Abstract
High temperature reduces the growth and yield of most of the agriculturally important crops. Elicitors have been reported to modulate the thermotolerance of crops under abiotic stresses. Here, we studied the effect of salicylic acid (SA) on thermotolerance level of C306 (thermotolerant) and PBW343 (thermosusceptible) wheat cultivars grown under heat stress. Pilot experiment confirmed spraying of 100 mM SA prior to heat stress (38 °C, 2 h) as the most effective treatment. Numerous protein spots were observed in C306 under SA + HS compared to PBW343 during post-anthesis stage by 2-DE. Differentially expressed proteins were identified as signaling molecule, heat-responsive transcription factors (HSFs) and heat shock proteins (HSPs) using MALDI-TOF–TOF/MS analysis. Abundance of transcripts of HSFs, HSPs, CDPK, SOD, RCA, etc. was observed in C306 in response to SA + HS, as compared to PBW343. C306 showed better accumulation of transcript of SAGs, osmolyte and total antioxidant capacity under SA treatment compared to PBW343. SA was observed to reduce the detrimental effect of HS on soluble starch synthase (SSS) activity as well as synthesis of starch granules in both the cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abiotic stresses adversely affect the growth and development of plants under the field condition. Plants have developed intricate mechanism to deal with such situations for their survival (Wang et al. 2004). Out of all abiotic stresses, heat stress has been predicted to be most damaging for agriculturally important cereal crops (Bita and Gerats 2013). High temperature interferes with the pollen growth, viability and fertilization process causes pseudo-seed setting problem in wheat (Kumar et al. 2013). Heat stress causes denaturation of key enzymes involved in various metabolic pathways, signaling and defense mechanism (Farooq et al. 2011). Immediately after exposure to high temperatures and perception of signals, changes occur at the molecular level altering the expression of genes and accumulation of transcripts, thereby leading to the synthesis of stress-associated proteins (SAPs) as a stress tolerance strategy (Iba 2002; Clarke et al. 2004). Variations in the expression of heat stress-associated proteins have been well documented in different cultivars of wheat (Kumar et al. 2012). It is the abundance of these SAPs which play an important role in modulating the defense mechanism of wheat under heat stress. The major problem in deciphering the tolerance mechanism in crops like wheat is the partially sequenced genome and many more missing links which need to be characterized.
In this regard, expression of SAPs like heat-responsive transcription factors (TFs), heat shock proteins (HSPs), antioxidant enzymes, signaling molecules, etc. is known to be an important adaptive strategy (Kumar et al. 2013). An abrupt increase in the expression of signaling molecules, HSPs (low and high molecular weight), antioxidant enzymes and accumulation of osmolytes and metabolites has been reported in response to HS in wheat (Kumar et al. 2013). Exogenous application of phytohormones has been reported to modulate the expression of SAPs in crops for enhancing the tolerance levels against stresses.
Salicylic acid (SA) is an endogenous growth regulator of phenolic nature, which participates in the regulation of physiological processes in plants. SA is known as signal molecule involved in the induction of defense mechanism since long back (Halim et al. 2006). In higher plants, SA is synthesized from the shikimate-phenylpropanoid pathway (Lee et al. 1995). There are experimental data indicating participation of SA in signal regulation of gene expression in the course of leaf senescence in Arabidopsis (Morris et al. 2000). SA has been reported to protect the plants from the heat stress, but the mechanism behind this is not known (Wang et al. 2014). SA has been reported to play a key role in plant growth and development and in plant responses to abiotic stresses. It modulates secondary metabolites pathway in plant under abiotic stresses (Morris et al. 2000). Khan et al. (2013) reported that SA interacts with proline metabolism and ethylene formation to alleviate the adverse effects of heat stress on photosynthesis in wheat. Similarly, exogenous application of SA through rooting medium has been reported to modulate the growth and photosynthetic rate of wheat under salt stress (Arfan et al. 2007). Wang et al. (2014) reported increase in the activities of different antioxidant enzymes in wheat treated with foliar SA. Similarly, Janda et al. (2014) reported that exogenous SA alleviates the damaging effect of various abiotic stresses and modulates the photosynthetic process of the plant. Few literatures have also established the role of exogenous SA in enhancing the resistance of wheat to biotic stresses (Underwood et al. 2014).
The present investigation has been designed to assess the effect of SA applied exogenously on thermotolerance capacity of wheat (T. aestivum cv C306 and PBW343) exposed to terminal heat stress during grain-filling stage.
