Abstract
Salinity is a major factor negatively affecting plant growth and agricultural productivity. To gain a better insight into Basella alba responses to different salt stress, some physiological parameters were investigated on this species after 15-day exposure to 200 mM NaCl or 100 mM Na2SO4 stress. Plant growth was significantly suppressed under salinity and a more pronounced impairment induced by NaCl instead of Na2SO4 was observed. A high level of water content was maintained in salt-treated shoot. Salinity stress caused marked increase in Na+, Ca2+, Cl− and SO4 2− concentrations and decrease in K+ level and K+/Na+, Ca2+/Na+ and Mg2+/Na+ ratios in plants. The absorptive abilities of K+, Ca2+ and Mg2+ in plants were improved significantly under salinity. Plants suffered a deeper oxidative stress in the presence of NaCl than Na2SO4 as evidenced by the higher increase in foliar superoxide anions (O ·−2 ) and malondialdehyde (MDA) production as well as electrolyte leakage. No salt-induced alterations were observed on foliar hydrogen peroxide (H2O2) level. B. alba responded to the oxidative stress by enhancing antioxidant capacity involving ascorbate, reduced glutathione as well as antioxidant enzymes. Superoxide dismutase (SOD), ascorbate peroxidase (APX) and glutathione reductase (GR) were all involved in the detoxification of reactive oxygen species (ROS) in plants exposed to salt stress, whereas catalase (CAT) only functioned in the Na2SO4-treated plants. The ability of water maintenance in shoot and improvement of cation absorbability as well as enhanced foliar antioxidant capability all contribute to the salt adaptation of B. alba, whereas a more efficient cation transport system and antioxidant mechanisms may be responsible for the better acclimation of this species to Na2SO4 than NaCl.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity imposes a severe threat on plant growth and global food security. In saline conditions, soil salinity is normally a mixture of different salts where NaCl and Na2SO4 often dominate (Devinar et al. 2013). High concentrations of Na+ and Cl− impose osmotic stress, ionic toxicity, nutritional stress and oxidative damage on the plants, which lead to a series of metabolic disorders. Till now, much of the research concerning salt tolerance of plant species has been based on experiments in which NaCl is the predominant salt, whereas Na2SO4 has received little attention (Colla et al. 2012). In normal conditions, sulfur toxicity is rare, but can occur in saline soils with high levels of sulfate salts (Maathuis 2009) due to accumulation of toxic metabolites in plant tissues (Shevyakova 1981).
It is indicated that chloride and sulfate types of salinities differ in their effects on growth, development and other physiological processes of the plant (Reinoso et al. 2005). Chloride salt inhibited plant yield, shoot and root biomass production more than sulfate salt in cucumber (Colla et al. 2012). With salinity level at the same equimolar concentration, sulfate is less toxic to jack pine (Franklin et al. 2002), pea (Hamdia and Shaddad 1996) and lucerne (Medicago sativa L.) (Rogers et al. 1998). On the contrary, growth of Cornus stolonifera Michx (Renault et al. 2001), Ocimum basilicum (Tarchoune et al. 2010) and Prosopis strombulifera (Devinar et al. 2013) decreased strongly in plants treated with Na2SO4 than in plants treated with NaCl of the same molar concentration.
Basella alba L., a member of the Basellaceae family, is extensively cultivated as an annual ornamental, edible or important medicinal plants in the tropics. The fruit of B. alba had been identified as a rich source of betalains (Lin et al. 2010), which is a good antioxidant component and is well correlated with plant salt tolerance (Chauhan et al. 2013). In fact, B. alba had been listed as a halophyte species in the Haloph database (Aronson 1989). Despite that a large body of work has been carried out on this species for its medicinal purposes (Dong et al. 2011), few information exists on its salt tolerance.
Because different salts occur in soils worldwide, and the plant species B. alba is important for multiple purposes and its good antioxidant traits, we conducted this study to know in more detail how this plant responds and adapts to saline conditions. We hypothesized that B. alba plants may respond differently to NaCl and Na2SO4 in growth and other physiological processes. The biomass production, inorganic ions uptake, membrane permeability as well as antioxidant defense capabilities in B. alba were investigated under NaCl or Na2SO4 stress in terms of equimolar concentrations of Na+.
Materials and methods
Plant material and salinity treatments
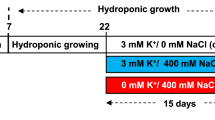
Seeds of B. alba were allowed to germinate in trays and irrigated daily with distilled water. After germination, 10-day-old seedlings were transplanted to plastic pots filled with sand. The seedlings were grown in a plant incubator under controlled environmental conditions with a relative humidity of 60–80 %, temperature 30/20 °C (day/night) and 14/10 h photoperiod at 480 μmol m−2 s−1. Plants were arranged in a randomized block design with three replicates per treatment, which consisted of 45 single plant pots (15 pots per replicate). The seedlings received half-strength Hoagland solution (Hoagland and Arnon 1950) which was renewed every 2 days. Twenty-five days after transplanting, salinity stress was imposed by adding NaCl or Na2SO4 at the 200 mM Na+ level, which is equivalent to 200 mM NaCl or 100 mM Na2SO4, respectively. Nutrient solution without salt addition served as the control. Plants were harvested after 15 days of salt treatments.
Measurements of growth parameters
Twelve plants were randomly selected for growth measurements. At harvest, plants were immediately washed with distilled water and separated into shoots and roots to determine the fresh weights. Subsequently, shoots and roots were dried in an aerated oven at 75 °C to constant weight. The dry weights of roots and shoots were recorded and prepared for inorganic ion content analysis. The water content (WC, %) of the plant was calculated with the following formula: WC (%) = (FW − DW) × 100/FW.
