Abstract
Soil salinity is one of the m ajor abiotic stresses affecting crop growth and yield worldwide. Barley is a species with higher salt tolerance among cereal plants and rich in genetic variation. It is quite important to understand the physiological mechanisms of genotypic difference in salt tolerance. In this study, physiological and biochemical responses of a Tibetan wild barley genotype XZ16 (salt tolerant) and a cultivated cultivar Yerong (salt sensitive) to salt stress were investigated. The results showed that the two genotypes differed dramatically in their responses to salt stress (150 and 300 mM NaCl) in terms of plant biomass, Na+ accumulation and Na+/K+ ratio in roots and shoots, chlorophyll content, xylem sap osmolarity and electrolyte leakage. XZ16 showed less biomass reduction, lower Na+/K+ ratio and electrolyte leakage, higher xylem sap osmolarity, and vacuolar H+-ATPase and H+-PPase activities than Yerong under 300 mM NaCl. The higher salt tolerance of XZ16 may be attributed to its lower concentration of Na+ influx or more sequestration into the vacuoles. The results indicate that the Tibetan wild barley is useful for improvement of cultivated barley in salt stress tolerance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil salinization is a major factor affecting crop productivity and becomes a serious issue in agricultural systems, particularly for those that rely heavily on irrigation (Flower 2004). High salinity may inhibit plant growth, causing reduced crop yield. The growth inhibition in plants is attributed to detrimental effects of salinity on many physiological activities, including ion toxicity, osmotic stress and nutrient disorder (Blumwald et al. 2000; Tammam et al. 2008). Obviously, the mechanism of salt tolerance in plants is quite complex. The elucidation of biochemical and physiological responses of plants to salinity is imperative to improve the salt tolerance of crops.
Adaptation of plants to salinity is involved in changes of many cellular, physiological and metabolic processes. Several mechanisms of salt tolerance in plants have been proposed, including exclusion of surplus Na+ or compartmentalization and ion homeostasis, etc. Na+ excretion and compartmentalization in plant cells are regulated by Na+/H+ antiporters in both plasma membranes and tonoplasts (Li et al. 2007). Normally, plant cells depend on proton gradients to sequester the toxic ions into vacuoles (Dietz et al. 2001) by increasing V-ATPase activity (Kluge 2003). It has been known that vacuolar H+-ATPase as well as H+-pyrophosphatase (V-PPase) plays an important role in vacuole acidification, which allows the active transport of ions across the tonoplast (Maeshima 2000; Sze et al. 2002).
Barley (Hordeum vulgare L.) is a widely cultivated crop in the world and is the most tolerant to salinity among cereal crops (Steppuhn et al. 2005). Therefore, barley is used not only as a model plant in salinity research, but also as a candidate crop for cultivation in salt lands. However, the narrow genetic diversity in the cultivated barley has increased sensitivity to salinity stress (Zhu 2001). On the other hand, wild barley shows a wider genetic variation in salt tolerance as compared to cultivated barley (Shavrukov et al. 2010; Qiu et al. 2011). Tibetan wild barley (Hordeum sponteneum) is considered as an ancestor of cultivated barley (Dai et al. 2012) and is rich in genetic variation in abiotic stress tolerance, e.g., drought (Zhao et al. 2010), aluminum toxicity (Dai et al. 2011) and salinity (Wu et al. 2011). However, few researches have been done on the difference in physiological and biochemical responses to salt stress between the wild and cultivated barleys. Hence, the current study was conducted aiming at (i) determining the difference between the wild and cultivated barleys in their responses to salt stress and (ii) understanding the mechanisms of salinity tolerance in wild barley.
