Abstract
In this study, we used poplar as a model plant and investigated the effects of gaseous nitrogen dioxide (NO2, 4 µl 1−1) on stomatal conductance, photosynthesis, dark- and photorespiration of Populus alba × Populus berolinensis hybrid leaves using the photosynthesis system and scanning electron microscope technique. The results showed that net photosynthetic rates were significantly reduced in leaves exposed to 4 µl 1−1 NO2 for 48 h as compared with leaves exposed to ambient carbon dioxide 380 µl 1−1 and ambient NO2 <0.1 µl 1−1 (the control) and the leaves exposed for 14 h. The decline of net photosynthetic rate was caused mainly by NO2 treatment. Dark respiration rates were dependent on co-action of the two factors (leaf temperature and NO2 treatment time). Post-illumination carbon dioxide burst in the exposed leaves occurred at 13–15 s after turning the light off, whereas this phenomenon was absent in the control leaves.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Foliar uptake of NO2 gas mainly occurs through stomata, with a small fraction of cuticular deposition (Rennenberg and Gessler 1999). Stomatal characteristics such as stomatal dimension and conductance (Chaparro-Suarez et al. 2011; Breuninger et al. 2013), epidermis physicochemical properties such as cuticular chemical compositions and water films (Lendzian and Kerstiens 1988; Ramge et al. 1993; Jetter et al. 1996) and related environmental factors (Geβler et al. 2000; Chaparro-Suarez et al. 2006) have influences on foliar NO2 uptake. On the other hand, gaseous NO2, due to its corrosive and highly oxidizing nature, also has impacts on stomatal dynamics and stomata-related physiological and biochemical processes, particularly photosynthesis and dark respiration. The effects of gaseous NO2 on stomatal aperture seem to be species-specific, so while for Ilex rotunda, there was a positive correlation between NO2 concentration and stomatal aperture (Takagi and Gyokusen 2004); for Raphanus sativus, the correlation was negative (Mazarura 2012). Studies on gaseous NO2 effects on leaf photosynthesis and/or respiration process are scarce and mainly focused on herbaceous plants with only a few studies in woody plants (Pallardy 2007). Inhibitory effects of NO2 on photosynthesis and dark respiration have been reported for Phaseolus vulgaris (Srivastava et al. 1974a, b), Glycine max. Merr (Carlson 1983), Vulpia microstachys (Vallano et al. 2012), and Raphanus sativus L. (Mazarura 2012); whereas stimulatory effects of NO2 on dark respiration have been found for Glycine max. Merr. cv. Williams (Sabaratnam et al. 1988) and Lolium multiflorum (Vallano et al. 2012). Oleksyn (1984) showed that in Pinus sylvestris the net photosynthesis, dark respiration, and photorespiration declined after 30 min exposure to gaseous NO2; however, these parameters increased or were unaffected by a longer time of fumigation. The results of Van Hove et al. (1992) showed that NO2 reduced dark respiration rate of Douglas fir, but did not affect net photosynthetic rate as compared with the control. Eller et al. (2011) also reported no significant difference in photosynthetic rates of the sugar maple leaves exposed to 40 nl l−1 NO2 and the control (ambient CO2 365 μl l−1 and ambient NO2 <1 nl l−1).
Populus species are commonly planted along roadside in urban areas of many countries for amenity purposes, and has been reported to have high capacity of absorption of NO2 and resistance to its effects (Okano et al. 1989) when compared with the other broad-leaved tree species. Morikawa et al. (1998) investigating 107 woody plants showed that Populus nigra had the second highest NO2–N assimilation capacity (Morikawa et al. 1998). Takahashi et al. (2005) also found that P. nigra had a higher NO2 assimilation capability when compared to the other roadside trees. The physiological mechanisms of the high tolerance to NO2 are still not well known. Several earlier publications focused on NO2 effects on poplar growth, and found that low concentrations (0.1 μl l−1) of NO2 had no significant influence on height, leaf area and dry weight of 1-year-old yellow-poplar seedlings (Dochinger and Jensen 1985), and 0.5 μl l−1 NO2 had a significant stimulation on leaf growth of Carolina poplar and Lombardy poplar, but a higher concentration (1 μl l−1) significantly decreased the stem growth (Eastham and Ormrod 1986). To date, only limited studies on NO2 effects on photosynthesis or respiration of Populus trees have been conducted. These studies showed that low concentrations (80–135 nl l−1) of NO2 gas had no significant impacts on leaf CO2 assimilation and stomatal conductance of hybrid Populus × euramericana (Siegwolf et al. 2001) and Populus × euramericana ‘Dorskamp’ (Schmutz et al. 1995) poplar clone cuttings. Recently we found exogenous sodium sulfide improves morphological and physiological responses of a hybrid Populus species to nitrogen dioxide (Hu et al. 2014a). In spite of these studies, the relationship between instantaneous plant performance (dark respiration and photorespiration) and foliar NO2 uptake (Sparks 2009), and adaptability of ultrastructure of mesophyll tissue adjacent to stomata and chemical elements composition (such as Nitrogen, Phosphorus, Calcium, and Magnesium) of leaf surface to gaseous NO2 and nitrate transporters of NO2-treated leaves (Hu et al. 2014a, b) are still unknown.