Analysis of inorganic ions and calculation of ion selectivity ratio of absorption and translocation
Dried plant tissues (roots and shoots) were ground to a fine powder for the determination of K+, Na+, Ca2+, Mg2+, Cl− and SO4 2−. A 50 mg sample of root or shoot was ashed in a muffle stove, then the ash was dissolved in concentrated nitric acid and diluted to 100 mL with distilled water (Zheng et al. 2009). The concentrations of K+ and Na+ were determined with a digital flame photometer (Cole-Parmer Instrument Company Model 2655-00, Chicago), while Ca2+ and Mg2+ concentrations were measured using an atomic-absorption spectrometer (Hitachi Z-5000, Japan). Analysis of Cl− and SO4 2− contents was performed according to the method of Kalbasi and Tabatabai (1985) with some modifications. A sample (50 mg) of oven-dried plant material was extracted with 20 ml deionized water in boiling water for 1.5 h. After cooling, the extraction was filtered and then added with deionized water to 50 ml. Cl− and SO4 2− were determined using an ion chromatograph (DX-120 ion chromatograph, DIONEX).
The selectivity ratio of absorption (AS) and translocation (TS) for K+, Ca2+ and Mg2+ over Na+ was calculated according to the formula of Flower and Yeo (1988) with some modifications: AS [X/Na] = ([X+]/[X+ + Na+]) whole plant/([X+]/[X+ + Na+]) medium, TS [X/Na] = ([X+]/[X+ + Na+]) shoot/([X+]/[X+ + Na+]) root, where [X+] represents K+, Ca2+ or Mg2+ in concentration of mmol g−1 DW.
Analysis of electrolyte leakage, malondialdehyde (MDA), O ·−2 and H2O2
The foliar electrolyte leakage was estimated as described by Dionisio-Sese and Tobita (1998). Six randomly chosen plants per treatment (one mature leaf per plant) were taken and cut into 1-cm segments which were then placed in individual test tube containing 25 ml distilled water. The tubes were incubated in a water bath at 25 °C for 2 h, and the initial electrical conductivity (EC1) of the solution was measured with a DDS-11A conductivity meter (Hongyi Company, Shanghai, China). The same samples were then autoclaved at 120 °C for 20 min and a second reading of the EC (EC2) was recorded after cooling the solution to room temperature (25 °C). Electrolyte leakage was calculated as EC1/EC2 and expressed as percentage.
The degree of membrane lipid peroxidation can be reflected by malondiadehyde (MDA) content, which was determined with the method of Madhava Rao and Sresty (2000). MDA was extracted from fresh leaf samples with 0.1 % trichloroacetic acid and calculated using an extinction coefficient of 155 mM−1 cm−1.
The H2O2 content was measured with the colorimetric method at 415 nm (Murkherje and Choudhuri 1983) and the result expressed as µmol g−1.
The reduction of nitroblue tetrazolium (NBT) method was adopted to determine the O ·−2 level in the leaf sample (Kubis 2008). The absorbance was measured at 580 nm and the O ·−2 content was defined as nmol g−1 protein.
Extraction and determination of antioxidant enzymes
All manipulations were performed under ice bath conditions. Fresh leaves (0.5000 g) were ground to fine powders with liquid nitrogen and extracted with prechilled 50 mM phosphate buffer (pH7.8), containing 1 mM EDTA-Na2 and 1 % polyvinylpyrrolidone (PVP). The homogenates were centrifuged at 10,000×g for 20 min at 4 °C and the supernatants were prepared for the determination of protein content and enzyme activities. Total soluble protein contents in the crude extracts were measured according to the method of Bradford (1976) employing bovine serum albumin as a standard. Five replicates per treatment were performed for analysis of antioxidant enzymes.
The superoxide dismutase (SOD) activity was assayed with the nitroblue tetrazolium (NBT) method (Dionisio-Sese and Tobita 1998). A final volume of 3 ml reaction mixture contained 50 mM potassium phosphate buffer (pH 7.8), 13 mM l-methionine, 75 µM nitroblue tetrazolium (NBT), 0.1 mM EDTA-Na2, 2 µM riboflavin and 0.1 ml of the enzyme extract. To start the reaction, the glass test tubes were exposed to 300 µmol m−2 s−1 light intensity at 25 °C for 20 min and the absorbance was measured at 560 nm. One unit of SOD was defined as the amount of enzyme that induced 50 % inhibition in the photochemical reduction of NBT. The SOD activity was expressed as the SOD units min−1 mg−1 protein.
Peroxidase (POD) activity was measured based on the guaiacol oxidation method (Polle et al. 1994). The reaction solution in a total volume of 3 ml consisted of 50 mM phosphate buffer (pH 7.0), 20 mM guaiacol, 10 mM H2O2 and 0.15 ml crude enzyme. The increase of absorbance was measured at 470 nm. A unit POD activity was defined as the POD units min−1 mg−1 protein.
The activity of catalase (CAT) was determined by monitoring the degradation of H2O2 at 240 nm based on the method of Aebi (1984) with some modifications. The assay mixture (3 ml) consisted of 50 mM phosphate buffer (pH 7.0), 15 mM H2O2 and 0.1 ml enzyme extract. One enzyme unit was recorded as CAT units min−1 mg−1 protein.
The determination of APX activity depends on the decrease in absorbance at 290 nm during the process of ascorbate oxidation. According to the method described by Nakano and Asada (1981), the assay medium of 3 ml contained 50 mM phosphate buffer (pH 7.0), 0.5 mM ascorbate, 0.1 mM EDTA and 1.2 mM H2O2. The enzyme activity was expressed as APX units min−1 mg−1 protein.