Materials and methods
Plant materials and growth conditions
Two barley genotypes, a Tibetan wild barley XZ16 (salt tolerant) and a cultivated barley Yerong (salt sensitive), were used in this study according to the previous experiment (Qiu et al. 2011). The experiment was conducted in 2012 at the Zijingang campus, Zhejiang University, Hangzhou, China. The seeds of the two barley genotypes were sterilized in 3 % H2O2 for 20 min and rinsed thoroughly with distilled water three times. Then the seeds were germinated in moist sand under an incubator (22/18 °C, day/night). After 10 days of germination (2nd leaf stage), uniform seedlings were selected and transplanted into 35_L plastic containers, which were covered with a polystyrol plate with 48 holes and placed in a greenhouse. Two seedlings were fixed in each hole. The composition of the nutrient solution was (mg L−1): (NH4)2SO4 48.2, MgSO4 154.88, K2SO4 15.9, KNO3 18.5, KH2PO4 24.8, Ca (NO3)2 86.17, Fe citrate 7, MnCl2·4H2O 0.9, ZnSO4·7H2O 0.11, CuSO4·5H2O 0.04, HBO3 2.9 and H2MoO4 0.01. On the 10th day of seedling transplantation, NaCl was added to the above solution to form three treatments: (1) 0 (control); (2) 150; and (3) 300 mM NaCl. The treatments lasted for 27 days. The nutrient solution was renewed every 7 days and continuously aerated with pumps during the whole experiment. A completely randomized design was used for the experiment with three replicates.
Measurement of leaf chlorophyll content
For measurement of chlorophyll content, 0.5 g leaves from each treatment were homogenized under 80 % acetone and then centrifuged at 12,000g for 10 min. The clear extract was used for measurement of absorbance values at 663 and 645 nm, and chlorophyll content was calculated as described by Porra et al. (1989).
Measurement of Na+ and K+ contents
On the 27th day of treatment, plant tissues (shoot and root) were sampled, then washed three times with deionized water and dried at 70 °C for 72 h. The dried tissues samples were ground. Approximately, 0.1 g sample was mixed with 10 mL HNO3 and digested in a microwave digestion instrument(Microwave 300, Anton PAAR, Graz, Austria). The digested solution was used for determination of Na+ and K+ contents by a flame atomic absorption spectrometry (AA6300, Shimadzu, Japan).
Preparation of tonoplast vesicles
Tonoplast_enriched membrane vesicles were isolated according to Zhang et al. (2004), with slight modification. All steps of the procedure were performed at 4 °C. About 1.5 g fresh shoots or roots were homogenized in a mortar with the pestle, adding the solution containing 30 mmolL−1 Hepes_Tris (pH 7.5), 250 mmolL−1 D_mannitol, 3 mmolL−1 EGTA, 1 mmolL−1 DTT and 0.1 % (w/v) bovine serum albumin. The obtained homogenate was filtered through two layers of cheesecloth and then centrifuged at 480g for 10 min. The supernatant was centrifuged at 12,000g for 15 min, and the obtained supernatant was re-centrifuged at 60,000g for 30 min. The pellet was gently suspended in the buffer which contained 2.5 mmolL−1 Hepes_Tris (pH 7.5), 250 mmolL−1 D_mannitol and 0.5 mmolL−1 DTT. The suspension was layered over a 1 %/8 % (w/v) dextran (T 70) dissolved in the buffer and centrifuged at 70,000g for 2 h. The membrane vesicle fraction located at the dextran interface was carefully collected.
Measurement of protein content and enzyme activity
Protein content in membrane vesicles was determined as described by Bradford (1976), using bovine serum albumin as a standard. The activities of H+-ATPase and H+-PPase were determined by measuring the amount of inorganic phosphate, which was released from ATP and pyrophosphate, respectively (Yoshiyuki et al. 1992). The reaction mixture for assaying H+-ATPase activity contained 30 mmolL−1 Hepes_Tris (pH 7.5), 6 mmolL−1 MgSO4, 50 mmolL−1 KCl, 0.1 mmolL−1 ammonium molybdate, 0.5 mmolL−1 Na azide, 0.1 mmolL−1 Na orthovanadate and 3 mmolL−1 Na2ATP, with the final volume being 2 ml. The mixture for assaying H+-PPase activity contained 30 mmolL−1 Hepes_Tris (pH 8.0), 2 mmolL−1 MgSO4, 50 mmolL−1 KNO3, 0.1 mmolL−1 ammonium molybdate, 0.5 mmolL−1 Na azide, 0.1 mmolL−1 Na orthovanadate and 1 mmolL−1 Na4PPi, with the final volume being also 2 mL. The reaction was initiated by adding 0.5 ml of enzyme solution and terminated by adding 1.0 ml of 15 % cold TCA after incubation at 37 °C for 20 min. The released Pi was measured by Fiske–Subbarow reagent, with KH2PO4 being used as a standard.