In this study, we assumed that high concentration (4 µl 1−1) of NO2 fumigation has significant impact on epidermis and stomata-related physiochemical characteristics of hybrid poplar leaves; photorespiration plays a potential role in the plant response to gaseous NO2. So we investigated gaseous NO2 effects on stomatal dynamics, photosynthetic and respiratory characteristics of the hybrid poplar clone cuttings and discussed the potential mechanisms of the interactions between populus trees and gaseous NO2.
Materials and methods
Plant materials and growth conditions
Twelve two-year-old seedlings of hybrid poplar clonal cuttings (Populus alba × P. berolinensis) were grown in pots (20 cm in diameter, 30 cm in height) filled with a soil/sand mixture (3:1 v/v) at outdoor conditions. Environmental conditions were monitored over a time period of 4 h (8:30–12:30 am) when measurements were taken. The mean temperature was 30.8 °C (max/min temp of 38.5 °C/22.95 °C); air relative humidity was 65 %; air CO2 concentration was approximately 380 μl L−1 and photosynthetic photon flux density (PPFD) was 1,000–1,900 μmol m−2 s−1. Seedlings were watered using tap water every day.
NO2 fumigation
Open-top glass chambers (0.6 cm × 0.40 cm × 0.80 cm) were used for NO2 fumigation. Stocks of NO2 source were supplied by the Special Gas Products Co., Ltd. (Dalian city, China). NO2 concentration in this study was set to 4 µl l−1, following Morikawa et al. (1998), Takahashi et al. (2005), and Kondo et al. (2008). 4 µl l−1 NO2 was supplied directly from a 40-L cylinder. Mean concentration of NO2 within the chamber was monitored using an NO2 analyzer (MIC-500-NO2, Shenzhen Yiyuntian Electrical Co., Ltd., China). The control was not fumigated by NO2 and the treated seedlings (six replicates) were fumigated by 4 µl l−1 NO2 for averaged 6 h per day. Leaf stomata and physiology (such as net photosynthetic rate, Pn) were measured when the leaves were exposed to NO2 during 48 h.
CO2 exchange rate–time curve
Net photosynthetic rate (P n), dark respiration rate (R dark), transpiration rate (T r), and stomatal conductance (G s) were measured using LI-6400XT Portable Photosynthesis System (LI-COR Biosciences, United States). Concentration of CO2, relative humidity, and photosynthetic photon flux density (PPFD) were controlled via an automatic control device of LI-6400XT. The parameters P n, R dark, T r, and G s were measured as follows. Leaves were pre-lighted at 1,200 μmol m−2 s−1 [under CO2 concentration of 370–380 μl l−1, air temperature (32–39 °C), and 65 % relative humidity conditions] for 8 min to obtain a stable photosynthesis rate. Then, the light was turned off for about 180 s to automatically record air temperature (T a), leaf temperature (T l), P n, R dark, T r, and G s at an interval of 2 s. Post-illumination CO2 burst (PIB) was determined according to the method of Laisk and Sumberg (1994). Decker (1955) concluded that this phenomenon “Post-illumination CO2 burst” was a product of light respiration. Six to eight fully expanded leaves from different seedlings were measured repeatedly for each fumigation time.
Measurement of stomatal dynamics
Scanning Electron Microscope was used to estimate stomatal dynamics of the leaves. Mature leaves exposed to gaseous NO2 for different times were collected, then washed with tap water and cut into 5 mm2 fragments. The fragments were fixed by 2.5 % glutaraldehyde for 6 h, and air was exhaled to immerse the fragments below the fluid level. After dehydration through a grades alcohol series, the samples were critical point dried, mounted on stubs, and coated with gold in a high-vacuum evaporation unit (Lin et al. 2001). Samples were examined at 5 kV acceleration voltages under a Hitachi S-4800 scanning electron microscope.