Measurement of glutathione reductase (GR) activity was conducted following the modified method of Foyer and Halliwell (1976). The reaction system (1 ml) contained 100 mM phosphate buffer (pH 7.5), 1 mM EDTA, 0.5 mM oxidized glutathione (GSSG), 0.12 mM NADPH-Na4 and 0.1 ml crude enzyme. The decrease of absorbance was determined at 340 nm. One unit of GR was defined as GR units min−1 mg−1 protein.
Measurement of nonenzymatic antioxidants
Ascorbate (ASA) and dehydroascorbate (DHA)
2 g of fresh leaf samples was extracted in 4 ml of 5 % (w/v) trichloroacetic acid in an ice bath followed by centrifugation at 10,000×g for 30 min at 4 °C. The supernatant was used for determination of ascorbate and total ASA (ASA + DHA) content according to the modified method of Wang et al. (1991). For ASA analysis, a final reaction mixture of 2.5 ml contained 0.5 ml absolute ethanol, 0.06 M trichloroacetic acid, 3 mM bathophenanthroline, 4 mM H3PO4, 0.18 mM FeCl3 and 0.5 ml of the sample extract. The mixture was allowed to react for 60 min under 30 °C and the produced red-colored solution was assayed at 534 nm. As for total ASA determination, the reaction mixture contained 0.5 ml of the extracted supernatant, 0.75 ml 3.89 mM dithiothreitol and 1.75 ml of absolute ethanol in a total volume of 3 ml. The reaction solution was placed in room temperature for 10 min to allow the reduction of DHA to ASA. After DHA was fully reduced, the following reagents were added in the sequence: 1 ml of 20 % trichloroacetic acid, 0.75 ml of 0.4 % (v/v) H3PO4–ethanol, 1.5 ml of 0.5 % (w/v) bathophenanthroline–ethanol and 0.75 ml of 0.03 % (w/v) FeCl3–ethanol. The final reaction volume was 7.0 ml which was then incubated at 30 °C for 60 min and the absorbance was read at 534 nm. The content of DHA was calculated from the difference of the total ASA and ASA. The results were expressed as μmol g−1 of fresh weight (FW).
Reduced and oxidized glutathione
Measurements of the reduced glutathione (GSH) and oxidized glutathione (GSSG) were carried out with the modified method of Smith (1985) as reported by Akbari et al. (2011). Leaf samples (0.5 g) were homogenized in 5 ml of 5 % (w/v) sulfosalicylic acid in an ice bath followed by centrifugation at 10,000×g for 30 min at 4 °C. The supernatant (1 ml) was taken and neutralized by adding 1.5 ml of 0.5 M potassium phosphate buffer (pH 7.5), which was prepared for the determination of total glutathione. An additional sample (1 ml) of neutralized supernatant was added to 0.2 ml of 2-vinylpyridine and incubated for 90 min at 25 °C to determine the GSSG. Both samples were extracted twice with 5 ml diethylether. The incubated solution consisted of the following components: 0.5 ml of 0.1 M sodium phosphate buffer (pH 7.5) containing 5 mM EDTA, 0.2 ml of 6 mM 5, 5′-dithiobis-(2-nitrobenzoic acid), 0.1 ml of 2 mM NADPH, 0.1 ml glutathione reductase type III (sigma) and 0.1 ml extract. The change in the absorbance at 412 nm was measured at 25 °C. The amount of GSH was calculated by subtracting GSSG from total glutathione concentrations. A standard curve was prepared from varying concentrations of GSH.
Statistical analysis
All data were presented as mean value ± SD. One-way analysis of variance (ANOVA) was applied to examine the salinity effects on each parameter using the SAS 9.2 statistical software package. Significant differences between means were determined by Fisher’s least-significant difference test (LSD) at P ≤ 0.05.
Results
Biomass and water content
All plants survived after a 15-day duration of 200 mM NaCl or 100 mM Na2SO4 stress. Growth of plants was significantly impaired under salt treatments and a more pronounced inhibition induced by NaCl instead of Na2SO4 was observed (Table 1). As compared to the control, NaCl decreased the shoot weight by 70 and 57 % on fresh and dry basis, whereas the weight reduction in shoot induced by Na2SO4 was 43 and 16 %, respectively. Similarly, NaCl stress caused 75 and 55 % decrease of root biomass (based on fresh and dry weight), while 29 and 9 % reductions were recorded in Na2SO4 treatments when compared to their controls. The root water content showed significant reduction of 16 and 8 % under NaCl and Na2SO4 treatments, respectively (Table 1). However, shoot water content maintained 93 % of fresh weight in salinity treatments, although significant reduction relative to the control was observed (Table 1).
Ion uptake and transportation
The K+ content in the plants decreased significantly under salt treatments, and the reductions of 44 and 40 % in root and 59 and 56 % in shoot were recorded in the treatment of NaCl and Na2SO4, compared to the control (Fig. 1). Conversely, salt stress caused remarkable increase in Na+ content in plant tissues (Fig. 1). As compared to the control, the content of Na+ was increased to 7.2 and 18.6 times in root and shoot with the treatment of NaCl, whereas the increment of Na+ in root and shoot was 7.4 and 14.7 times under Na2SO4 stress. A higher Na+ content in shoot of NaCl treatment than the Na2SO4 treatment was observed (Fig. 1). The Ca2+ content in shoot showed significant increase of 143 and 86 % in the treatment of NaCl and Na2SO4, respectively (Fig. 1), whereas no significant difference was observed for Ca2+ content in root, irrespective of the treatment. The salinity of Na2SO4 decreased the Mg2+ concentration both in root and shoot, while NaCl showed no inhibition on Mg2+ content compared to the control group (Fig. 1). The Cl− content both in shoot and root increased significantly under NaCl stress (Fig. 1), which was 4.78 and 4.95 times that of control. Similarly, a considerable amount of SO4 2− in plants treated with Na2SO4 stress was observed (Fig. 1). The SO4 2− level in the shoot and root was 5.08 and 3.47 times that of control. As compared to the control, plant Cl− content was not affected by Na2SO4 stress and plant SO4 2− level was also unaffected by NaCl stress. A higher content of inorganic ions (K+, Na+, Ca2+, Mg2+, Cl− and SO4 2−) in shoot than in root was recorded irrespective of treatment.