Measurement of electrolyte leakage
Root electrolyte leakage (EL) was measured as described by Radoglou et al. (2007) with some modification. In brief, plant roots were cut into 2 cm segments and then rinsed three times with deionized water. About 0.2 g root sample was placed in a test tube with 20 ml deionized water. The tube was agitated on a shaker for 24 h, and the conductivity of the solution (EC1) was determined using a conductivity meter (DDSJ_308A). The root samples were then treated in an autoclave at 121 °C for 20 min, and again conductivity of the solution was determined after the tubes were cooled down to room temperature (EC2). The EL value was calculated according to the equation: (EC1/EC2) × 100.
Measurement of osmolarity in the xylem sap
Osmolarity in the xylem sap was determined using the method reported by Steudle et al. (1980). A disc of glass microfiber paper (Whatman GF/B) was placed in a barrel of a 2 cm3 plastic syringe, so as to cover the outlet hole. The plant shoots were cut at 80 mm above the junction of the root and shoot and then placed into the barrel. The syringe was frozen in liquid nitrogen and then thawed to ambient temperature. When temperature equilibration was reached, the plunger and Blu_Tack were taken off. The barrel with the syringe was placed into a 15 cm3 centrifuge bucket. After centrifugation at 2500g for 10 min, clear sap was collected and added into another centrifuge tube to keep the tissue residue remaining in the barrel. The osmolarity of each sample was measured by a vapor pressure osmometer (Model 5600).
Statistical analysis
All data were analyzed using statistical analysis software SAS V8. The correlation coefficients (r) were evaluated with a Fisher’s two_tailed test. LSD test was used for multiple comparisons of the means at P ≤ 0.05.
Results
Growth parameters
Root fresh weight was increased under low salinity level (150 mM NaCl) for both genotypes, relative to the control. Under high salinity (300 mM), root fresh weight was significantly reduced for both genotypes; however, the level of reduction differed between the two genotypes, with XZ16 showing less reduction (15 %) than Yerong (36 %) (Fig. 1a). Shoot fresh weight was reduced under the two salinity levels. There were 10 % and 22 % reductions at 150 mM NaCl for XZ16 and Yerong as compared to control, respectively. But at 300 mM NaCl, the reduction was much greater in Yerong (79 %) than in XZ16 (48 %) (Fig. 1b).
Under 150 mM NaCl, the two genotypes exhibited increase in root dry weight in comparison with the control. Under 300 mM NaCl, 50 % reduction in root dry weight was observed in Yerong, while only 25 % was reduced in XZ16 compared with their respective control (Fig. 1c). Shoot dry weight of XZ16 and Yerong decreased by the same percentage (28 %) under 150 mM NaCl relative to the control (Fig. 1d). Under 300 mM NaCl, Yerong and XZ16 reduced by 64 and 38 %, respectively (Fig. 1d).
Under 150 mM NaCl, no apparent change was observed in the root length of XZ16, while Yerong showed 10 % reduction relative to the control. Up to 36 and 17 % reduction was observed in Yerong and XZ16 under 300 mM NaCl, respectively (Fig. 2a). Under 150 mM NaCl, 11 % decrease was observed in shoot height of both genotypes compared with control. On the other hand, under 300 mM NaCl, 40 and 29 % reduction in shoot height was detected in Yerong and XZ16, respectively (Fig. 2b).
Na+ and K+ contents in plants
Na+ contents in both roots and shoots of the two genotypes increased significantly (P ≤ 0.01) with increasing NaCl level in the growth medium (Table 1). By contrast, K+ contents in both roots and shoots of the two genotypes showed dramatic reduction with increasing NaCl level. Moreover, the increased Na+ content and reduced K+ content in Yerong were markedly larger than those in XZ16. Consequently, Yerong had significantly higher Na+/K+ ratio than XZ16 in both roots and shoots, except for a slight increase in shoot Na+/K+ ratio in XZ16 under 150 mM NaCl, in comparison with Yerong (Table 1).
Chlorophyll content
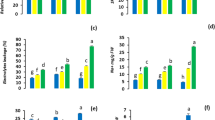
The chlorophyll a, b and total (a + b) contents in the two genotypes increased when plants were exposed to 150 mM NaCl, with 16, 12 and 14 % increase in Yerong and 13, 12 and 13 % increase in XZ16, respectively. Under 300 mM NaCl, Yerong showed a reduction of 33, 38 and 36 % in chlorophyll a, b and (a + b) contents, respectively, while only 6 % decrease was observed in XZ16 for chlorophyll a content, and little change for chlorophyll b and [(a + b) contents (Fig. 3a–c].
