Abstract
Wheat, a glycophyte grown in tropical and subtropical regions, is frequently being subjected to soil salinity ultimately affecting the plant growth and yield. Focus of the present study was to evaluate the ameliorative efficiency of different seed priming methods including hydropriming and halopriming [KCl and CaCl2 (100 mM)] by observing change in the expression of antioxidant defense system and accumulation of phenolic as well as proline in the spring wheat Lu26s (salt tolerant) and Lasani-06 (salt sensitive), grown under salt stress of 100 mM NaCl. Results showed that salt stress provoked a marked decline in germination, growth and yield parameters as well as increased lipid peroxidation and hydrogen peroxide (H2O2) contents. However, higher accumulation of proline and low H2O2 contents were recorded in both cultivars under halopriming followed by hydropriming. Halopriming induced a significant increase in antioxidant enzyme activities (CAT, POD, APX) of salt-tolerant cultivar Lu26s, whereas such pattern of enhanced activities of antioxidant enzymes in cultivar Lasani-06 was also found but the content of these activities was less than control under saline regime. The cultivar Lu26s (salt tolerant) maintained lower Na+ and higher K+/Na+ ratio in leaves than salt-sensitive cultivar Lasani-06. Reason behind the loss of grain yield under salinity was found due to the reduction in the grain spike−1 in cultivar Lasani-06, while, in cultivar Lu26s, it was due to decrease in the size of grains. Enhanced germination, low proline and Na+ contents stimulated antioxidant activities as well as phenolic contents associated with improved salt tolerance in haloprimed plants. These results suggest that halopriming is an efficient approach for imparting tolerance in wheat against salinity stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity is an important land degradation problem that affects almost 800 million hectares of cultivated farmland, consequently poses detrimental impact on crop production across the globe (Rengasamy 2006). In arid and semi-arid environment, limited rainfall, high evapotranspiration and temperature are the main causes of salinity that result in poor germination and seedling establishment, inducing a notable reduction in germination rate and delay in the initiation of germination (Almansouri et al. 2001; Li et al. 2011). Water stress, nutritional instability and accumulation of sodium ion (Na+) are regarded as the plant growth associated impacts of saline stress (Shannon 1997), causing adverse pleiotropic effects at physiological, biochemical and molecular level (Parida and Das 2005). Accumulation of the Na+ in leaves restricts the plant growth by decreasing life of photosynthetic tissues (Munns 2002). The regulation of Na+ and its active efflux in leaves is one of the significant adaptations for salinity tolerance which includes osmotic adjustment and compartmentalization of toxic ions and scavenging of reactive oxygen species (ROS) by the antioxidant defense system (Sekmen and Takio 2007; Miller et al. 2010). Under optimum conditions, ROS production and destruction are the routine processes but under stress condition, antioxidant defense system protects the cell from the cytotoxic effects of ROS (Foyer and Noctor 2011).
Among different approaches to cope with salinity stress in plants, seed priming (pre-sowing seed treatment such as hydropriming, halopriming, osmopriming, hardening, osmohardening and hormonal priming) is an easy, low-cost and low-risk technique that has recently been used to accelerate synchronized seed germination, vigorous seedling establishment, stimulated vegetative growth and yield in many grass, vegetable and field crops, to overcome the adverse effects of salinity in agricultural lands (Iqbal and Ashraf 2006; Iqbal et al. 2006). The improved seed performance might be due to the osmotic adjustment, metabolic repair processes or stimulation of germination metabolites during priming trea tments (Haghpanah et al. 2009). It has been proposed that hydropriming is a cost-effective technique having beneficial role on the growth of maize, rice, soybean and chickpea (Ashraf and Foolad 2005). Priming phenomen has been known for more than two decades but is appreciated recently. It improves plant defense responses by boosting crop’s resistance in biotic and abiotic stresses (Beckers and Conrath 2007). This approach has proven its effectiveness to improve crop establishment on saline soil (Afzal et al. 2005).
Wheat is the main crop that is cultivated in Pakistan both in irrigated and arid zones. It is one of the staple foods of the country. Its production (hectare−1) is less than the ideal yield because of many reasons which also include salinization. However, halopriming (with CaCl2 and KCl) is known to increase salt tolerance in different crops (Ahmadv et al. 2012) but these studies are limited to the germination studies only. Moreover, the biochemical and physiological basis of halopriming-induced salt tolerance are still unclear. Therefore, the present study was planned to investigate the effect of KCl and CaCl2 priming on germination, chlorophyll contents, ions distribution and activities of antioxidant enzymes of two wheat cultivars differing in salt tolerance under salt stress.