Concentrations of inorganic ions in shoot (a) and root (b) of B. alba under salt treatments. Plants cultured in sand were irrigated with half-strength Hoagland solution with or without additional salt (200 mM NaCl or 100 mM Na2SO4) for 15 days (see details in “Materials and methods”). Six plants in each treatment were selected for salt ion analysis. The data in the figure represent mean ± SD. Different letters in the same cluster indicate statistical difference according to Fisher’s least-significant difference test (P ≤ 0.05)
The ratios of K+/Na+, Ca2+/Na+ and Mg2+/Na+ both in roots and shoots were reduced considerably by two salinities (Table 2). Particularly, the ratio of K+/Na+ decreased to 2 and 3 % of the control in shoot and 7 and 8 % of the control in root on treatment with NaCl and Na2SO4, respectively.
Salinity stress significantly increased the AS ratio of K+, Ca2+ and Mg2+ over Na+ (Fig. 2). A stronger selective absorption capability of K+ over Na+ in plants treated with Na2SO4 than NaCl was recorded, whereas, for AS ratio of Mg2+ over Na+, the higher selective ability was found in NaCl-treated plant than in Na2SO4-treated one. There was no significant difference for AS ratio of Ca2+ over Na+ between treatment of NaCl and Na2SO4.
The selectivity ratio of absorption (a) and translocation (b) for K+, Ca2+ and Mg2+ over Na+ in B. alba under salt treatments. Plants cultured in sand were irrigated with half-strength Hoagland solution with or without additional salt (200 mM NaCl or 100 mM Na2SO4) for 15 days (see details in “Materials and methods”). Six plants in each treatment were selected for salt ion analysis. The data in the figure represent mean ± SD. Different letters in the same cluster indicate statistical difference according to Fisher’s least-significant difference test (P ≤ 0.05)
The TS ratio for K+ and Mg2+ over Na+ decreased significantly under NaCl stress (Fig. 2). It showed no significant difference for the TS ratio of K+ and Mg2+ over Na+ between control and Na2SO4 treatment. The TS ratio of Ca2+ over Na+ for translocation was not affected by NaCl or Na2SO4 salinity.
Lipid peroxidation, electronic leakage, hydrogen peroxide and active oxygen
The foliar MDA content increased significantly in plants treated with NaCl, while no significant difference was observed in plants with Na2SO4 treatment when compared with the control (Table 3). The foliar electrolyte leakage increased significantly on treatment with two types of salinity (Table 3). However, a higher value of electrolyte leakage was recorded in NaCl stress than with Na2SO4 stress. In contrast, H2O2 concentration was not affected by NaCl or Na2SO4 stress in comparison with the control (Table 3). The considerable increase of 290 and 141 % in O ·−2 content was observed under treatment of NaCl and Na2SO4, respectively (Table 3).
Enzyme activities
Salinity led to significant increase of SOD activity compared to the control (Fig. 3), which was more remarkable in the presence of Na2SO4 (70 %) than in the presence of NaCl (33 %). In comparison with the control, the activity of POD exhibited a significant decrease of 51 % in the NaCl medium (Fig. 3), whereas no significant difference of POD was observed for plants treated with Na2SO4. A marked increase of 40 % in CAT activity was induced by Na2SO4 stress (Fig. 3); however, no significant change in CAT was measured in the NaCl treatment relative to that of the control. For APX (Fig. 3), remarkable increase either in the medium of NaCl (32 %) or Na2SO4 (80 %) was shown compared to the control. Similarly, GR activity also showed increase in plants exposed to salinity stress (Fig. 3).
Activities of SOD, POD, CAT, GR and APX in leaves of B. alba under salt treatments. Plants cultured in sand were irrigated with half-strength Hoagland solution with or without additional salt (200 mM NaCl or 100 mM Na2SO4) for 15 days (see details in “Materials and methods”). The fresh leaves were harvested from five plants in each treatment for analysis of enzyme (SOD, POD, CAT, GR and APX) activities. The data in the figure represent mean ± SD. Different letters in the same cluster indicate statistical difference according to Fisher’s least-significant difference test (P ≤ 0.05)
Nonenzymatic antioxidants
The foliar ASA content appeared to be 38 and 73 % higher in plants treated with NaCl and Na2SO4 (Fig. 4), respectively, when compared with the control. Similarly, a marked increase of total ascorbate (ASA + DHA) level was also recorded under the salinity treatments (Fig. 4). Salt treatment increased the foliar total glutathione (GSH + GSSG) level markedly (Fig. 4). However, the GSH content showed significant increase of 42 % in Na2SO4 treatment and no significant change in GSH content was observed in NaCl treatment as compared to the control (Fig. 4). The ratio of ASA/DHA and GSH/GSSG both decreased significantly in the presence of salinity; moreover, a more pronounced reduction in the GSH/GSSG ratio was observed with NaCl stress than with Na2SO4 stress (Fig. 4).