Statistic analysis
Standard deviation (SD) in graphs was conducted using Microsoft Office Excel Statistical Package (Microsoft Office Excel, 2013). The comparison of averages and correlation coefficients was based on t test at a significance level of 5 % (p < 0.05) or 1 % (p < 0.01).
Results
Effects of NO2 on photosynthesis and respiration processes
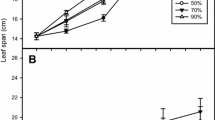
At the steady status of photosynthesis, the net photosynthetic rate of leaves exposed to NO2 was significantly lower than that of the control leaves (CK) (Fig. 1). After turning the light off, CO2 exchange rates of CK and NO2-exposed leaves sharply decreased for about 10 s, and then slowly reached a relatively steady state in subsequent 60 s (Fig. 1). For CK leaves, post-illumination CO2 burst (PIB) was absent and it was lower than dark respiration rate (R dark). In contrast, NO2-exposed leaves showed a typical characteristic of PIB where the maximum PIB occurred at 13–15 s after turning the light off (Fig. 1); PIB value was higher than R dark. Both R dark and PIB (absolute values) of NO2-exposed leaves were lower than that in CK leaves. These results indicated an inhibitory effect of gaseous NO2 on photosynthesis and dark respiration processes.
Post-illumination transients in CO2 exchange rates. Populus alba × P. berolinensis leaves were exposed to 380 μl l−1 CO2, 21 % O2, and PPDF of 1200 μmol m−2 s−1 in a steady photosynthesis state. At time = 10 s, light was switched off. Values of the post-illumination CO2 burst of the leaves exposed to gaseous NO2 for 48 h (TNO2) (black circle) and CK leaves (white circle) were read at arrow
We statistically analyzed the results of P n and R d in different leaf temperatures/the same NO2 treatment time and in different treatment times at similar leaf temperatures. The results showed that in a given PPFD of 1,200 μmol m−2 s−1, net photosynthetic rates (P n) of the NO2-treated leaves were dependent on the treatment time (R 2 = 0.5966); P n values declined with the increase in treatment time. In contrast, leaf temperature had a weak correlation with P n values (R 2 = 0.0151). The t test analysis showed a significant difference in the effect of NO2 treatment time and leaf temperature on P n values (P = 0.048, at the significance level of 0.05). The t test analysis also showed that there was no significant difference in the effect of NO2 treatment time (R 2 = 0.4022) and leaf temperature (R 2 = 0.5093) on R d values (P = 0.504, at the significance level of 0.05). Dark respiration rates of the treated leaves were determined by the co-action of leaf temperature and NO2 treatment time. However, the two factors had an opposite effect on R d values: R d was correlated positively with leaf temperature, but negatively with NO2 treatment time.
Effects of gaseous NO2 on stomata morphology
Figure 3a showed a typical SEM image of Populus alba × P. berolinensis leaf, including upper and lower epidermis, palisade/spongy tissues, and epidermal hairs. Dense white trichomes in abaxial leaf surface are visible without any additional equipment. Stomatal distribution was observed in both abaxial and adaxial leaves (amphistomatic) (data as shown in Hu et al. 2014a). Significant differences in stomatal size (length × width μm) were found in the leaves exposed to 4 μl l−1 NO2 for 0 (Fig. 3b), 14 (Fig. 3c) and 48 h (Fig. 3d). Stomatal opening in the leaves exposed to NO2 for 48 h was lower than that of 0-h and 14-h exposed leaves. Exposure to the gaseous NO2 for 48 h resulted in a decline in T r (P = 0.054, at the significant level of 0.05) and G s (P = 0.028, at the significant level of 0.05) as compared with the control leaves (Fig. 4). 14 h exposure to NO2 led a slight decline in G s and increase in T r of the treated leaves compared with the control leaves.