Materials and methods
Plant material, growth and treatment conditions
Seeds of two wheat (Triticum aestivum L.) cultivars Lu26s (salt tolerant) and Lasani-06 (salt sensitive) were obtained from Ayub Agriculture Research Institute (AARI), Faisalabad, Pakistan. A two-factor factorial designed pot experiment was conducted with three replications consisting of four priming treatments and two salinity levels as factors. Total 75 healthy seeds were selected for each priming treatment (25 seeds per pot were sown, after emergence they were thinned by 7–8 plants pot−1). Solution of KCl (100 mM), CaCl2 (100 mM) and distilled water were used for seed priming. Before sowing, the seeds were soaked with KCl, CaCl2 as well as in distilled water, respectively, for 12 h at room temperature (20 ± 2 °C). After priming, seeds were air dried on filter paper for 12 h at room temperature (20 ± 2 °C). All the haloprimed (CaCl2 or KCl), hydroprimed (HP) and untreated seeds (UT) were grown in both control (0 mM NaCl) as well as in salinity stress (100 mM NaCl) (Iqbal et al. 2006). Plants were watered (with distill water and saline solution) as per need. All the pots were placed under natural light (sunlight) with air temperature ranging from 13 to 34 °C during the day and 10 to 25 °C during night, while, relative humidity was in range of 45 and 65 % at day and night, respectively.
Experiment was conducted in earthen pots lined with polythene layer, containing 10 kg of air dried soil with pH 7.5, electric conductivity 685 µS cm−1, total organic matter 0.6 %, total soil phosphorus 5.30 mg kg−1 and total nitrogen of 500 mg kg−1 soil. Each pot received N, P, and K fertilizer in quantity of 10, 6 and 6 g m−2, respectively, as per requirement/recommended dose. Before sowing, pots were irrigated with 2.5 dm3 of water (control) or saline solution of NaCl (100 mM) according to the treatment plan. The respective electrical conductivity (EC) of 100 mM of NaCl solutions was 12.5 dS m−1.
Germination percentage
Calculation of germination percentage of seeds from each treatment was recorded after every 5 days with the help of the following formula:
Days to 50 % germination
Days to 50 % germination (E50) were calculated following the formula developed by Coolbear et al. (1984) with slight modifications:
where N represents the final number of seeds emerged, and n i and n j represent the cumulative number of seeds emerged counted at times t i and t j , respectively, when n i < N/2 < n j .
Mean emergence time
Mean emergence time (MET) was worked out by following Ellis and Roberts (1981):
where n represents the number of seeds emerged on day D, and D represents the number of days from the onset of seed germination.
Coefficient of uniformity of emergence
Coefficient of uniformity of emergence (CUE) was calculated by following Bewley and Black (1985):
In the above equation, t represents the time in days, starting from the day of sowing, and n denotes the number of seeds that have completed emergence on day t, and t′ represents the MET.
Emergence index
Emergence index (EI) was calculated using following formula:
Germination energy
Germination energy (GE) of the seeds was calculated on the fourth day after sowing (DAS) of seeds following Ruan et al. (2002b). The percentage of germinating seeds at fourth DAS is relative to the total number of seeds tested.
Plant growth, yield and biomass
Growth traits were measured at flowering stage in terms of plant height, shoot and root dry weight. The samples were put in the forced hot air draft oven at 70 ± 5 °C for 5 days to measure shoot and root dry weight (g) after recording shoot/root lengths (cm) and fresh weight (g) of plant. For the determination of leaf area (cm2), length and maximum width of each leaf were measured and leaf area was calculated using the formula of Carleton and Foote (1965). At maturity (final harvest), the remaining plants (4–5 plants pot−1) from each pot were harvested and data regarding the number of spike, grains spike−1, grains plant−1 and 100-grain weight were recorded.
Chlorophyll contents, antioxidant enzyme activities and total phenolic
Middle section of leaf without midrib was used for all biochemical analysis. Total chlorophyll contents at flowering stage were determined according to the method of Arnon (1949). The extent of the salt-induced oxidative damage was evaluated through changes in lipid peroxidation by measuring the malondialdehyde (MDA) formation (Cakmak and Horst 1991) in the leaves of wheat cultivars. Briefly, 1 g of fresh leaves was ground in 20 mL of 0.1 % TCA (Trichloroacetic acid) solution and centrifuged for 10 min at 12,000g. One mL of the supernatant was mixed with 4 mL of TCA containing 5 % TBA (Thiobarbituric acid), heated for 30 min at 95 °C and cooled on ice. The mixer was centrifuged at 12,000g for 10 min and absorbance of the supernatant was taken at 532 and 660 nm.
The activity of POD was measured by the method of Maehly and Chance (1954) where the activity of APX was assessed following the method of Nakano and Asada (1981) with minor modifications. An aliquot of 200 µL enzyme extract was added to a mixture containing 800 µL of 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM ascorbic acid and 0.1 mM H2O2. Optical density (OD) of the mixture was recorded for one min (as decrease in absorbance at 290 nm). The total phenolic was measured by adding 0.5 mL of enzyme extract in 2.5 mL of Folin–Ciocalteu reagent (10 % v/v) and 2 mL of Na2CO3 (7.5 %). The mixture was heated at 45 °C for 40 min and the absorbance was measured at 765 nm. The gallic acid was used as standard and expressed as mg of Gallic acid equivalent/g extract. Proline contents were measured according to Bates et al. (1973). For the determination of sodium and potassium ions, leaves were dried and ground. About 0.1 g of the ground leaf was digested with H2SO4 and H2O2, and then sodium and potassium contents were analyzed through flame photometer.