Contents of ASA, total ascorbate (ASA + DHA), GSH, total glutathione (GSH + GSSG) and ratios of ASA/DHA and GSH/GSSG in leaves of B. alba under salt treatments. Plants cultured in sand were irrigated with half-strength Hoagland solution with or without additional salt (200 mM NaCl or 100 mM Na2SO4) for 15 days before being sampled for determinations of antioxidant molecules. The fresh leaves were collected from five plants in each treatment. Data in the figure represent mean ± SD. Different letters in the same cluster indicate statistical difference according to Fisher’s least-significant difference test (P ≤ 0.05)
Discussion
A number of plant species showed high salt tolerance traits, such as Cynara cardunculus (Benlloch-González et al. 2005), Gypsophila oblanceolata (Sekmen et al. 2012), Plantago crassifolia (Vicente et al. 2004) and Centaurea tuzgoluensis (Yıldıztugay et al. 2011). Aronson (1989) defined B. alba as a halophyte species based on the criterion in plants that survive to complete their life cycles in at least 80 mM NaCl. In the present study, B. alba survived under 200 mM NaCl or 100 mM Na2SO4 stress conditions with low growth rate, indicating it to be a salt-tolerant species. However, significant growth suppressions were observed in plants under salinity stress (Table 1), which could be partly due to osmotic stress. The results showed that the water content of salinity-stressed plants decreased significantly as compared to that in control plants implying an osmotic effect. In addition, a lower root water content in NaCl treatment than that of Na2SO4 can be explained by a difference in osmotic potential in growing medium (Table 1), as the osmotic potential of 200 mM NaCl was lower than that of 100 mM Na2SO4. There were no significant differences for shoot water content between the two salinity treatments (Table 1). Especially, the succulent shoot was able to maintain a water content of about 93 % of fresh weight under salinity (Table 1), which may contribute to the salt tolerance of this species through diluting the toxic effect of accumulated salt ions (Vicente et al. 2004).
Under saline conditions, high concentrations of Na+, Cl− or SO4 2− could produce a high uptake of these ions, showing that B. alba was unable to prevent the transport of these salt ions from the root to the shoot (Fig. 1). Growth reduction induced by salinity stress (Table 1) was likely associated with the toxicity of Na+, Cl− and/or SO4 2− that accumulated in plant tissues. Particularly, B. alba accumulated 2.79 and 2.21 mmol g−1 Na+ after 15 days of treatment with 200 mM NaCl or 100 mM Na2SO4 (Fig. 1), a higher Na+ accumulating level compared to that of other halophyte species such as Plantago crassifolia (Vicente et al. 2004) and Centaurea tuzgoluensis (Yıldıztugay et al. 2011). It was found that Na+ content was higher in the shoots stressed by NaCl than that stressed by Na2SO4 at equimolar Na+ (Fig. 1). Similar observations were reported in dogwood (Renault et al. 2001) and Gleditsia tricanthos (Dirr 1974). The data suggested that Cl− may affect Na+ uptake by altering plasma membrane lipid composition and thus changes in membrane permeability, according to the membrane response to chloride reported by Kuiper (1968). As compared with Na+, Cl− was reported to be more injurious in some cases (Kalaji and Pietkiewicz 1993). Xu et al. (2000) suggested that the normally nontoxic Cl− level in plants range from 1 to 20 mg g−1, being equivalent to 0.03 to 0.56 mmol g−1. The Cl− content in shoot and root of NaCl treatment was 1.85 and 0.61 mmol g−1 (Fig. 1), respectively, which was probably reaching a toxic level in plant tissues especially in the shoots, whereas the Cl− levels in plants, both of control (0.12–0.39 mmol g−1) and Na2SO4 treatment (0.19–0.39 mmol g−1), were within the normal range. The requirement of sulfur for plant optimal growth varies between 0.1 and 0.5 % of the plant dry weight (Marschner 1995). In 100 mM Na2SO4-stressed plants (Fig. 1), the content of SO4 2− was 0.33 mmol g−1 in shoot and 0.06 mmol g−1 in root, being equivalent to a sulfur content of 1.04 % in shoot and 0.19 % in the root, respectively. It was indicated that shoot SO4 2− level exceeded the normal requirement of plant optimal growth. However, it could not be determined whether the shoot suffered toxic effect under 100 mM SO4 2− stress. According to Shevyakova (1981), the excess of SO4 2− induces the synthesis of useful secondary-type S-metabolites such as 3′-phosphoadenosine 5′-phosphosulphate (PAPS) which is responsible for the survival of plants under sulfate salinity. Besides, some toxic metabolites can be degraded to the inorganic sulfate in the medium of moderately high sulfate ion concentration (50–100 mM Na2SO4) (Shevyakova 1981). In the current study, plant growth responded differently to NaCl and Na2SO4 stress (Table 1), which appeared to be species specific as observed in previous studies (Franklin et al. 2002; Colla et al. 2012; Devinar et al. 2013). It was found that Cl− posed a greater interfering effect on plant development and water balance than SO4 2− (Rogers et al. 1998). The greater biomass reduction in NaCl than in Na2SO4 was probably caused by higher Na+ content in the shoot as well as the higher toxicity of Cl− than SO4 2−.
As reported in previous studies concerning other plant species (Renault et al. 2001; Pagter et al. 2009), tissue concentrations of cationic nutrients were generally reduced in salt-treated plants. Differing from the K+ content in the plant, the root Ca2+ remained constant and shoot Ca2+ increased significantly in response to salt stress (Fig. 1). Similar results were found in jack pine (Franklin et al. 2002), Vica faba (Gadallah 1999) and Plantago crassifolia (Vicente et al. 2004). Our data indicate that the presence of Cl− or SO4 2− may impede the ability of the plant to restrict the movement of Ca2+, supporting the fact that anions play an important role in the absorption and transportation of Ca2+ (Fouldrin and Limami 1993; Halperin et al. 1997). The higher reduction of Mg2+ either in shoot or root treated with Na2SO4 than that with NaCl may be attributed to the antagonistic action between Mg2+ and SO4 2− rather than Cl− (Grattan and Grieve, 1999). Similar results were also observed in dogwood (Renault et al. 2001).