Materials and methods
Pilot experiment
Two popular wheat cultivars—C306 (thermotolerant) and PBW343 (thermosusceptible)—were selected from the mini-core subset of wheat developed after screening more than 1000 accessions for thermotolerance using Phenomics facility at Indian Agricultural Research Institute (IARI), New Delhi, India. The seeds of both the cultivars were procured from Division of Genetics, IARI. Pre-treated seeds (Bavistin @ 0.25 %) were sown inside BOD under regulated conditions (optimum temperature regime of 26/22 °C, humidity of 60 %, photoperiod of 16 h and light intensity of 350 µmol m−2 s−1). Thirty Petri plates (in two groups of 15 each) were used for germination of C306 and PBW343 cultivars of wheat in triplicates. The 10 days old seedlings were exposed to T 0—22 °C (control), T 1—heat stress (HS—38 °C, 2 h), T 2—SA (50 mM) + HS, T 3—SA (100 mM) + HS and T4—SA (500 mM) + HS treatments. Leafs were collected in triplicate after 24 h and were used for the estimation of fresh weight, total protein, total RNA and total antioxidant capacity (TAC). The treatment of 100 mM SA + HS showed better performance with respect to the above-selected parameters.
Sowing and stress treatments
Pre-treated seeds (Bavistin @ 0.25 %) were sown in pots (10″ × 10″) filled with vermiculite and sand-mix in equal quantity. Twenty-four pots in group of two (12 each) were sown with equal seeds of C-306 and PBW343 cultivars of wheat. The pots were kept inside regulated chamber (optimum temperature regime of 26/22 °C, humidity of 60 %, photoperiod of 16 h and light intensity of 350 µmol m−2 s−1) at National Phytotron Facility, IARI. Proper irrigation was provided at regular interval. Plants were randomly divided into four groups (3 pots each) at seedling stage (10 days old) with 10 seedlings in each pot. Both the cultivars were exposed to T 1—heat stress (HS—38 °C for 2 h), T 2—SA (100 mM), and T 3—SA (100 mM) + HS at pre-anthesis stage. A plant without treatment was used as control (22 °C). Foliar spray of SA was provided thrice inside closed chamber at an interval of 4 h. Heat stress treatment was imposed as mentioned by Kumar et al. (2013). Leaf samples were harvested at 24 h (anthesis; Feekes scale—10.51), 10 (milky ripe; Feekes scale—11.1), 20 (mealy ripe; Feekes scale—11.2) and 30 (kernel hardening; Feekes scale—11.3) days after the treatments (Large 1954). The collected samples (in triplicates) were immediately frozen in liquid nitrogen for subsequent analysis.
Differential protein profiling using two dimensional electrophoresis (2-DE)
Briefly, frozen sample (0.5 g) was finely ground in liquid nitrogen and homogenized on ice (2 min) in an extraction solution (5.0 ml) consisting of equal volumes of PCI (49 % phenol, 49 % chloroform, 2 % isoamyl alcohol) and NTES (10 mM NaCl, 1 mM EDTA, 1 % SDS, 10 mM Tris–HCl, pH 8.5). Other steps were followed as per the protocol previously mentioned by Kumar et al. (2014). The protein concentration was measured by Bradford method (Bradford 1976).
Isoelectric focusing and one dimensional electrophoresis (1-DE)
Isoelectric focusing (IEF) was performed using the IEF100 electrophoresis system (Hoefer, USA) and 18 cm SERVA immobilized pH gradient (IPG) blue Strip of 3–10 linear pH gradients (SERVA, Germany). The protocol for the IEF and 1D SDS-PAGE was followed as mentioned by Kumar et al. (2014). The stained gels were scanned using HP Scanjet G4010 (Hewlett-Packard). The image analysis of the gels was carried out using Image Master 2D Platinum (GE healthcare) version 7.0.6.
MALDI-TOF–TOF analysis for the identification of protein spots
The selected spots were manually picked up one by one using 200 µl tip by matching the selected match/spot ID to the actual gel. The silver stained spots were processed for in gel digestion by trypsin and the samples were analyzed on a MALDI-TOF–TOF mass spectrometer (Ultraflex III, Bruker Daltonics) following the protocol as mentioned by Kumar et al. (2014). The Flex analysis 3.3 software (Bruker Daltonics) was used to extract and process the peptide mass peaks from the spectrum. The post-analysis processed spectrum was used for searching protein against the non-redundant NCBI (NCBInr) database using MASCOT 2.2 (Matrix Science) search engine (http://www.matrixscience.com/).
Transcript profiling of stress-associated genes (SAGs) by qRT-PCR
Total RNA was extracted from the collected samples using Trizol method (Invitrogen, UK) and quantified by QuantIT RNA quantification Qubit (Invitrogen, UK). First-strand cDNA synthesis was performed using oligo dT primers and the Superscript II reverse transcriptase (Invitrogen, UK), according to the manufacturer’s instructions. Primers for RT-qPCRs were designed using Prime 3 primer designing software (Premier Biosoft, USA) (Table 1). Quantitative real-time PCR was performed as per the protocol mentioned by Kumar et al. (2014) using two biological and three technical replicates. The expression levels of wheat actin gene (accession no. AF282624) were used as internal standards for the normalization of data. The comparative Ct (2−ΔΔCt) method was used to calculate relative fold expression (Pfaffl et al. 2002).