Statistical analysis
All values reported in this study are mean of at least three replicates. A two-way analysis of variance (ANOVA) was performed using a statistical package, SPSS version 16.0 (SPSS, Chicago, IL). Duncan’s test was done to determine the significant difference among treatments.
Results
Seed germination parameters
Data regarding different germination parameters showed that seed pre-sowing treatment with CaCl2 or KCl significantly increased final germination, germination energy, coefficient of uniformity of emergence and germination index, while, significant decrease was recorded in the mean emergence time and days to 50 % emergence as compared to hydroprimed and unprimed seeds under 100 mM NaCl (Fig. 1). It was also observed that seed pre-sowing treatment with CaCl2 primed seeds performed well followed by KCl primed seeds. Among the wheat cultivars, values of germination parameters of Lu26s were higher than cultivar Lasani-06 (Fig. 1).
Plant biomass and chlorophyll contents
All growth characters were found to be significantly affected by salinity. However, varied response of sensitivity to salinity was recorded in different treatments. The seeds primed with CaCl2 or KCl maintained significantly higher fresh and dry weight of root and shoot followed by hydroprimed seeds over unprimed control. Overall, the effect of halopriming was more pronounced in Lu26s (salt tolerant) than Lasani-06 (salt sensitive). Root/shoot ratios (in both cultivars) were higher in salt stress as compared to untreated control. It was found that shoot was more affected than root under salt stress (Table 1). Salt stress negatively affected the leaf area of both wheat cultivars. Indeed, under control conditions, the leaf area ranged from 281 to 320 cm2 in Lu26s and 318 to 357 cm2 in Lasani-06. The leaf area of salt-sensitive variety (Lasani-06) was more affected (57 % decrease) than the salt-resistant cultivar (34 % decrease) under salt stress (Table 1). Data regarding chlorophyll contents presented a significant variation among the cultivars (Table 1). In general, a decline trend was observed in both cultivars under salinity stress. Maximum reduction was recorded in Lasani-06; while, Lu26s retained high amount of chlorophyll, however, haloprimed treatments exhibited less reduction in chlorophyll contents under salinity. However, a significant decrease in chlorophyll contents was found in untreated and hydroprimed treatment (Table 1).
Yield attributes
Yield attributes such as the number of spikes, grains plant−1, grains spike−1 and 100-grains weight were reduced significantly under salinity stress (Table 2), though the change was higher in Lasani-06 than Lu26s. Among the priming treatments, yield components of the haloprimed seeds were significantly higher than those of hydroprimed and control treatments. Under saline stress, cultivar Lu26s decreased 100-grains weight by 65 and 35 % under unprimed and hydroprimed treatments, while 21 and 30 % reduction in 100-grains weight was recorded under KCl and CaCl2 treatments, respectively. The decrease in all other described yield attributes was more pronounced in salt-sensitive cultivar (Lasani-06) as compared to Lu26s. However, among seed treatments, hydroprimed seeds were more affected than haloprimed seeds.
Effects of seed priming and salinity on oxidative stress
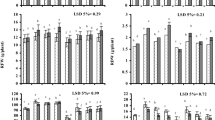
A significant increase in H2O2 accumulation was observed in the leaves of both wheat cultivars after salt exposure as compared to control plants, but to greater extent in Lasani-06 as compared to Lu26s cultivar. Lasani-06 showed highest H2O2 level in untreated and hydroprimed plants; while, significant decrease was recorded in haloprimed plants (Fig. 2a, b). Salt-stressed leaves had more MDA contents in both wheat cultivars under all priming treatments as compared to control plants. MDA contents were significantly higher in salt-sensitive cultivar (Lasani-06) as compared to salt-resistant cultivar (Lu26s). Priming with KCl or CaCl2 significantly reduced the MDA contents under salt stress as compared to hydroprimed and untreated plants (Fig. 2c, d).