Xylem sap osmolarity and electrolyte leakage
Xylem sap osmolarity was increased in the plants treated with salt stress, indicating the occurrence of osmotic adjustment in response to the stress. Under 150 mM NaCl, both genotypes showed 40 % increase in xylem sap osmolarity, in comparison to the control. Under 300 mM NaCl, 62 and 65 % increases were observed in Yerong and XZ16, respectively (Fig. 4a).
The amount of electrolyte leakage from the roots of Yerong and XZ16 subjected to salinity stress is shown in Fig. 4b. Salinity treatment increased the amount of electrolyte leakage in the two genotypes, with Yerong showing more increase than XZ16.
Vacuolar H+-ATPase and H+-PPase activity
The two barley genotypes showed significant (P < 0.01) increase of vacuolar H+-ATPase activity when plants were treated by salt stress. However, the vacuolar H+-PPase activity in Yerong was reduced under salt stress. Under 150 mM NaCl, vacuolar H+-ATPase activity of Yerong and XZ16 in roots showed 55 and 63 % increase, respectively, compared with the control. Under 300 mM NaCl, Yerong and XZ16 showed an increase of 70 and 79 %, respectively (Fig. 5a). For vacuolar H+-ATPase activity in shoots, under 150 and 300 mM NaCl, Yerong showed 46 and 50 % increase relative to the control, while XZ16 showed 47 and 56 % increase, respectively (Fig. 5b). In contrast, vacuolar H+-PPase activities in roots and shoots were significantly reduced under 150 mM NaCl for both genotypes relative to the control. Interestingly, increases of 31 % in roots and 26 % in shoots for H+_PPase activity was detected in XZ16 plants exposed to 300 mM salinity, while such an increase was not detected in Yerong (Fig. 5c, d).
Discussion
Up to date, a lot of wild barley accessions from Middle East have been used in modern barley breeding (Ellis et al. 2000), while Tibetan wild barley has been less explored (Qiu et al. 2011). Recent researches have shown that Tibetan wild barley has a wide genetic diversity (Qiu et al. 2011; Wu et al. 2011). This study demonstrated that XZ16 was less affected under 300 mM NaCl in terms of all growth parameters in comparison to Yerong (Fig. 1a, b), supporting our previous findings that some Tibetan wild barley accessions had higher salt tolerance than cultivated barley (Qiu et al. 2011).
In general, high Na+ level inside cells inhibits K+ uptake and, as a result, leads to increase in Na+/K+ ratio, which is commonly regarded as an indicator of salt tolerance in plants (Munns 2005, Chinnusamy et al. 2005). In the current study, salt stress lowered K+ concentrations in both roots and shoots of XZ16 and Yerong. However, the reduced extent was much less in XZ16 than in Yerong (Table 1). Hence, ZX16 had a lower Na+/K+ ratio than Yerong. It was reported that high Na+ level or high Na+/K+ ratio could result in a disorder of many enzymatic processes in plant tissues (Tester and Davenport 2003; Razmjoo et al. 2008), and a suitable Na+/K+ ratio is also important for maintaining normal growth and metabolic activities (Shabala et al. 2003). Thus, higher Na+ and lower K+ levels in Yerong caused greater growth inhibition relative to XZ16.
Signaling to shoot via xylem sap is an important activity by which plants respond to abiotic stress (Fernandez et al. 2011). Salt-tolerant genotypes showed increase in osmolarity of the xylem sap (Shabala et al. 2003; Chen et al. 2005; Ayalew and Katsuhara 2009). It is worth noting that XZ16 showed more increase in xylem sap osmolarity in comparison with Yerong under salt stress. Obviously, it is not possible that more Na accumulation in the mesophyll cells of salt-sensitive genotypes is attributed to the difference in leaf osmolarity. Therefore, it is necessary for us to determine the elemental composition of other inorganic ions and organic osmolytes which potentially contributed to osmotic adjustment.