Discussion
In this study, we found that 4 μl l−1 NO2 had negative influence on net photosynthetic rate (P n) and dark respiration rate (R dark) of leaves of hybrid poplar clone cuttings (Populus alba × P. berolinensis) (Fig. 1). Significant declines in P n and R dark occurred in the leaves exposed to 4 μl l−1 NO2 for 48 h. Our results are in agreement with previous research that showed inhibitory effect of gaseous NO2 (7.39 μl l−1, exposed for 2 h) on net photosynthetic rate of the leaves of Populus euramericana (Furukawa 1991), but in disagreement with other researchers that hybrid poplar clone cuttings showed stimulatory effects of gaseous NO2 on CO2 assimilation rates of the leaves of Populus × euramericana ‘Dorskamp’ (Schmutz et al. 1995) and Populus × euramericana (Siegwolf et al. 2001). Studies with other species such as Phaseolus vulgaris (Srivastava et al. 1974a, b) and Glycine max. Merr (Carlson 1983; Sabaratnam et al. 1988) also showed inconsistent results of gaseous NO2 effects on CO2 assimilation rates. The inconsistent results may be caused mainly by differences in gaseous NO2 concentration and environmental conditions under which the plants were grown. For example, in the research indicated above, where the stimulatory effects of NO2 occurred, the plants were exposed to low concentrations (80–135 nl l−1) of gaseous NO2. Besides, it did not evaluate the effects of gaseous NO2 on respiration process. Oleksyn (1984) have investigated the effects of gaseous NO2 on photosynthesis and respiration processes of Pinus sylvestris leaves, and found that 30 min of NO2 fumigation led to the decline of net photosynthesis rate, dark respiration rate, and photorespiration rate, but long-term NO2 fumigation either increased or unchanged these parameters. In the present study, we only tested the gaseous NO2 effects on photosynthesis and respiration processes for 48 h, and our results showed a declining trend with the increasing time of fumigation (Fig. 2).
Correlation between P n and leaf temperature (a) and NO2 fumigation time (b)/between R dark and leaf temperature (c) and NO2 fumigation time (d) in the poplar leaves exposed to 4 μl l−1 NO2. P n, photosynthesis; R dark, dark respiration. Statistics analysis was according to the results of t test at a significance level of 5 % (P = 0.05). Asterisk means significant difference at P = 0.05
Temperature is an important factor affecting plant photosynthesis and dark respiration (Layne et al. 1991). So we analyzed the effects of leaf temperature on photosynthesis and dark respiration of the NO2-treated leaves. There were low correlation coefficients between leaf temperature and P n in the same NO2 fumigation time (Fig. 2a). Leaf temperature had a significantly positive impact on R d of the treated leaves. It agrees with the results of Silim et al. (2010), who found the maximum net photosynthesis rate of Populus balsamifera are insensitive to growth temperature relatively to dark respiration rate. The results of Carlson (1983) also showed an inhibitory effect of gaseous NO2 on dark respiration and apparent photorespiration. In fact, in the present study, there was a high correlation coefficient (R 2 > 0.91) between leaf temperature and R d in 0–14 h NO2 treatment, but it declined to a low value (R 2 = 0.32) in 48-h NO2 treatment. So the decline in P n and R d of 48-h treated leaves was mainly caused by NO2 fumigation. The phenomenon of post-illumination CO2 burst (PIB) was significantly present in the leaves exposed to gaseous NO2 for 48 h. Although we observed PIB in a few leaves exposed to gaseous NO2 for 0 and 14 h, this phenomenon was absent in most of the tested leaves (data not shown). The absence of PIB in control leaves might be caused by the masked effects of variable amount of CO2 assimilation (Sharkey 1988) and strong mitochondrial respiration during the post-illumination period (Azcon-Bieto and Osmond 1983). Absence of PIB was found at low temperature (13–15 °C) (Kaše and Čatskŷ 1983) and 1 % O2, with the temperature in the range of 15–35 °C (Parys and Romanowska 2000). In our present study, the maximum rate of CO2 evolution occurred after 13–15 s of turning the light off. This result was in agreement with the findings of Kaše and Čatskŷ (1983), who reported that at atmospheric O2 concentration, the maximum rate of CO2 evolution (PIB) of Phaseolus vulgaris leaves in each temperature generally occurred at 12–30 s after darkening the leaf. A potential role of photorespiration in foliar uptake of gaseous NO2 has been proposed by Hu and Sun (2010). The present study can only demonstrate the inhibitory effects of gaseous NO2 on CO2 evolution in the light and/or dark, but fails to explain the relationship between photorespiration and foliar NO2 uptake. Further research is needed to compare the differences in NO2–N metabolisms among species having different photorespiration capacities or to evaluate NO2–N metabolisms in species treated with substances that stimulates or inhibits photorespiration.