Changes of H2O2 (ng g−1 FW) (a, b), malonaldehyde (MDA, nmol g−1 FW) (b, c), catalase (CAT) (e, f), peroxidase (POD) (g, h), ascorbate peroxidase (APX) (i, j), phenolic (mg g−1 Gallic Acid Equivalent) (k, l) and proline (µg−1f.wt) (m, n) in untreated control (UT), hydroprimed (HP), CaCl2 and KCl primed wheat plants under different levels of salinity stress. Data presented are means from three replicates with standard errors. Within each treatment different letters indicate significant differences by Duncan’s multiple range test at P < 0.05
Antioxidant enzyme activities
A significant difference in CAT activity (Fig. 2e, f) and APX activity (Fig. 2i, j) was observed among two wheat cultivars under salt stress. In general, salt-resistant cultivar Lu26s showed higher contents of CAT and APX enzyme activities over all priming treatments. However, CAT and APX activities were significantly decreased in salt-sensitive cultivar (Lasani-06) under all priming treatments. This decrease was more pronounced in hydroprimed and untreated control as compared to haloprimed plants. Salinity stress induced a greater level of POD activity in leaves of Lu26s cultivar as compared to control and more increase was pronounced in haloprimed treatment (Fig. 2g, h). However, POD activity significantly decreased in salt-sensitive cultivar. Moreover, significantly lower value of POD activity was recorded in all priming treatments.
Proline and phenolic contents, and K+ and Na+ accumulation
Results showed that leaf proline and phenolic contents were significantly higher under salt stress in both cultivars. However, haloprimed treatment in salt-resistant cultivar Lu26s and sensitive cultivar Lasani-06 induced a significant increase in proline (Fig. 2k, l) and phenolic (Fig. 2m, n) contents under salinity stress. Further, leaf chemical analysis of wheat cultivars indicated that Na+ contents ameliorated under salinity stress (Table 3). The cultivar Lasani-06 had relatively higher Na+ contents than Lu26s as compared to control. Relatively less concentration of Na+ was found in haloprimed seed plants in both cultivars, while reduced K+ contents were recorded in all priming treatments under salinity stress in cultivar Lasani-06. Cultivar Lu26s maintained high concentration of K+ in all priming treatments under salinity stress, whereas relatively reduced K+/Na+ ratio was observed in Lasani-06 as compared to Lu26s (Table 3).
Discussion
The plant growth and development are adversely affected attributes of the plant under salinity stress which result in ultimate loss of yield (Ashraf and Harris 2004). Pre-sowing treatment with inorganic salt resulted in amelioration of salinity stress as studied in different plants (Cayuela et al. 1996; Sivritepe et al. 2003). Present study conducted under salinity stress elaborates that seed emergence rate, germination index and energy are the crucial contributors of seed vigor. Higher seed emergence rate is the primary factor that ensures overall seedling performance. Data regarding germination parameters demonstrated that salinity and seed priming significantly influenced the wheat germination as previously found by Kaya et al. (2006). This may be due to the metabolic activities in primed seed during germination that had started much earlier than the appearance of radicle and plumule; thus, primed seeds have better efficiency of water absorption from growth media compared to unprimed seed (Hopper et al. 1979). Seed priming in fact initiates the metabolic processes (Bewley and Black 1985) such as cell division, which reduces seed germination time (Sivritepe et al. 2003). Indeed, priming is an effective technique that increases seed vigor and improves germination and seedling growth (Jumsoon et al. 1996) which is also confirmed by our findings.
On the other hand, increased saline stress inhibits the germination which may be due to toxic effects of Na+ and Cl− on germination (Khajeh-Hosseini et al. 2003), which ultimately limits the water absorption ability of seed (Dodd and Donovan 1999). Significant rise in mean germination time of unprimed seeds and early germination of primed seeds (with KCl or CaCl2) under salinity stress is in line with the findings of Elouaer and Hannachi (2012) and Shehzad et al. (2012). Ruan et al. (2002a) also demonstrated the improved germination index in rice seed primed with KCl and CaCl2. Greater efficiency of seed priming with KCl is possibly related to the osmotic advantage of K+ that acts as co-factor in the activities of numerous enzymes (Taiz and Zeiger 2002). Potassium and calcium ions actually mitigate the harmful effects of Na+ on plant metabolism by reducing the Na+ uptake (Greenway and Munns 1980). In the present study, variation in growth and development of wheat cultivars in response to saline stress might be due to the variations in physiological and biochemical characteristics that have occurred in the process of salt tolerance, i.e., antioxidants enzymes, ion balance and accumulation of compatible solutes. Photosynthesis is another critical attribute for assessment because it is responsible for plant productivity under both normal and stress conditions (Natr and Lawlor 2005; Athar et al. 2008). Decrease in the contents of chlorophyll in studied plants may be due to the production of proteolytic enzyme, i.e., chlorophyllase, which reduces the chlorophyll and damages the photosynthetic machinery (Sabater and Rodrguez 1978) or this reduction may be attributed to the instability of the pigment protein complex. Saline stress in the present investigation also decreased the root, shoot dry and fresh weight of primed and unprimed seeds under salinity stress but magnitude of reduction was relatively less in primed seeds. Reduction in root and shoot fresh weight can be supported by the findings of Jaleel et al. (2007) who demonstrated that reduction in the shoot fresh and dry weight was attributed to decrease in photosynthesis due to less leaf area that resulted a reduction in plant biomass (Basra et al. 2003).