Estimation of osmolyte accumulation and total antioxidant capacity
Proline content was determined according to method of Bates (Bates et al. 1973) with modification. The chromophore containing toluene was separated and absorbance is then read at 520 nm in spectrophotometer against toluene blank. The FRAP (Ferric reducing antioxidant power assay) procedure described by Benzie and Strain (Benzie and Strain 1999) was followed for the total antioxidant activity assay of collected samples. The values were expressed as the concentration of antioxidants having a ferric reducing ability equivalent to that of 1 mmol L−1 FeSO4.
Scanning electron microscopy (SEM) of starch granule
For SEM, mature seeds (collected at same time based on Feekes scale) were cut into small pieces of less than 1 mm and the dissected tissues were transferred to 0.1 M phosphate buffer (pH 7.2). The primary and secondary fixations of the samples were carried out as per the protocol mentioned by Kumar et al. (2013). The dried samples were mounted on to suitable stubs with appropriate tape or glue. Sputter was coated with 24 ηm palladium for high vacuum imaging. Images were taken in Carl Zeiss scanning electron microscopy at 20 kV.
Soluble starch synthase (SSS) activity assay
0.5 g of sample was homogenized with a pestle in a pre-cooled mortar containing 5 ml frozen extraction medium [100 mmol L−1 HEPES–NaOH (4-2-hydroxyethyl-1-piperazine ethane sulfonic acid—sodium hydroxide)], pH 7.5, 2 mmol L−1 EDTA, 50 mmol L−1 2-mercaptoethanol, 12.5 % (v/v) glycerol). The supernatant obtained after centrifugation (10,000g, 25 min, 4 °C) was directly used for the activity assay of SSS as per the protocol mentioned by Kumar et al. (2013). The activity was measured as the increase in absorbance at 340 nm after the addition of 1.4 IU hexokinase and 0.35 IU glucose-6-phosphate dehydrogenase. The standard curve of sodium pyruvate was used to calculate the activity of SSS in nmole min−1 mg−1 protein.
Estimation of starch content
Oven-dried mature endospermic tissue (100 mg) was extracted with 70 % hot ethanol, centrifuged at 12,000 rpm for 15 min and the residue was dried in oven. Residue was re-suspended in 5.0 ml water and 7.5 ml of 52 % perchloric acid, centrifuged and the supernatant was collected in fresh centrifuge tube. The sample was re-extracted using 5 ml perchloric acid and the supernatant was pooled and volume was made to 100 ml with water. Suitable aliquot was used for the glucose estimation using anthrone reagent. The intensity of the color formed at 620 nm was used for the glucose estimation using standard curve and the value observed was multiplied by 0.9 to get the starch yield.
Data
The experiment was conducted in a completely randomized design (CRD). Data were analyzed using one-way analysis of variance (one-way ANOVA). The standard errors were given in histograms.
Results
Pilot experiment for standardizing the concentration of salicylic acid
Pilot experiment was laid down to standardize the concentration of SA to be used for further investigation. We observed significant increase in the fresh weight with increase in the concentration of SA (50 to 100 mM); maximum was observed in response to 100 mM SA (Fig. 1a). A decrease in the fresh weight was observed in response to SA treatment of 500 mM. Under HS treatment prior to SA application, maximum fresh weight was observed in response to 100 mM SA (T6). Similar pattern of increase in fresh weight in response to SA was observed in case of PBW343 cultivar of wheat (Fig. 1b). Similarly, we observed increase in the total soluble protein content in both the cultivars under HS; maximum was observed in response to 100 mM SA (T 3) in both the cultivars (Fig. 1c, d). Under HS, exogenous application of different concentration of SA does not show any significant differences in the total soluble protein in C306, whereas PBW343 showed maximum soluble protein in response to 100 mM SA prior to HS (Fig. 1d). A significant increase in the total RNA content was observed in response to SA and HS treatment in both the cultivars; abundance was more in C306 compared to PBW343. Total RNA content was observed maximum under 100 mM of SA in C306 and 50 mM SA in PBW343 (Fig. 1e, f). We observed significant decrease in the RNA content in both the cultivars with increase in the SA concentration from 100 to 500 mM. SA and HS showed significant increase in the total antioxidant capacity in C306; maximum was observed in response to SA (100 mM) + HS treatment (Fig. 1g). Similarly, PBW343 cultivar showed marked change in the total antioxidant capacity under SA and HS treatment (Fig. 1h). Maximum total antioxidant capacity in PBW343 was observed in response to SA (100 mM) + HS (38 °C, 2 h). Parameters (physiological, biochemical and molecular) studied in the present investigation showed better results in C306 (thermotolerant), as compared to PBW343 (thermosusceptible) under different treatments of SA and HS. To conclude, 100 mM SA + HS were observed to be the treatment of choice for further investigation.
