperation of all three authors.
Abbreviations
- DT:
-
Doubling time (h or day)
- RGR:
-
Relative growth rate (day−1)
- RY:
-
Relative yield (g g−1 week−1)
References
Appenroth K-J, Augsten H, Liebermann B, Feist H (1982) Effects of light quality on amino acid composition of proteins in Wolffia arrhiza (L.) Wimm. using a specially modified Bradford method. Biochem Physiol Pflanzen 177:251–258
Appenroth K-J, Teller S, Horn M (1996) Photophysiology of turion formation and germination in Spirodela polyrhiza. Biol Plantarum 38:95–106
Appenroth K-J, Borisjuk N, Lam E (2013) Telling duckweed apart: genotyping technologies for Lemnaceae. Chin J Appl Environ Biol 19:1–10
Bhantumnavin K, McGarry MG (1971) Wolffia arrhiza as a possible source of inexpensive protein. Nature 232:495
Bog M, Schneider P, Hellwig F, Sachse S, Kochieva EZ, Martyrosian E, Landolt E, Appenroth K-J (2013) Genetic characterization and barcoding of taxa in the genus Wolffia Horkel ex Schleid. (Lemnaceae) as revealed by two plastidic markers and amplified fragment length polymorphism (AFLP). Planta 237:1–13
Borisjuk N, Chu P, Gutierrez R, Zhang H, Acosta K, Friesen N, Sree KS, Garcia C, Appenroth KJ, Lam E (2015) Assessment, validation and deployment strategy of a two-barcode protocol for facile genotyping of duckweed species. Plant. Biol. 17(Suppl. 1):42–49
Cui W, Cheng JJ (2015) Growing duckweed for biofuel production: a review. Plant Biol 17(Suppl. 1):16–23
Fedler CB, Duan R (2011) Biomass production for bioenergy using recycled wastewater in a natural waste treatment system. Resour Conserv Recy 55:792–800
Fujita M, Mori K, Kodera T (1999) Nutrient removal and starch production through cultivation of Wolffia arrhiza. J Biosci Bioeng 87:194–198
Kuehdorf K, Jetschke G, Ballani L, Appenroth K-J (2014) The clonal dependence of turion formation in the duckweed Spirodela polyrhiza—an ecogeographical approach. Physiol Plant 150:46–54
Lam E, Appenroth K-J, Michael T, Mori K, Fakhoorian T (2014) Duckweed in bloom: the 2nd international conference on duckweed research and applications heralds the return of a plant model for plant biology. Plant Mol Biol 84:737–742
Landolt E (1986) The family of Lemnaceae—a monographic study. vol 1. Biosystematic Investigations in the Family of Duckweeds (Lemnaceae). Veröffentlichungen des Geobotanischen Institutes der ETH, Stiftung Rübel, Zurich
Landolt E, Kandeler R (1987) The family of Lemnaceae—a monographic study. vol 2. Biosystematic Investigations in the Family of Duckweeds (Lemnaceae). Veröffentlichungen des Geobotanischen Institutes der ETH, Stiftung Rübel, Zurich
Naumann B, Eberius M, Appenroth K-J (2007) Growth rate based dose-response relationships and EC-values of ten heavy metals using the duckweed growth inhibition test (ISO 20079) with Lemna minor L. clone St. J Plant Physiol 164:1656–1664
Poorter H, Remkes C, Lambers H (1990) Carbon and nitrogen economy of 24 wild-species differing in relative growth rate. Plant Physiol 94:621–627
Schenk RU, Hildebrandt AC (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot 50:199–204
Sree KS, Appenroth K-J (2014) Increase of starch accumulation in the duckweed Lemna minor under abiotic stress. Albanian J Agric Sci 13(Special edition):11–14
Sree KS, Adelmann K, Garcia C, Lam E, Appenroth K-J (2015a) Natural variance in salt tolerance and induction of starch accumulation in duckweeds. Planta 749:169–182
Sree KS, Keresztes Á, Mueller-Roeber B, Brandt R, Eberius M, Fischer W, Appenroth K-J (2015b) Phytotoxicity of cobalt ions on the duckweed Lemna minor—morphology, ion uptake, and starch accumulation. Chemosphere 131:149–156
Sree KS, Maheshwari SC, Boka K, Khurana J, Keresztes A, Appenroth K-J (2015c) The duckweed Wolffia microscopica: a unique aquatic monocot. Flora 210:31–39
Suppadit T, Jaturasitha S, Sunthorn N, Poungsuk P (2012) Dietary Wolffia arrhiza meal as a substitute for soybean meal: its effect on the productive performance and egg quality of laying Japanese quails. Trop Anim Health Prod 44:1479–1486
Venkataraman R, Seth PN, Maheshwari SC (1970) Studies on the growth and flowering of a short-day plant, Wolffia microscopica. I. General aspects and induction of flowering by cytokinins. Z Pflanzenphysiol 62:316–327
Verma R, Suthar S (2014) Synchronized urban waste water treatment and biomass production using duckweed Lemna gibba L. Ecol Eng 64:337–343
Zelitch I (1982) The close relationship between net photosynthesis and crop yield. Bioscience 32:796–802
Zetina-Cordoba P, Ortega-Cerilla ME, Ortega-Jimenez E, Herrera-Haro JG, Sanchez-Torres-Esqueda MT, Reta-Mendiola JL, Vilaboa-Arroniz J, Munguia-Ameca G (2013) Effect of cutting interval of Taiwan grass (Pennisetum purpureum) and partial substitution with duckweed (Lemna sp. and Spirodela sp.) on intake, digestibility and ruminal fermentation of Pelibuey lambs. Livestock Science 157:471–477
Zhao Y, Fang Y, Jin Y, Huang J, Bao S, Fu T, He Z, Wang F, Wang M, Zhao H (2015) Pilot-scale comparison of four duckweed strains from different genera for potential application in nutrient recovery from wastewater and valuable biomass production. Plant Biol 17(Suppl. 1):82–90
Ziegler P, Adelmann K, Zimmer S, Schmidt C, Appenroth K-J (2015) Relative in vitro growth rates of duckweeds (Lemnaceae)—the most rapidly growing higher plants. Plant Biol 17(Suppl. 1):33–41
Acknowledgments
We thank Prof. Dr. Peter Schopfer, University of Freiburg, Freiburg, Germany and Prof. Dr. Bisanti Biswal, Sambalpur University, Jyotovihar, Orissa, India for critical comments. We also thank the students Christopher Koterew and André Noack, Jena for their experimental support. KSS acknowledges the Science and Engineering Research Board, Govt. of India, for its support through the Fast Track Young Scientist project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. A. Kleczkowski.
Electronic supplementary material
Below is the link to the electronic supplementary material.

11738_2015_1951_MOESM1_ESM.jpg
Fig. S1 Relative yield (RY) of clones belonging to all 11 species of the genus Wolffia. Column height depicts mean of the relative yield (g g−1 week−1) of all the clones of a particular species or that of a single clone of a species as per the investigation. The range of relative yield of the clones belonging to a particular species is shown, where applicable, by a vertical line with limiting cross bars. All plants were cultivated in Schenk-Hildebrandt medium. For the details about the investigated clones, see Table 1 (JPEG 405 kb)
Rights and permissions
About this article
Cite this article
Sree, K.S., Sudakaran, S. & Appenroth, KJ. How fast can angiosperms grow? Species and clonal diversity of growth rates in the genus Wolffia (Lemnaceae). Acta Physiol Plant 37, 204 (2015). https://doi.org/10.1007/s11738-015-1951-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1951-3