High salt uptake disrupts ionic homeostasis and leads to nutrient deficiency. The decreased ratios of K+/Na+, Ca2+/Na+ and Mg2+/Na+ (Table 2) in plant were largely due to the excessive accumulation of Na+. As a result, the Na+-induced K+ deficiency and ion imbalance could be a major factor that resulted in growth inhibition. To alleviate the specific ion toxicity induced by Na+, B. alba developed strong selectivity for K+, Ca2+ and Mg2+ absorption under salinity stress (Fig. 2). Moreover, the normal selective translocation ability for different cations over Na+ was maintained in plants with Na2SO4 treatment (Fig. 2). The results indicated that a more efficient transport system for K+, Ca2+ and Mg2+ existed in Na2SO4-stressed plants than in NaCl-stressed plants.
Lipid peroxidation and the consequent increase in MDA levels are frequently reported in studies related to salinity toxicity (Panda and Khan 2009; Sekmen et al. 2012). The observed increase in MDA level and EL ratio (Table 2) in this study suggested that lipid peroxidation occurred as a result of ROS formation induced by NaCl or Na2SO4 stress. The rate of O ·−2 increased significantly under salinity whereas H2O2 remained stable irrespective of the treatment (Table 3), indicating that oxidative damage occurred in the O ·−2 burst stage before H2O2 generation. In accordance with the observations on growth parameters (Table 1), NaCl induced a higher production of O ·−2 and lipid peroxidation rate measured as MDA level and EL ratio compared to samples treated with Na2SO4 (Table 2).
SOD, in particular, constitutes the first line of defense against oxidative damage by catalyzing the detoxification of O ·−2 to H2O2 and O2 in chloroplast, mitochondrion and other organelles (Gill and Tuteja 2010). The enhanced activity of this enzyme under salinity implies that this species had efficient capacity for the removal of O ·−2 radicals, thus reducing its oxidative damage and cellular toxicity (Sabra et al. 2012). A more efficient O ·−2 scavenging ability was recorded in Na2SO4-stressed plants than in NaCl-stressed ones (Fig. 3). As the product of SOD activity, H2O2 is then eliminated by two groups of enzymes: catalases and peroxidases. CAT is suggested to be involved in mass scavenging of H2O2 whereas POD, APX and GR are suggested to be involved in fine regulation of H2O2 (Abogadallah 2010). Particularly, APX plays a key role in scavenging H2O2 through the ascorbate–glutathione cycle in the cytosol and chloroplast. The increased activity in APX was associated with the tolerance to oxidative stress in various plant species (Amor et al. 2006; Yıldıztugay et al. 2011). In the current study, the activities of APX and GR were induced by salinity in contrast to POD that was inhibited (Fig. 3). CAT activity increased in plants treated with Na2SO4, whereas it remained unchanged in plants treated with NaCl (Fig. 3). Our data indicated that a co-operation is activated between CAT, APX and GR for establishing a proper H2O2 or other ROS in plants exposed to Na2SO4 stress. In contrast, in plants treated with NaCl, the removal of H2O2 mainly depended on the function of APX and GR. The low basal rate and decreased POD activity in this species seem to indicate that this enzyme is not crucial in detoxification of ROS damage under salt stress. Additionally, it would be interesting to link the antioxidant capacity of B. alba with its Ca2+ status. Calcium has been suggested to increase the activities of antioxidant enzymes and reduce cell membrane lipid peroxidation, likely in relation with its cytosolic contents and its role in signal transduction (Amor et al. 2005). Overall, the detoxification of H2O2 is extremely important in regulating the levels of other ROS within plant systems (Hodges and Forney 2001), and the low level of H2O2 may act as a signal molecule involved in acclimatory signal triggering tolerance to salinity stress (Gill and Tuteja 2010).
Scavenging of ROS depends on both enzymatic and nonenzymatic components. In the ASA–GSH cycle, APX catalyzes the detoxification of H2O2 to water in the first step by using ASA as a reductant (Tarchoune et al. 2010). The increased foliar ASA and ASA + DHA level observed under salinity treatments (Fig. 4) indicated that ASA synthesis was stimulated or ASA catabolism was inhibited. The ASA or ASA + DHA in two types of salinity-stressed plants had no significant difference (Fig. 4). More important than the total ascorbate is the ASA/DHA ratio, which is considered as an index of the cell redox status and one of the first signs of oxidative stress. Significant reduction in ASA/DHA ratio in salt-stressed plants (Fig. 4) might be an indication of APX participation in ROS detoxification (Fig. 3). This is because sufficient ASA concentration is important for maintaining APX activity, which can be inactivated when ASA level drops down (Asada 1999).
Glutathione (GSH) is involved in both the direct and the indirect control of ROS concentrations. The protective and regulatory roles of GSH are based on changes in its redox state and the half-cell reduction potential of the GSH/GSSG couple (Szalai et al. 2009). In the leaves of plants grown in NaCl medium, the GSH level (Fig. 4) was not induced, although the GR activity (Fig. 3) and total glutathione (Fig. 4) increased, indicating that the reduction of GSSG by GR was not sufficient to counterbalance GSH oxidation (Fig. 3). In contrast, the GSH content increased markedly in leaves of the Na2SO4-treated plants (Fig. 4), which was mainly attributed to the function of sulfate as the synthesis of GSH is regulated by the sulfur-assimilatory pathway (Fatma et al. 2013). According to Fatma et al. (2014), excess sulfur supplementation improved salt tolerance of mustard through increased production of glutathione. The decrease of GSH/GSSG ratio in leaves of plants treated with NaCl or Na2SO4 could be due to GSH degradation. A higher GSH level and GSH/GSSG ratio in Na2SO4-stressed plant than in NaCl-treated plant indicates once more that B. alba reacted better to Na2SO4 than to NaCl.