Pilot experiment showing the effect of exogenous salicylic acid (SA) and heat stress (HS) on physiological, biochemical and molecular parameters of C-306 (thermotolerant) and PBW343 (thermosusceptible) cultivars of wheat (10 days old seedling); a, b fresh weight per seedling, c, d total protein content g−1 fresh weight tissue, e, f total RNA content, and g, h total antioxidant capacity (FRAP); seedlings were exposed to T 0—22 °C, T 1—HS of 38 °C, 2 h, T 2 —SA (50 mM), T 3 —SA (100 mM), T4 —SA (500 mM), T5 —SA (50 mM) + HS (38 °C, 2 h), T6 —SA (100 mM) + HS (38 °C, 2 h), T7 —SA (500 mM) + HS (38 °C, 2 h); ANOVA significance levels: *, P < 0.05; **, P < 0.001; ***, vertical bars indicate s.e. (n = 3)
Differential protein profiling and protein identification
We observed the expression of numerous heat-responsive proteins in HS-treated (38 °C, 2 h) gels of C306 and PBW343 compared to control (22 °C) (Fig. 2b, d). The HS-responsive proteins were observed more in C306 compared to PBW343 on characterizing the gels using software (Fig. 2). Gel characterization using Image Master 2D Platinum (IMP7) software (GE Healthcare) showed the presence of unique proteins in both the control and HS-treated gels of C306 and PBW343 cultivars of wheat.
Differential protein profiling of C306 (thermotolerant) and PBW343 (thermosusceptible) cultivars of wheat using 2-dimensional polyacrylamide gel electrophoresis (2-DE), a C306-control, b C306-treated (SA100mM + HS), c PBW343-control, d PBW343-treated (SA100mM + HS); C—22 °C, HS—38 °C, 2 h; IPG strip (Serva, Germany) pH 3.0–10 was used for the isoelectric focusing; 12 % gel was used for the second dimension (SDS-PAGE)
Six differentially expressed protein spots were randomly selected from both the cultivars and were subjected to MALDI-TOF–TOF/MS for their identification. The mass spectrum of the selected protein spots has been presented in Online Resource 1 (ESM_1). Based on the Mascot search analysis (Matrix Science), the protein spots were identified as small HSP (HSP17), oxygen evolving enhancer protein, ATP synthase small subunit, rubisco activase (RCA), calcium dependent protein kinase (CDPK), and superoxide dismutase (SOD). The selected protein spots have already been functionally characterized in different plant species, for example, sHSP plays dual role of catalytic chaperone as well as protects the nascent proteins from denaturation under stress conditions (Kumar et al. 2014). Oxygen evolving enhancer protein regulates the photosystem II stabilization and in turn modulates the photosynthetic activity (Murakami et al. 2002). ATP synthase provides the energy to the cell through the synthesis of ATP. RCA acts as chaperone converting the inactive form of Rubisco into active form and augments the carbon assimilatory process. CDPK is involved in signaling process and SOD is an important antioxidant defense system of the cells under different stress conditions. We also predicted some of the other SAPs like TFs, signaling molecule and pathways-related enzymes based on the homology search.
Transcript profiling of heat-responsive TFs under SA and HS
Transcript profiling of heat shock transcription factor 4 (HSF4), heat shock transcription factor 7 (HSF7) and dehydration responsive element binding (DREB) was carried out in C306 and PBW343 cultivars exposed to SA and HS treatments (Fig. 3). The HSF4 expression was observed maximum in response to SA + HS, though the abundance of transcript was more in C306 (thermotolerant) compared to PBW343 (thermosusceptible) with the increase in number of days after treatment (Fig. 3a, b). Similar pattern of expression of HSF7 and DREB was observed in response to SA + HS. The transcript of HSF7 showed decrease in the accumulation pattern measured on 10th, 20th and 30th days after treatment (Fig. 3c, d). DREB showed down-regulation in PBW343 and up-regulation in C306 in response to SA treatment with number of days after treatment (Fig. 3e, f). Relative expression of HSF7 was high compared to HSF4 and DREB in both the cultivars under SA + HS treatments.