In this study, we used the SEM/EDS technique to analyze gaseous NO2 effects on stomatal dynamics of the hybrid poplar clone cuttings. Our results showed that 4 μl l−1 NO2 resulted in stomatal dysfunction (partial closure of stomata and the decline of stomatal conductance, Figs. 3, 4). Several previous studies have ever reported the impacts of air pollutants (such as NO2 and/or SO2) on the ultrastructure of mesophyll tissues adjacent to stomata. For example, Holopainen et al. (1992) found that air pollutants (SO2 and NO2) have significant impact on the ultrastructure of conifer needles, especially the chloroplasts of mesophyll tissue adjacent to stomata. Gaseous SO2 or NO2 led to swollen thylakoids and a reduction in the number of grana stacks as compared with the control (Schiffgens-Gruber and Lutz 1992). The results of Rantanen et al. (1994) also demonstrated the significant effects of mixed gaseous SO2 + NO2 on ultrastructure of mesophyll cells: swelling and slight reduction of thylakoids, enhanced translucence of the plastoglobuli, reduced length of chloroplasts and starch grains, and increased number of plastoglobuli. Moreover, the deleterious effects of SO2, NO, NO2, and O3 on spruce needles was related to membrane rupture and higher amounts of vacuolar tannins (Tjoelker et al. 2007). In the present study, we only observed the responses of stomata to NO2 gas; NO2 effects on ultrastructure of guard cells and mesophyll tissue adjacent to stomata was not involved. Toxic effects of gaseous NO2 may be mainly caused by generation and accumulation of NO2-derived NO -2 in apoplastic and symplastic space (Yoneyama and Sasakawa 1979; Wellburn 1990; Hu and Sun 2010). However, we did not investigate NO2 − accumulation in the exposed leaves, so the relationship between stomatal behavior and NO2 − accumulation is uncertain.
Stomatal conductance (G s) and transpiration rate (T r) of the leaves of hybrid poplar clone cuttings (Populus alba × P. berolinensis) exposed to 4 μl l−1 NO2 for 0, 14 and 48 h. Values were presented as the mean and standard deviation. Significant differences between exposure times were marked with different letters at the P values of 0.05 (a and bc) or the P values of 0.01 (ab and ac)
Conclusions
This study demonstrated that 4 μl l−1 gaseous NO2 has significant negative influence on stomata-related physiological processes of Populus alba × P. berolinensis leaves, particularly photosynthesis and dark- and photo-respiration processes. Photorespiration has a close relation with carbon/nitrogen metabolism. Thus, further research is needed to investigate the relationship between photorespiration, foliar uptake of gaseous NO2, and NO2–N assimilation and compare the differences in NO2–N metabolisms between the species with different photorespiration capacities using photorespiration-stimulatory or -inhibitory substances. Further, estimation of photorespiration rate of leaf exposed to gaseous NO2 with more accurate techniques should also be considered in future research.
Author contribution statement
Dr. Yanbo Hu, corresponding author for experiment design, project conduction, and paper writing; Dr. Mulualem Tigabu and Dr. Bellaloui Nacer, co-authors for project discussion and paper writing; Dr. Jinghong Wang, Jian Diao, Ke Wang, Rui Yang, co-authors for project conduction; Dr. Guangyu Sun, corresponding author for project discussion.