Results of the present study also showed higher root/shoot ratio under salinity stress. Saline stress often reduces shoot growth more than root growth (Läuchli 1990) and can reduce the number of florets per ear, increase sterility and affect the time of flowering and maturity in both wheat (Maas and Grieve 1990) and rice (Khatun et al. 1995). Thus, the biomass percentage allocated to roots, stems and leaves was found widely different and related to time of exposure, species/varieties and ontogenetic stages under saline conditions (Munns and Tester 2008). It was observed that decrease in grain yield in Lu26s cultivar was related to the decline in 100-grains weight but in Lasani-06 it was due to decrease in grain number plant−1. The decrease in grain number plant−1 in Lasani-06 cultivar may be due to the lack of photo-assimilates accumulation that reduces the number of grain plant−1. This shows that availability of photo-assimilates is the rate limiting factor to grain number. Availability of the assimilates during saline stress determined the grain size (Poustini and Siosemardeh 2004). In cultivar Lu26s, there might be no limitation of photo-assimilates accumulation due to this reason more grain numbers were recorded in Lu26s as compared to Lasani-06, but at grain-filling stage less availability of assimilates due to prolonged saline stress reduced the weight of grains. Our results may support the earlier findings (Rahnama et al. 2011; Husain et al. 2003; Houshm et al. 2005) who observed similar phenomena with wheat grains.
To understand the salt tolerance mechanism in plant, ions regulation is another important critical parameter. Thus, the assessment of ion accumulation, especially Na+ and K+ in different plant organs is essential to infer the salt tolerance mechanisms (Noreen et al. 2010). In the present investigation, high K+ and K+/Na+ ratio and low Na+ ion helped Lu26s to maintain high growth while among the seed treatments, haloprimed seed plants maintained high contents of K+ and K+/Na+ ratio thus revealing that salt stress also imbalances homeostasis that leads to the ionic and osmotic stresses in plant cells (Athar et al. 2008). Higher concentration of Na+ in Lasani-06 may be due to increased amounts of Na+ in root, passive Na+ diffusion through damaged membranes and decreased efficiency of exclusion mechanisms as described by Bajji et al. (2001).
Plants under stress also produce some defense mechanisms to protect themselves from the harmful effects of salinity-induced oxidative stress. ROS are involved in the initiation of a number of auto-oxidative chain reactions, including lipid peroxidation, DNA damage and protein degradation (Mittler 2002). In the present study, lipid peroxidation was assessed by measuring amount of MDA in the leaves of wheat cultivars, because salt stress is known to result in extensive lipid peroxidation, which is an effective indicator of salt-induced oxidative damage at the cellular level (Islam et al. 2014a, b; Hernández and Almansa 2002). Alleviation of oxidative damages and maintaining integrity of the cellular membranes is a key mechanism in salinity tolerance (Stevens et al. 2006). In the present study, significant increase in MDA contents in salt-sensitive cultivar Lasani-06 than salt-tolerant cultivar Lu26s under salt stress indicates that Lu26s may have a better protection against oxidative damage. However, among priming treatments, CaCl2 and KCl primed plants showed lower MDA contents than hydroprimed and untreated control under salinity stress. The same phenomenon was found by Masood et al. (2006) while studying the effect of salinity in Azolla cultivars. The lesser MDA contents in haloprimed plants may be due to Ca+ and K+ ions that prevent the electrolyte leakage and lipid peroxidation and play crucial role against salinity tolerance (Nedjimi and Daoud 2009; Wu et al. 2013). It is evident that priming can increase the activities of free radical scavenging enzymes, i.e., catalase (CAT), ascorbate (APX) and peroxidase (POD) in seeds (Ashraf and Ali 2008; Chiu et al. 1995; Chang and Sung 1998). Thus, seed priming in this study has increased the activity of scavenging enzymes and improved the seedling vigor as indicated by increased POD, APX and CAT activities in the leaves of salt-tolerant wheat cultivar. Improved protection in Lu26s as compared to Lasani-06 under halopriming may reflect a more efficient antioxidative system. Though, activity of antioxidant enzymes was reduced significantly in salt-sensitive cultivar but this decrease was most pronounced in hydroprimed and untreated control as compared to CaCl2 or KCl primed plants. Catalase is a key enzyme in scavenging H2O2 by breaking it down to form H2O and O2 (Mittler 2002). A decrease in CAT activity after priming in salt-sensitive cultivar confirmed the findings of Srinivasan and Saxena (2001) who reported that CAT activity was not increased after priming in radish; however, the decrease/increase was closely related to the genetic background of cultivars (sensitive/tolerant), level of salt stress (NaCl concentration and duration) and type of pre-sowing seed treatments. Therefore, it is expected that enhanced antioxidant enzyme activity in wheat cultivars due to halopriming is a key component against tolerance to NaCl stress (Afzal et al. 2006) as observed in the present study. These results suggest that salt-tolerant cultivar Lu26 s may have a better protection against reactive oxygen species (ROS) by increasing the activity of antioxidant enzymes (APX, POD and CAT) under salt stress. Similar observations have also been reported for salt-tolerant and -sensitive cultivars of potato by Rahnama and Ebrahimzadeh (2005).