Transcript profiling of heat-responsive transcription factor (TFs) in C306 (thermotolerant) and PBW343 (thermosusceptible) cultivars of wheat in response to salicylic acid and heat stress treatment; HSF4, HSF7 and DREB genes were used for the expression profiling; relative expression was estimated on 1st, 10th, 20th and 30th days after treatment during anthesis stage; T 0 —22 °C, T 1 —HS of 38 °C, 2 h, T 2 —SA (100 mM), T 3 —SA (100 mM) + HS (38 °C, 2 h); β-actin gene (Accession No. AF282624) was used as endogenous control for normalizing the data, CFX96 platform (Bio Rad) was used for the qRT-PCR; all data are presented as mean ± SE of three replicates; different letters above each bar indicate a significant difference between treatments (P < 0.05, one-way ANOVA)
Expression profiling of stress-associated genes (SAGs) under SA and HS
Expression studies of SAGs (CDPK, RCA-A, HSP17 and SOD) were carried out in C306 and PBW343 cultivars of wheat using qRT-PCR (Fig. 4). The expression of CDPK was very high on 1st day after SA + HS treatment in C306 compared to PBW343 (Fig. 4a, b). Similar was the case with respect to other treatments like HS and SA on 1st day. Expression analysis on 10th, 20th and 30th days after treatments showed non-significant changes in the expression in both the cultivars. SOD showed high expression in C306 compared to PBW343 in response to SA + HS and further decrease in the expression was observed with number of days after treatments (Fig. 4c, d). HSP17 (sHSP) showed maximum transcript accumulation compared to other SAGs identified in the present investigation (Fig. 4e, f); relative expression was 30 (C306) and 25 (PBW343) fold in response to SA + HS on 1st days after treatment. In case of RCA-A, maximum expression was observed in response to SA + HS treatment in both the cultivars on 1st day (Fig. 4g, h). Increases in the expression of RCA-A were observed with number of days after treatment in C306 compared to down-regulation in case of PBW343.
Transcript profiling of stress-associated genes (SAGs) in C306 (thermotolerant) and PBW343 (thermosusceptible) cultivars of wheat in response to salicylic acid and heat stress treatment; CDPK, SOD, HSP17 and RCA-A genes were used for the expression profiling; relative fold expression was estimated on 1st, 10th, 20th and 30th days after treatment during anthesis stage; T 0 —22 °C, T 1 —HS of 38 °C, 2 h, T 2 —SA (100 mM), T 3 —SA (100 mM) + HS (38 °C, 2 h); β-actin gene (Accession No. AF282624) was used as endogenous control for normalizing the data; CDPK calcium dependent protein kinase, HSP heat shock protein, RCA rubisco activase, SOD superoxide dismutase; CFX96 platform (Bio Rad) was used for the qRT-PCR; all data are presented as mean ± SE of three replicates; different letters above each bar indicate a significant difference between treatments (P < 0.05, one-way ANOVA)
Proline accumulation and total antioxidant capacity in wheat under SA and HS
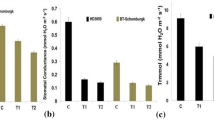
Increase in the proline accumulation under HS was observed more in C306, as compared to PBW343 (Fig. 5a). Under SA treatment, the percentage increase in proline was high in C306. The maximum proline accumulation was observed in response to SA + HS in both the cultivars; C306 showed maximum accumulation compared to PBW343. Under individual treatment of HS and SA, the TAC observed in C306 was high compared to PBW343 (Fig. 5b). Maximum TAC was observed in C306 in response to SA + HS compared to PBW343, which is in conformity with our earlier results. SA + HS induce the accumulation of proline as well as TAC, more in thermotolerant cultivar compared to thermosusceptible.
Estimation of biochemical parameters associated with thermotolerance in C306 (tolerant) and PBW343 (susceptible) cultivars of wheat in response to salicylic acid and heat stress treatment; a proline accumulation (µmole/g fresh weight), b total antioxidant capacity (µmole Fe/100 µl), c soluble starch synthase activity assay (nmole/min/mg protein), d starch content (% dry weight basis); estimation was carried out during grain-filling stage, samples were collected based on Feekes scale; C—22 °C, HS—38 °C, 2 h, SA—100 mM; different letters above each bar indicate a significant difference between treatments (one-way ANOVA significance levels: *, P < 0.05;**, P < 0.001;***); vertical bars indicate s.e. (n = 3)
Variations in the soluble starch synthase (SSS) activity and starch content under SA and HS
We observed decrease in the soluble starch synthase activities in C306 and PBW343 cultivars in response to HS (38 °C, 2 h). Under HS, C306 showed ~45 % decrease in the SSS activity compared to control (22 °C) (Fig. 5c). We observed ~ 12.3 % increase in the activity of SSS in C306 treated with 100 mM SA compared to the control (22 °C). Similarly, C306 treated with SA + HS showed ~23 % decrease in the activity of SSS compared to control; percent decrease in the activity was less compared to HS treatment (Fig. 5c). In case of PBW343, we observed ~51 % decrease in the activity of SSS under HS compared to control. Similarly, ~7.6 % increase (under 100 mM SA treatment) and ~22 % decrease (under SA + HS) in the SSS activity were observed in PBW343 compared to control. The variations in the SSS activity in response to SA and HS treatments in both the cultivars were observed highly significant. The SA treatment prior to HS was observed to mitigate the effect of HS on SSS activity; the specific activity was observed high in C306 compared to PBW343 under different treatments (Fig. 5c).
The photosynthates are accumulated inside the endospermic tissue in the form of complex starch which is synthesized by the SSS along with other enzymes of starch biosynthesis pathway. We observed significant variations in the starch content of C306 and PBW343 under SA and HS treatment. Under HS, we observed ~26 % (C306) and ~35 % (PBW343) decrease in the starch content compared to control (Fig. 5d). Similarly, plants treated with 100 mM SA showed ~7 % (C306) and ~1.8 % (PBW343) increase in the starch content compared to control. When plants were treated with 100 mM SA prior to HS, we observed ~4.9 % (C306) and ~15.8 % (PBW343) decrease in the starch content compared to control; percent decrease was less compared to HS treatment in both the cultivars (Fig. 5d).