Conclusions
The current study showed that B. alba was tolerant both to NaCl and Na2SO4 stress. Growth suppression was observed due to osmotic and ion-specific effect as well as oxidative damage induced by salinity, with NaCl being more toxic than Na2SO4. Several defensive strategies including the ability of water maintenance in succulent shoot, increased absorptive capability for cations (K+, Ca2+ and Mg2+) as well as the enhancement of enzymatic and nonenzymatic components all contributed to the salt adaptation of B. alba, allowing it to withstand high salinity conditions. In general, a more efficient cation transport system and ROS scavenging capacity and protection mechanism may be responsible for the better adaptation of this species to Na2SO4 than NaCl.
Author contribution statement
Jianfeng Ning designed the research and wrote the manuscript. Shaoying Ai helped to design and revise the manuscript and provide analytical instrument. Shaohai Yang contributed analysis tools. Lihua Cui helped to design the experiments and contributed some reagents/materials. Yong Chen and Lili Sun performed the experiments. Ronghui Wang and Mengjun Li contributed to the data analysis, Zhaobing Zeng helped to compile the manuscript. All authors have read and approved the final manuscript.
References
Abogadallah GM (2010) Antioxidative defense under salt stress. Plant Signal Behav 5:369–374
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Akbari GA, Hojati M, Modarres-Sanavy SAM, Ghanati F (2011) Exogenously applied hexaconazole ameliorates salinity stress by inducing an antioxidant defense system in Brassica napus L. plants. Pestic Biochem Physiol 100:244–250
Amor NB, Hamed KB, Debez A, Grignon C, Abdelly C (2005) Physiological and antioxidant responses of the perennial halophyte Crithmum maritimum to salinity. Plant Sci 168:889–899
Amor NB, Jimenez A, Megdiche W, Lundqvist M, Sevilla F, Abdelly C (2006) Response of antioxidant systems to NaCl stress in the halophyte Cakile maritima. Physiol Plant 126:446–457
Aronson JA (1989) Haloph: a data base of salt tolerant plants of the world. Office of Arid Land Studies, University of Arizona, Tucson
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Ann Rev Plant Physiol Mol Bio 50:601–639
Benlloch-González M, Fournier JM, Ramos J, Benlloch M (2005) Strategies underlying salt tolerance in halophytes are present in Cynara cardunculus. Plant Sci 168:653–659
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chauhan SP, Sheth NR, Rathod IS, Suhagia BN, Maradia RB (2013) Analysis of betalains from fruits of Opuntia species. Phytochem Rev 12:35–45
Colla G, Rouphael Y, Rea E, Cardarelli M (2012) Grafting cucumber plants enhance tolerance to NaCl and sulfate salinization. Sci Hortic Amst 135:177–185
Devinar G, Llanes A, Masciarelli O, Luna V (2013) Different relative humidity conditions combined with chloride and sulfate salinity treatments modify abscisic acid and salicylic acid levels in the halophyte Prosopis strombulifera. Plant Growth Regul 70:247–256
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Dirr MA (1974) Tolerance of honeylocust seedlings to soil-applied salts. Hort Sci 9:53–54
Dong CX, Hayashi K, Mizukoshi Y, Lee JB, Hayashi T (2011) Structures of acidic polysaccharides from Basella rubra L. and their antiviral effects. Carbohydr Polym 84:1084–1092
Fatma M, Khan MIR, Masood A, Khan NA (2013) Coordinate changes in assimilatory sulfate reduction are correlated to salt tolerance: involvement of phytohormones. Annu Rev Res Biol 3:267–295
Fatma M, Asgher M, Masood A, Khan NA (2014) Excess sulfur supplementation improves photosynthesis and growth in mustard under salt stress through increased production of glutathione. Environ Exp Bot 107:55–63
Flower TJ, Yeo AR (1988) Ion relation of salt tolerance. In: Baker DD, Hall JL (eds) Solute transport in cell and tissues. John Wiléy and Sons Inc, New York, pp 392–416
Fouldrin K, Limami A (1993) Calcium (45Ca) mobility in chicory root (Cichorium intybus L.) as affected by the anionic composition of the nutrient solution during forcing. J Amer Soc Hort Sci 118:587–592
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplast: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Franklin JA, Zwiazek JJ, Renault S, Croser C (2002) Growth and elemental composition of jack pine (Pinus banksiana) seedlings treated with sodium chloride and sodium sulfate. Trees 16:325–330
Gadallah MAA (1999) Effects of proline and glycinebetaine on Vicia faba responses to salt stress. Biol Plant 42:249–257
Gill S, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Grattan SR, Grieve CM (1999) Salinity-mineral nutrient relations in horticultural crops. Sci Hort 78:127–157
Halperin SJ, Kochian LV, Lynch JP (1997) Salinity stress inhibits calcium loading into the xylem of excised barley (Hordeum vulgare) roots. New Phytol 135:419–427
Hamdia M, Shaddad MAK (1996) Comparative effect of sodium carbonate, sodium sulphate, and sodium chloride on the growth and related metabolic activities of pea plants. J Plant Nutr 19:717–728
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. California Agricultural Experiment Station. Circular 347:1–32