Phenolic being carbon-rich secondary metabolites, involves in the scavenging of free radicals and oxidative species, regulation of osmotic pressure, protection of membrane integrity, stabilization of enzymes/proteins, maintaining appropriate NADP+/NADPH ratios and improvement of antioxidant activity of saline-stressed plants. Proline’s ROS-scavenging ability helps in minimizing oxidative damage produced by salinity and protects membrane integrity (Fidalgo et al. 2004). In the present investigation, a substantial increase in proline levels might be attributed to the strategies adapted by plants to cope with salinity stress. Under salinity stress, plants produce jasmonic acid and its methylated derivatives ultimately result in the accumulation of phenolic contents in stressed plant (Pedranzani et al. 2007) to combat with reactive oxygen species. However, the production of phenolic contents in saline-stressed plant is species dependent as broccoli (López-Pérez et al. 2009) and Romanian lettuce (Kim et al. 2008) were unable to produce phenolic under saline stress, whereas phenolic production was found higher under salinity stress in maize (Hichema et al. 2009) and Spanish lettuce (Blasco et al. 2013). However, in the present investigation, a significant increase in phenolic contents was found in haloprimed wheat plants in both cultivars. This increase played functional role to protect the plant against salt-induced oxidative stress, resulting better growth and yield production in haloprimed plants than untreated control and hydroprimed plants.
Conclusions
Findings of present study suggest that halopriming ameliorates the salinity stress by upregulating the growth, photosynthesis, production of proline, phenolic contents and activities of antioxidant enzymes (POD, APX and CAT). However, it was found that the precise impact of salinity was genotype dependent. The increased tolerance of cultivar Lu26s was due to the lower concentration of Na+ and higher K+/Na+ ratio in shoot, while KCl or CaCl2 priming helped the plant to lower Na+ in shoot and increase K+/Na+ ratio both in control and saline stress. However, a further study is needed to explore the underlying molecular and physiological mechanisms of seed priming on germination in relation to plant hormones and transcription. Moreover, the mechanism of halopriming was observed against a single level of salinity stress (NaCl 100 mM) in this preliminary investigation which needs to be evaluated against certain levels to make an authenticated statement for the expressions of wheat cultivars with halopriming with KCl or CaCl2.
Author contribution statement
This work was designed by F. Islam, T. Yasmeen and S. Ali. Experiments and data analyses were performed by F. Islam, B. Ali, M. A. Farooq and S. Ali. However, manuscript was written by F. Islam, T. Yasmeen, R. A. Gill and B. Ali.
References
Afzal I, Basra SM, Iqbal A (2005) The effects of seed soaking with plant growth regulators on seedling vigor of wheat under salinity stress. J Stress Physiol Biochem 1:6–14
Afzal I, Basra SMA, Hameed A, Farooq M (2006) Physiological enhancements for alleviation of salt stress in wheat. Pak J Bot 38:1649–1659
Ahmadv G, Soleymani F, Saadatian B, Pouya M (2012) Effects of seed priming on seed germination and seedling emergence of cotton under salinity stress. World App Sci J 20:1453–1458
Almansouri M, Kinet JM, Lutts S (2001) Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.). Plant Soil 231:243–254
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Ashraf M, Ali Q (2008) Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L.). Environ Exp Bot 63:266–273
Ashraf M, Foolad MR (2005) Pre-sowing seed treatment—a shotgun approach to improve germination, plant growth, and crop yield under saline and non-saline conditions. Adv Agron 88:223–271
Ashraf M, Harris P (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Athar HUR, Khan A, Ashraf M (2008) Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Envir Exp Bot 63:224–231
Bajji M, Lutts S, Kinet JM (2001) Water deficit effects on solute contribution to osmotic adjustment as a function of leaf ageing in three durum wheat (Triticum durumDesf.) cultivars performing differently in arid conditions. Plant Sci 160:669–681
Basra S, Zia M, Mehmood T, Afzal I, Khaliq A (2003) Comparison of different invigoration techniques in Wheat (Triticum aestivum L.) seeds. Pak J Arid Agri 5:6–11
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beckers G, Conrath U (2007) Priming for stress resistance: from the lab to the field. Curr Opin Plant Bio 10:425–431