It was found that salt stress reduced photosynthetic pigment content (Khan 2003). In this study, we found that low NaCl level (150 mM) increased, while high NaCl level (300 mM) reduced chlorophyll a, b and total contents in both genotypes. However, XZ16 was less affected by salt stress than Yerong. Lower chlorophyll content in Yerong seedlings might lead to smaller photosynthetic rate, and, as a result, smaller plant biomass (Fig. 1). By contrast, XZ16 maintained higher chlorophyll content under salt stress, which might be one of the important factors contributing to its less inhibition in plant growth under salt stress. Electrolyte leakage could be used to measure the intactness of cell membranes (Verslues et al. 2006). In this study, Yerong showed more electrolyte leakage than XZ16 under salt stress, again indicating its more sensitivity to salt stress.
H+-ATPase and H+-PPase share the task of vacuolar acidification, which helps in the regulation of pH in cellular compartments during salt stress (Walter et al. 2009). The compartmentation of Na+ into the vacuoles is mediated by an Na+/H+ antiporter, which is dependent on the electrochemical gradient of H+ produced by H+-ATPase and H+-PPase. A major concern of the current study was to evaluate protein pump activity in plant cells for coping with high salt stress in wild and cultivated barleys. The results showed that XZ16 had significantly higher activities of vacuolar H+-ATPase and H+-PPase relative to Yerong, which may be attributed to proper sequestration of sodium ion inside the vacuole for ZX16 (Queirós et al. 2009). Thus, the regulation of H+ATPase and H+-PPase activities might be an important cellular mechanism for salt tolerance in the wild barley.
In summary, wild barley genotype XZ16 displayed higher tolerance to salt stress with less cell damage based on physiological and biochemical characters. This genotype showed a lower Na+/K+ ratio in roots and shoots, lower electrolyte leakage, higher xylem sap osmolarity, and vacuolar H+-ATPase and H+-PPase activities in comparison with Yerong, a salt-sensitive cultivar. The results presented here have shed new light on the significance of Tibetan wild barley in improving salt stress tolerance of cultivated barley.
Authors contribution statement
JZ and ZG: experiment design and paper writing; JZ and HN: performance of the experiment and analysis; WD, HY and SI: data analysis; WF: experiment arrangement
References
Ayalew L, Katsuhara M (2009) Insights into the salt tolerance mechanism in barley (Hordeum vulgare) from comparisons of cultivars that differ in salt sensitivity. J Plant Res 123:105–118
Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta 1465:140–151
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgam quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S (2005) Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ 28:1230–1246
Chinnusamy V, Jagendore A, Zhu JK (2005) Understanding and improving salt tolerance in plants. Crop Sci 45:437–448
Dai HX, Shan WN, Zhao J, Zhang GP, Li C, Wu FB (2011) Difference in response to aluminum stress among Tibetan wild barley genotypes. J Plant Nutr Soil Sci 174:952–960
Dai F, Nevo E, Wu DZ, Comadran J, Zhou MX, Qiu L, Cheng ZH, Belles A, Chen GX, Zhang GP (2012) Tibet is one of the centers of domestication of cultivated barley. Proc Natl Acad Sci USA 109:16969–16973
Dietz KJ, Tavakoli N, Kluge C, Mimura T, Sharma SS, Harris GC (2001) Significance of the V-type ATPase for the adaptation to stressful growth conditions and its regulation on the molecular and biochemical level. J Exp Bot 52:1969–1980
Ellis R, Foster B, Handley L, Gordon D, Russell J, Powell W (2000) Wild barley: a source of genes for crop improvement in the 21st century. J Exp Bot 51:9–17
Fernandez GN, Hernandez M, Casado-Vela J, Bru R, Elortza F, Hedden P, Olmos E (2011) Changes to the proteome and targeted metabolites of xylem sap in Brassica oleracea in response to salt stress. Plant Cell Environ 34:821–836
Flower TJ (2004) Improving crop salt tolerance. J Exp Bot 55:307–319
Khan NA (2003) NaCl_inhibited chlorophyll synthesis and associated changes in ethylene evolution and antioxidative enzyme activities in wheat. Biol Planta 47:43–74