Scanning electron microscopy of starch granule
The thermotolerant (C306) cultivar showed bold, globular, structured and intact granules under control condition compared to defragmented and small granules with pleated structure in response to HS (Fig. 6a, b). Endospermic tissue of C306 exposed to SA prior to HS showed intact and globular granules with large size; the effect of HS was observed minimal (Fig. 6c). Similarly, PBW343 (thermosusceptible) cultivar showed intact granule under control condition, whereas HS causes numerous cracks on the granule structure with granules stacked upon each other (Fig. 6d, e). PBW343 sample treated with SA prior to HS showed less damaging effect of HS compared to heat shock-treated sample; the extent of damage in response to HS was more pronounced in PBW343 (Fig. 6e, f). The tolerance initiated by the SA against the HS was quite visible in the thermotolerant cultivar compared to thermosusceptible.
Scanning electron microscopy (SEM) of starch granule in C306 (thermotolerant) and PBW343 (thermosusceptible) cultivars of wheat under salicylic acid and heat stress treatments; starch granule synthesized in C306 under a control, b heat stress (HS), c SA + HS; starch granule synthesized in PBW343 under d control, e heat stress (HS), f SA + HS; C—22 °C, HS—38 °C, 2 h, SA—100 mM; samples for SEM were collected based on Feekes scale and dissection of endosperm was carried out using microtome; scanning electron microscopy was performed on Carl Zeiss instrument
Discussion
Heat stress has severe effect on the photosynthetic system and activities of various metabolic pathways associated enzymes. Exogenous application of elicitors has been reported to enhance the tolerance mechanism of wheat under different abiotic stresses (Kumar et al. 2012, Clarke et al. 2004). Many researchers have reported the potential role of SA against biotic and abiotic stresses in different crops; though controversial reports contradicting the positive effect of SA are also there. Few reports says that SA has negative effect on the germination and seedling growth of wheat (Yang et al. 2002), whereas other observed the positive effect of SA on physiological and biochemical parameters (Singh et al. 2010). Our experiment showed improvement in the physiological and biochemical parameters in response to 100 mM SA + HS (Fig. 1). The concentration of SA has been observed to be crucial in the regulation of stress responses (Horvath et al. 2002). Acetyl salicylic acid significantly attenuated the deleterious impacts of heat stress to different tissues of plants as observed by Khan et al. (2014). Treatment with 0.5 mM salicylic acid (SA) significantly alleviated growth inhibition induced by drought in wheat seedlings (Kang et al. 2013).
Numerous SA-responsive proteins were observed on 2-DE gel; C306 showed more number of differentially expressed proteins compared to PBW343 (Fig. 2). Wu et al. (2013) using proteomic tool identified 56 differential expressed protein spots in response to SA and ABA in maize and reported that they were mainly involved in photosynthesis and defense response which is in conformity with our observation. Similarly, Wang et al. (2014) reported that foliar supplementation of SA in wheat elevates the activities of antioxidant enzymes and modulates the tolerance level under heat and high light stress; findings are in concurrent with our observation. Abundance of SAGs transcript was observed on 1st day after treatment which subsequently decreases further except in case of DREB, HSP17 and RCA, where transcripts were observed till kernel hardening stage (Figs. 3, 4). The presence of sHSP and RCA during post-anthesis and grain-filling protects the photosynthetic system as well as enzymes of starch biosynthesis pathway for balancing the source to sink ratios under terminal HS. SA was shown to play a role in the induction of thermotolerance in Arabidopsis (Larkindale and Knight 2002). Addition of SA was observed to ameliorate the oxidative stress in barley and mustard-stressed plants (Fayez and Bazaid 2014; Nazar et al. 2015). Similarly, CDPK has been shown to be involved in abiotic stress responses and induced by SA (Leclercq et al. 2005) as observed in the present investigation (Fig. 3). Findings suggest that SA plays a regulatory role in calcium-mediated signal transduction pathways. Heat stress causes deactivation of Rubisco enzyme which in turn reduces the rate of photosynthesis (Salvucci and Crafts-Brandner 2004); RCA acts as catalytic chaperones and converts inactive form of Rubisco into active form under stress condition. Here, RCA expression under HS was observed maximum in SA-treated leaves than in the controls, indicating that SA may alleviate Rubisco inactivation under HS and augment the carbon assimilatory process of the plant. The finding is in conformity with the observation of Nazar et al. (2015), who reported improvement in the photosynthesis and growth parameters of mustard treated with SA under drought stress. Application of SA has been reported to modulate the photosynthetic performance and acclimation of plants to different stresses (Janda et al. 2014).