Bewley JD, Black M (1985) Seeds physiology of development and germination. Springer, Berlin
Blasco BA, Leyva R, Romero L, Ruiz JM (2013) Iodine effects on phenolic metabolism in lettuce plants under salt stress. J Agri Food Chem 61:2591–2596
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Carleton AE, Foote WH (1965) A comparison of methods for estimating total leaf area of barley plants. Crop Sci 5:602–603
Cayuela E, Pérez-Alfocea F, Caro M, Bolarin M (1996) Priming of seeds with NaCl induces physiological changes in tomato plants grown under salt stress. Physiol Plant 96:231–236
Chang SM, Sung JM (1998) Deteriorative changes in primed sweet corn seeds during storage. Seed Sci Technol 26:613–626
Chiu KY, Wang CS, Sung JM (1995) Lipid peroxidation and peroxide-scavenging enzymes associated with accelerated aging and hydration of watermelon seeds differing in ploidy. Physiol Plant 94:441–446
Coolbear P, Francis A, Grierson D (1984) The effect of low temperature pre-sowing treatment on the germination performance and membrane integrity of artificially aged tomato seeds. J Exp Bot 35:1609–1617
Dodd GL, Donovan LA (1999) Water potential and ionic effects on germination and seedling growth of two cold desert shrubs. Am J Bot 86:1146–1153
Ellis R, Roberts E (1981) The qualification of aging and survival in orthodox seeds. Seed Sci Technol 9:373–409
Elouaer MA, Hannachi C (2012) Seed priming to improve germination and seedling growth of safflower (Carthamus tinctorius) under salt stress. Eur Asian J Bio Sci 6:76–84
Fidalgo F, SantosA Santos I, Salema R (2004) Effects of long-term salt stress on antioxidant defence systems, leaf water relations and chloroplast ultrastructure of potato plants. Ann App Bio 145:185–192
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Greenway H, Munns R (1980) Mechanisms of salt tolerance in nonhalophytes. Ann Rev Plant Physiol 31:149–190
Haghpanah A, Younesi O, Moradi A (2009) The effect of priming on seedling emergence of differentially matured sorghum (Sorghum bicolor L.) seeds. J Appl Sci Res 5:729–732
Hernández JA, Almansa MS (2002) Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol Plant 115:251–257
Hichema H, Mounir D, Naceurc E (2009) Differential responses of two maize (Zea mays L.) varieties to salt stress: changes on polyphenols composition of foliage and oxidative damages. Ind Crops Pro 30:144–151
Hopper N, Overholt J, Martin J (1979) Effect of cultivar, temperature and seed size on the germination and emergence of soya beans (Glycine max (L.) Merr.). Ann Bot 44:301–308
Houshm S, Arzani A, Maibody SAM (2005) Evaluation of salt-tolerant genotypes of durum wheat selected from in vitro and field experiments. Field Crop Res 9:345–354
Husain S, Munns R, Condon AG (2003) Effect of sodium exclusion trait on chlorophyll retention and growth of durum wheat in saline soil. Aus J Agr Res 54:589–597
Iqbal M, Ashraf M (2006) Wheat seed priming in relation to salt tolerance: growth, yield and levels of free salicylic acid and polyamines. Ann Bot Fenn 43:250–259
Iqbal M, Ashraf M, Jamil A, Ur-Rehman S (2006) Does seed priming induce changes in the levels of some endogenous plant hormones in hexaploid wheat plants under salt stress? J Integ Plant Biol 48:181–189
Islam F, Yasmeen T, Ali Q, Ali S, Arif MS, Hussain S, Rizvi H (2014a) Influence of Pseudomonas aeruginosa as PGPR on oxidative stress tolerance in wheat under Zn stress. Ecotox Environ Safe 104:285–293
Islam F, Yasmeen T, Riaz M, Arif MS, Ali S, Raza SH (2014b) Proteus mirabilis alleviates zinc toxicity by preventing oxidative stress in maize (Zea mays) plants. Ecotox Environ Safe 110:143–152
Jaleel C, Manivannan P, Lakshmanan G, Sridharan R, Panneerselvam R (2007) NaCl as a physiological modulator of proline metabolism and antioxidant potential in Phyllanthus amarus. Com Ren Biol 330:806–813
Jumsoon K, Jeuonlai C, Ywonok J (1996) Effect of seed priming on the germinability of tomato (Lycopersicon esculentum Mill.) seeds under water and saline stress. J Kor Soc Horti Sci 37:516–521
Kaya MD, Okçu G, Atak M, Çıkılı Y, Kolsarıcı Ö (2006) Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annus L.). Euro J Agro 24:291–295
Khajeh-Hosseini M, Powell A, Bingham I (2003) The interaction between salinity stress and seed vigour during germination of soyabean seeds. Seed Sci Tech 31:715–725
Khatun S, Rizzo CA, Flowers TJ (1995) Genotypic variation in the effect of salinity on fertility in rice. Plant Soil 173:239–250
Kim HJ, Fonseca JM, Choi JH, Kubota C, Kwon DY (2008) Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J Agri Food Chem 56:3772–3776