Kluge C (2003) New insight into the structure and regulation of the plant vacuolar H+-ATPase. J Bioenerg Biomembr 35:377–388
Li JY, Jiang GQ, Huang P, Ma J, Zhang FC (2007) Over expression of the Na+/H+ antiporter gene from Suaeda salsa confers cold and salt tolerance to transgenic Arabidopsis thaliana. PCTOC 90:41–48
Maeshima M (2000) Vacuolar H+ pyrophosphatase. Biochim Biophys Acta 1465:37–51
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663
Porra RJ, Thompson WA, Kriedmann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochem Biophys Acta 975:384–394
Qiu L, Wu DZ, Shafaqat A, Cai SG, Dai F, Jin XL, Wu FB, Zhang GP (2011) Evaluation of salinity tolerance and analysis of allelic function of HvHKT1 and HvHKT2 in Tibetan wild barley. Theor Appl Genet 122:695–703
Queirós F, Fontes N, Silva P, Almeida D, Maeshima M, Gerós H, Fidalgo F (2009) Activity of tonoplast proton pumps and Na+/H+ exchange in potato cell cultures is modulated by salt. J Exp Bot 60:1363–1374
Radoglou K, Cabral R, Repo T, Hasanagas N, Sutinen ML, Waisel Y (2007) Appraisal of root leakage as a method for estimation of root viability. Plant Biosyst 141:443–459
Razmjoo K, Heydarizadeh P, Sabzalian MR (2008) Effect of salinity and drought stresses on growth parameters and essential oil content of Matricaria chamomile. Int J Agric Biol 10:451–454
Shabala S, Shabala L, Volkenburgh E (2003) Effect of calcium on root development and root ion fluxes in salinized barley seedlings. Funct Plant Biol 30:507–514
Shavrukov Y, Gupta NK, Miyazaki J, Baho MN, Chalmers KJ, Tester M, Langridge P, Collins NC (2010) HvNax3—a locus controlling shoot sodium exclusion derived from wild barley (Hordeum vulgare ssp. spontaneum). Funct Integr Genomic 10:277–291
Steppuhn H, Genuchten MT, Grieve CM (2005) Root-zone salinity selecting a product-yield index and response functions for crop tolerance. Crop Sci 45:209–220
Steudle E, Smith JAC, LuÈttge U (1980) Water relations parameters of individual mesophyll cells of the CAM plant KalanchoeÈ daigremontiana. Plant Physiol 66:1155–1163
Sze H, Schumacher K, Müller ML, Padmanaban S, Taiz L (2002) A simple nomenclature for a complex proton pump: VHA genes encode the vacuolar H+-ATPase. Trends Plant Sci 7:157–161
Tammam AA, Alhamd MFA, Hemeda MM (2008) Study of salt tolerance in wheat Triticum aestivum L. cultivar Banysoif. Aust J Crop Sci 3:115–125
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–527
Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45:523–539
Walter V, Spelt CD, Vermeer J, Reale L, Koes FR, Quattrocchio F (2009) An H+ P-ATPase on the tonoplast determines vacuolar pH and flower colour. Nat Cell Biol 10:1456–1462
Wu DZ, Qiu L, Xu LL, Ye LZ, Chen MX, Sun DF, Chen ZH, Zhang HT, Jin XL, Dai F, Zhang GP (2011) Genetic variation of HvCBF genes and their association with salinity tolerance in Tibetan annual wild barley. PLoS One 6:e22938
Yoshiyuki N, Kasamo K, Shimosato N, Makoto S, Eiji O (1992) Stimulation of the extrusion of protons and H+-ATPase activities with the decline in pyrophosphatase activity of the tonoplast in intact mung bean roots under high-NaCl stress and its relation to external levels of Ca2 + ions. Plant Cell Physiol 33:139–149
Zhang WH, Yu BJ, Chen Q, Liu YL (2004) Tonoplast H+-ATPase activity in barley roots is regulated by ATP and pyrophosphate contents under NaCl stress. J Plant Physiol Mol Biol 30:45–52
Zhao J, Sun HY, Dai HX, Zhang GP, Wu FB (2010) Difference in response to drought stress among Tibet wild barley genotypes. Euphytica 172:395–403
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Acknowledgments
This research was supported by the Natural Science Foundation of China (31201166 and 31330055), China Agriculture Research System (CARS-05) and Jiangsu Collaborative Innovation Center for Modern Crop Production (JCIC-MCP).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Filek.
Rights and permissions
About this article
Cite this article
Jabeen, Z., Hussain, N., Wu, D. et al. Difference in physiological and biochemical responses to salt stress between Tibetan wild and cultivated barleys. Acta Physiol Plant 37, 180 (2015). https://doi.org/10.1007/s11738-015-1920-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1920-x