The exogenous application of SA (0.1 mM) has been reported to induce the synthesis of HSP70 and HSP17.6, which has protein refolding activity and increases the thermotolerance of pea (Pan et al. 2006). We also observed very high relative expression of HSP17 in the present investigation under SA and HS treatment which conforms to the findings of others (Fig. 4). We observed high osmolyte accumulation and TAC in response to SA + HS; the extent of increase was more in thermotolerant as compared to thermosusceptible (Fig. 5a, b). TAC of thermotolerant cultivar under HS has been reported to be very high compared to thermosusceptible and is used as a good indicator of thermotolerance capacity of any crop (Kumar et al. 2013). Exogenous application of SA or acetylsalicylate has been shown to enhance the thermotolerance in tobacco and Arabidopsis (Dat et al. 1998; Clarke et al. 2004). SA was observed to reduce the detrimental effect of HS on SSS activity as well as granule synthesis (Fig. 5c, f). Our earlier observation showed synthesis of defragmented starch granule and low SSS activity in wheat under HS (Kumar et al. 2013). C306, being thermotolerant cultivar, showed high relative expression of TFs and HSPs, accumulation of proline and high TAC compared to PBW343 (thermosusceptible) in response to 100 mM SA + HS treatment. SA has direct effect on the signaling molecules which in turn modulate the expression of stress-associated genes like TFs, HSPs, antioxidant enzymes, etc. and ultimately enhances the accumulation of osmolytes and overall thermotolerance capacity under HS.
Phytohormones have been identified as low-cost technology for mitigating the effect of abiotic stresses. Here, 100 mM SA prior to HS was observed most effective in mitigating the damaging effect of HS on various biochemical and molecular parameters associated with starch granule synthesis in wheat. Differential protein profiling showed appearance of many unique proteins in response to SA + HS predicted to be involved in the defense mechanism of the plant under HS; unique proteins were observed more in C306 compared to PBW343. Transcript profiling showed abundance of SAGs (CDPK, HSP17, RCA, etc.) in response to SA + HS treatment; expression was observed significantly high in thermotolerant compared to susceptible cultivars. Endospermic tissue of C306 exposed to SA prior to HS showed intact and globular granules with large size; the effect of HS was observed minimal on SSS activity and granule synthesis in thermotolerant compared to thermosusceptible cultivars. To conclude, 100 mM SA treatment has heat stress amelioration ability and can be used as substitute technology for the development of climate smart wheat crop.
Author contribution statement
RRK and SG declare equal participation in the execution of the experiment. RRK SKS PV lay down the pilot experiment and exposed the plants to different treatments. SG KS and CV executed the SSS activity assay, starch content and real-time PCR. RRK SKS PV executed osmolytes estimation and total antioxidant capacity. ND HP prepared the sample for the SEM. RRK HP CV RDR wrote the manuscript and edited for the language. All authors have read and approved the final version of the article.
References
Arfan M, Athar HR, Ashraf M (2007) Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress? J Plant Physiol 6(4):685–694
Bates LS, Waldren RP, Teari D (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Benzie IFF, Strain JJ (1999) Ferric reducing/antioxidant power assay: direct measure of the total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Meth Enzymol 299:15–27
Bita CE, Gerats T (2013) Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci 4:273
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Clarke SM, Mur LA, Wood JE, Scott IM (2004) Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J 38(3):432–447
Dat JF, Lopez-Delgado H, Foyer CH, Scott IM (1998) Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard plants. Plant Physiol 116:1351–1357
Farooq M, Bramley H, Palta JA, Siddique KHM (2011) Heat Stress in wheat during reproductive and grain-filling phases. Crit Rev Plant Sci 30:1–17
Fayez KA, Bazaid SA (2014) Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J Saudi Soc Agric Sci 13(1):45–55
Halim VA, Vess A, Scheel D, Roshal S (2006) The role of salicylic acid and jasmonic acid in pathogen defence. Plant Biol 8:307–313
Horvath E, Janda T, Szalai G, Paldi E (2002) In vitro salicylic inhibition of catalase activity in maize: difference between the isozymes and a possible role in the induction of chilling tolerance. Plant Sci 163:1129–1135
Iba K (2002) Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol 53:225–245
Janda T, Gondor OK, Yordanova R, Szalai G, Pal M (2014) Salicylic acid and photosynthesis: signaling and effects. Acta Physiol Plant 36(10):2537–2546