Läuchli A (1990) Calcium, salinity and the plasma membrane. In: Leonard RT, Hepler PK (eds) Calcium in plant growth and development. The American Society of Plan Physiologists Symposium Series, vol 4, pp 26–35
Li J, Yin L, Jongsma M, Wang C (2011) Effects of light, hydropriming and abiotic stress on seed germination, and shoot and root growth of pyrethrum (Tanacetum cinerariifolium). Ind Crops Prod 34:1543–1549
López-Pérez L, del Carmen Martínez-Ballesta M, Maurel C, Carvajal M (2009) Changes in plasma membrane lipids, aquaporins and proton pump of broccoli roots, as an adaptation mechanism to salinity. Phytochem 70:492–500
Maas EV, Grieve CM (1990) Spike and leaf development in salt-stressed wheat. Crop Sci 30:1309–1313
Maehly A, Chance B (1954) Catalases and peroxidases. Methods Biochem Anal 1:357–424
Masood A, Shah NA, Zeeshan M, Abraham G (2006) Differential response of antioxidant enzymes to salinity stress in two varieties of Azolla/(Azolla pinnata and Azolla filiculoides). Environ Exp Bot 58:216–222
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Biol 59:651–681
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Natr L, Lawlor D (2005) Photosynthetic plant productivity. In: Pessarakli M (ed) Photosynthesis handbook, 2nd edn. CRC Press, New York, pp 501–524
Nedjimi B, Daoud Y (2009) Cadmium accumulation in Atriplex halimus subsp schwein furthii and its influence on growth, proline, root hydraulic conductivity and nutrient uptake. Flora Morphol Distrib Funct Ecol Plants 204:316–324
Noreen Z, Ashraf M, Akram N (2010) Salt-induced regulation of some key antioxidant enzymes and physio-biochemical phenomena in five diverse cultivars of turnip (Brassica rapa L.). J Agro Crop Sci 196:273–285
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotox Environ Safe 60:324–349
Pedranzani H, Sierra-de-Grado R, Vigliocco A, Miersch O, Abdala G (2007) Cold and water stresses produce changes in endogenous jasmonates in two populations of Pinus pinaster Ait. Plant Growth Regul 52:111–116
Poustini K, Siosemardeh A (2004) Ion distribution in wheat cultivars in response to salinity stress. Field Crops Res 85:125–133
Rahnama A, Poustini K, Tavakkol-Afshari R, Ahmadi A, Alizadeh H (2011) Growth properties and ion distribution in different tissues of bread wheat genotypes (Triticum aestivum L.) differing in salt tolerance. J Agron Crop Sci 197:21–30
Rahnama H, Ebrahimzadeh H (2005) The effect of NaCl on antioxidant enzyme activities in potato seedlings. Biol Plant 49:93–97
Rengasamy P (2006) World salinization with emphasis on Australia. J Exp Bot 57:1017–1023
Ruan S, Xue Q, Tylkowska K (2002a) Effects of priming on germination and health of rice (Oryza sativa L.) seeds. Seed Sci Technol 30:451–458
Ruan S, Xue Q, Tylkowska K (2002b) The influence of priming on germination of rice (Oryza sativa L.) seeds and seedling emergence and performance in flooded soil. Seed Sci Tech 30:61–67
Sabater B, Rodrguez MT (1978) Control of chlorophyll degradation in detached leaves of barley and oat through effect of kinetin on chlorophyllase levels. Physiol Plant 43:274–276
Sekmen H, Takio S (2007) Differential responses of antioxidative enzymes and lipid peroxidation to salt stress in salt-tolerant Plantago maritima and salt-sensitive Plantago media. Physio Plant 131:399–411
Shannon MC (1997) Adaptation of plants to salinity. Adv Agron 60:75–120
Shehzad M, Ayub M, Ahmad A, Yaseen M (2012) Influence of priming techniques on emergence and seedling growth of forage sorghum (Sorghum bicolor L.). J Ani Plant Sci 22:154–158
Sivritepe N, Sivritepe H, Eris A (2003) The effects of NaCl priming on salt tolerance in melon seedlings grown under saline conditions. Sci Hortic 97:229–237
Srinivasan K, Saxena S (2001) Priming seeds for improved viability and storability in Raphanus sativus cv. Chinese pink. Indian J Plant Physiol 6:271–274
Stevens J, Senaratna T, Sivasithamparam K (2006) Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): associated changes in gas exchange, water relations and membrane stabilisation. Plant Growth Reg 49:77–83
Taiz L, Zeiger E (2002) Plant Physiology (2002). Sinauer Associates Inc., Massachusetts
Wu H, Shabala L, Barry K, Zhou M, Shabala S (2013) Ability of leaf mesophyll to retain potassium correlates with salinity tolerance in wheat and barley. Physiol Plant 149:515–727
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Wang.
Rights and permissions
About this article
Cite this article
Islam, F., Yasmeen, T., Ali, S. et al. Priming-induced antioxidative responses in two wheat cultivars under saline stress. Acta Physiol Plant 37, 153 (2015). https://doi.org/10.1007/s11738-015-1897-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1897-5