Hodges DA, Forney CF (2001) Antioxidant responses in harvested leaves of two cultivars of spinach differing in senescence rates. J Amer Soc Hort Sci 126:611–617
Kalaji MH, Pietkiewicz S (1993) Salinity effects on plant growth and other physiological processes. Acta Physiol Plant 15:89–124
Kalbasi M, Tabatabai MA (1985) Simultaneous determination of nitrate, chloride, sulfate, and phosphate in plant materials by ion chromatography. Commun Soil Sci Plant Anal 16:787–800
Kubis J (2008) Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. J Plant Physiol 165:397–406
Kuiper PJC (1968) Lipids in grape roots in relation to chloride transport. Plant Physiol 43:1367–1371
Lin SM, Lin BH, Hsieh WM, Ko HJ, Liu CD, Chen LG, Chiou RY (2010) Structural identification and bioactivities of red-violet pigments present in B. alba fruits. J Agr Food Chem 58:10364–10372
Maathuis FJM (2009) Physiological functions of mineral macronutrients. Curr Opin Plant Biol 12:250–258
Madhava Rao KV, Sresty TVS (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Institute of Plant Nutrition, University of Hohenheim, Academic Press, Germany
Murkherje SP, Choudhuri MA (1983) Implication of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogenperoxide in Vigna seedings. Physiol Plant 58:166–170
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Pagter M, Bragato CM, Alagoli M, Brix H (2009) Osmotic and ionic effects of NaCl and Na2SO4 salinity on Phragmites australis. Aquat Bot 90:43–51
Panda SK, Khan MH (2009) Growth, oxidative damage and antioxidant responses in greengram (Vigna radiata L.) under short-term salinity stress and its recovery. J Agron Crop Sci 195:442–454
Polle A, Otter T, Seifert F (1994) Apoplastic peroxidases and lignification in needles of Norway (Picea abies L.). Plant Physiol 106:53–60
Reinoso H, Sosa L, Reginato M, Luna V (2005) Histological alterations induced by sodium sulfate in the vegetative anatomy of Prosopis strombulifera (Lam.) Benth. World J Agric Sci 2:109–119
Renault S, Croser C, Franklin JA, Zwiazek JJ (2001) Effects of NaCl and Na2SO4 on red-osier dogwood (Cronus stolonifera Michx) seedlings. Plant Soil 233:261–268
Rogers ME, Grieve CM, Shannon MC (1998) The response of lucerne (Medicago sativa L.) to sodium sulphate and chloride salinity. Plant Soil 202:271–280
Sabra A, Daayf F, Renault S (2012) Differential physiological and biochemical responses of three Echinacea species to salinity stress. Sci Hortic Amst 135:23–31
Sekmen AH, Turkan I, Tanyolac ZO, Ozfidan C, Dinc A (2012) Different antioxidant defense responses to salt stress during germination and vegetative stages of endemic halophyte Gypsophila oblanceolata Bark. Environ Exp Bot 77:63–76
Shevyakova NI (1981) Transport and metabolism of sulphate under salt stress. In: Brouwer R, Gašparíková O, Kolek J, Loughman BC (eds) Structure and function of plant roots. Developments in plant and soil sciences, vol 1, pp 351–353
Smith IK (1985) Stimulation of glutathione synthesis in photorespiring plants by catalase inhibitors. Plant Physiol 79:1044–1047
Szalai G, KellŐs T, Galiba G, Kocsy G (2009) Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J Plant Growth Regul 28:66–80
Tarchoune I, Sgherri C, Izzo R, Lachaal M, Ouerghi Z, Navari-Izzo F (2010) Antioxidative responses of Ocimum basilicum to sodium chloride or sodium sulphate salinization. Plant Physiol Biochem 48:772–777
Vicente O, Boscaiu M, Naranjo MÁ, Estrelles E, Bellés JM, Soriano P (2004) Responses to salt stress in the halophyte Plantago crassifolia (Plantaginaceae). J Arid Environ 58:463–481
Wang SY, Jiao HJ, Faust M (1991) Changes in ascorbate, glutathione, and related enzymes activities during thidiazuron-induced bud break of apple. Physiol Plant 82:231–236
Xu G, Magen H, Tarchitzky J, Kafkafi U (2000) Advances in chloride nutrition of plants. Adv Agron 68:97–150
Yıldıztugay E, Sekmen AH, Turkan I, Kucukoduk M (2011) Elucidation of physiological and biochemical mechanisms of an endemic halophyte Centaurea tuzgoluensis under salt stress. Plant Physiol Biochem 49:816–824
Zheng QS, Liu L, Liu ZP, Chen JM, Zhao GM (2009) Comparison of the response of ion distribution in the tissues and cells of the succulent plants Aloe vera and Salicornia europaea to saline stress. J Plant Nutr Soil Sci 172:875–883
Acknowledgments
The authors are very grateful to Dr. Bin Guo (Institute of Soil and Fertilizer, Zhejiang Academy of Agricultural Sciences, Hangzhou, China) for his helpful comments on this manuscript. This work was financially supported by the Science and Technology Planning Project of Guangdong Province (2010B030800009), the Agricultural Science and Technology Planning Project of Guangzhou City (GZCQC1002FG08015) and the project of the Special Fund for Agro-scientific Research in the Public Interest (201003014-2).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by L. A. Kleczkowski.
Shaoying Ai and Lihua Cui contributed equally.
Rights and permissions
About this article
Cite this article
Ning, J., Ai, S., Yang, S. et al. Physiological and antioxidant responses of Basella alba to NaCl or Na2SO4 stress. Acta Physiol Plant 37, 126 (2015). https://doi.org/10.1007/s11738-015-1860-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1860-5