Kang GZ, Li GZ, Liu GQ, Xu W, Peng XQ, Wang CY, Zhu YJ, Guo TC (2013) Exogenous salicylic acid enhances wheat drought tolerance by influence on the expression of genes related to ascorbate-glutathione cycle. Biol Plant 57(4):718–724
Khan A, Waqas M, Hamayun M, Al-Harrasi A, Al-Rawahi A, Lee IJ (2013) Co-synergism of endophyte Penicillium resedanum LK6 with salicylic acid helped Capsicum annuum in biomass recovery and osmotic stress mitigation. BMC Microbiol 13:51
Khan AR, Cheng Z, Ghazanfar B, Khan MA, Yongxing Z (2014) Acetyl salicylic acid and 24-epibrassinolide enhance root activity and improve root morphological features in tomato plants under heat stress. Acta Agric Scand Sect B 64(4):304–311
Kumar RR, Goswami S, Sharma SK, Singh K, Gadpayle KA, Kumar N, Rai GK, Singh M, Rai RD (2012) Protection against heat stress in wheat involves change in cell membrane stability, antioxidant enzymes, osmolyte, H2O2 and transcript of heat shock protein. Int J Plant Physiol Biochem 4(4):83–91
Kumar RR, Sharma SK, Goswami S, Singh GP, Singh R, Singh K, Pathak H, Rai RD (2013) Characterization of differentially expressed stress-associated proteins in starch granule development under heat stress in wheat (Triticum aestivum L.). Indian J Biochem Biophys 50:126–138
Kumar RR, Singh GP, Goswami S, Pathak H, Rai RD (2014) Proteome analysis of wheat (Triticum aestivum) for the identification of differentially expressed heat-responsive proteins. Aust J Crop Sci 8(6):973–986
Large EC (1954) Growth stages in cereals illustration of the Feeke scale. Plant Pathol 3(4):128–129
Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128:682–695
Leclercq J, Ranty B, Sanchez-Ballesta MT, Li ZG, Jones B, Jauneau A, Pech JC, Latche A, Ranjeva R, Bouzayen M (2005) Molecular and biochemical characterization of LeCRK1, a ripening-associated tomato CDPK-related kinase. J Exp Bot 56:25–35
Lee HI, Leon J, Raskin I (1995) Biosynthesis and metabolism of salicylic acid. Proc Natl Acad Sci USA 92:4076–4079
Morris K, Mackerness SAH, Page T, John CF, Murphy AM, Carr JP, Buchanan-Wollaston V (2000) Salicylic acid has a role in regulating gene expression during leaf senescence. Plant J 23(5):677–685
Murakami R, Ifuku K, Takabayashi A, Shikanai T, Endo T, Sato F (2002) Characterization of an Arabidopsis thaliana mutant with impaired psbO, one of two genes encoding extrinsic 33-kDa proteins in photosystem II. FEBS Lett 523:138–142
Nazar R, Umar S, Khan NA, Sareer O (2015) Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S Afr J Bot 98:84–94
Pan Q, Zhan J, Liu H, Zhang J, Chen J, Wen P, Huang W (2006) Salicylic acid synthesized by benzoic acid 2-hydroxylase participates in the development of thermotolerance in pea plants. Plant Sci 171:226–233
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucl Acids Res 30(9):e36–e36
Salvucci ME, Crafts-Brandner SJ (2004) Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plant 120:179–186
Singh PK, Chaturvedi VK, Bose B (2010) Effects of salicylic acid on seedling growth and nitrogen metabolism in cucumber (Cucumis sativus L.). J Stress Physiol Biochem 6:102–113
Underwood J, Moch J, Chen MS, Zhu L (2014) Exogenous salicylic acid enhances the resistance of wheat seedlings to Hessian fly (Diptera: cecidomyiidae) infestation under heat stress. J Econ Entomol 107(5):2000–2004
Wang WX, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252
Wang Y, Zhang H, Hou P, Su X, Zhao P, Zhao H, Liu S (2014) Foliar-applied salicylic acid alleviates heat and high light stress induced photoinhibition in wheat (Triticum aestivum) during the grain filling stage by modulating the psbA gene transcription and antioxidant defense. Plant Growth Regul 73(3):289–297
Wu L, Zu X, Wang X, Sun A, Zhang J, Wang S, Chen Y (2013) Comparative proteomic analysis of the effects of salicylic acid and abscisic acid on maize (Zea mays L.) leaves. Plant Mol Biol Rep 31(3):507–516
Yang CM, Lee CN, Chou CH (2002) Effects of three allelopathic phenolics on chlorophyll accumulation of rice (Oryza sativa) seedlings: i. Inhibition of supply-orientation. Bot Bull Acad Sin 43:299–304
Acknowledgments
The financial support received from Indian Council of Agriculture Research (ICAR) under the National Initiative for Climate Resilient Agriculture (NICRA) project (12/115 TG 3079) and extra-mural research funding by SERB, Department of Science and Technology (DST) (Project no. SERB/SB/SO/PS/07/2014) is highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Communicated by Z.-L. Zhang.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, R.R., Sharma, S.K., Goswami, S. et al. Salicylic acid alleviates the heat stress-induced oxidative damage of starch biosynthesis pathway by modulating the expression of heat-stable genes and proteins in wheat (Triticum aestivum). Acta Physiol Plant 37, 143 (2015). https://doi.org/10.1007/s11738-015-1899-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1899-3