References
Azcon-Bieto J, Osmond CB (1983) Relationship between photosynthesis and respiration: the effect of carbohydrate status on the rate of CO2 production by respiration in darkened and illuminated wheat leaves. Plant Physiol 71:574–581
Breuninger C, Meixner FX, Kesselmeier J (2013) Field investigations of nitrogen dioxide (NO2) exchange between plants and the atmosphere. Atmos Chem Phys 13:773–790
Carlson RW (1983) Interaction between SO2 and NO2 and their effects on photosynthetic properties of soybean Glycine max. Environ Pollut Ecol Biol 32:11–38
Chaparro-Suarez IG, Thielmann A, Meixner FX, Kesselmeier J (2006) Re-investigation of the nitrogen dioxide (NO2) uptake by tree species. Geophys Res Abstr 8:706–716
Chaparro-Suarez IG, Meixner FX, Kesselmeier J (2011) Nitrogen dioxide (NO2) uptake by vegetation controlled by atmospheric concentrations and plant stomatal aperture. Atmos Environ 45:5742–5750
Decker JP (1955) A rapid, post-illumination deceleration of respiration in green leaves. Plant Physiol 30:82–84
Dochinger LS, Jensen KF (1985) Effect of acid mist and air pollutants on yellow-poplar seedling height and leaf growth. Research Paper. NE-572. U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station, Broomall, 4 p
Eastham AM, Ormrod DP (1986) Visible injury and growth responses of young cuttings of Populus Canadensis and P. nigra to nitrogen dioxide and sulphur dioxide. Can J Forest Res 16:1289–1292
Eller ASD, McGuire KL, Sparks JP (2011) Responses of sugar maple and hemlock seedlings to elevated carbon dioxide under altered above- and belowground nitrogen sources. Tree Physiol 31:391–401
Furukawa A (1991) Inhibition of photosynthesis of Populus euramericana and Helianthus annuus by SO2, NO2, O3. Ecol Res 6:79–86
Geβler A, Rienks M, Rennenberg H (2000) NH3 and NO2 fluxes between beech trees and the atmosphere—correlation with climatic and physiological parameters. New Phytol 147:539–560
Holopainen T, Anttonen S, Wulff A, Palomäki V, Kärentampi L (1992) Comparative evaluation of effects of gaseous pollutants, acidic deposition and mineral deficiencies: structural changes in the cells of forest plants. Agric Ecosyst Environ 42:365–398
Hu YB, Sun GY (2010) Leaf nitrogen dioxide uptake coupling apoplastic chemistry, carbon/sulfur assimilation, and plant nitrogen status. Plant Cell Rep 29:1069–1077
Hu YB, Bellaloui N, Sun GY, Tigabu M, Wang JH (2014a) Exogenous sodium sulfide improves morphological and physiological responses of a hybrid Populus species to nitrogen dioxide. J Plant Physiol 171:868–875
Hu YB, Fernández V, Ma L (2014b) Nitrate transporters in leaves and their potential roles in foliar uptake of nitrogen dioxide. Front Plant Sci 5:360. doi:10.3389/fpls.2014.00360
Jetter R, Riederer M, Lendzian KJ (1996) The effects of dry O3, SO2 and NO2 on reconstituted epicuticular wax tubules. New Phytol 133:207–216
Kaše M, Čatskŷ J (1983) Post-illumination burst of carbon dioxide in Phaseolus vulgaris L. as affected by leaf temperature. Biol Plant 25:225–230
Kondo K, Yamada K, Nakagawa A, Takahashi M, Morikawa H, Sakamoto A (2008) Molecular characterization of atmospheric NO2-responsive germin-like proteins in azalea leaves. Biochem Biophys Res Commun 377:857–861
Laisk A, Sumberg A (1994) Partitioning of the leaf CO2 exchange into components using CO2 exchange and fluorescence measurements. Plant Physiol 106:689–695
Layne DR, Flore JA, Hanover JW, Mebrahtu T (1991) Leaf temperature effects on net photosynthesis, dark respiration, and photorespiration of seedlings of black locust families with contrasting growth rates. Can J For Res 21:161
Lendzian KJ, Kerstiens G (1988) Interactions between plant cuticles and gaseous air pollutants. Asp Appl Biol 17:97–104
Lin JX, Jach ME, Ceulemans R (2001) Stomatal density and needle anatomy of Scots pine (Pinus sylvestris) are affected by elevated CO2. New Phytol 150:665–674
Mazarura U (2012) Effect of sequences of ozone and nitrogen dioxide on plant dry matter and stomatal diffusive resistance in radish. Afr Crop Sci J 20:371–384
Morikawa H, Higaki A, Nohno M, Takahashi M, Kamada M, Nakata M, Toyohara G, Okamura Y, Matsui K, Kitani S, Fujita K, Irifune K, Goshima N (1998) More than a 600-fold variation in nitrogen dioxide assimilation among 217 plant taxa. Plant Cell Environ 21:180–190
Okano K, Machida T, Totsuka T (1989) Differences in ability of NO2 absorption in various broad-leaved tree species. Environ Pollut 58:1–17
Oleksyn J (1984) Effects of SO2, HF and NO2 on net photosynthetic and dark respiration rates of Scots pine needles of various ages. Photosynthetica 18:259–262
Pallardy SG (2007) Physiology of Woody Plants, 3rd edn. Academic Press, San Diego 480
Parys E, Romanowska E (2000) Relationship between postillumination burst of CO2 and enhancement of respiration in tall fescue leaves. Acta Physiol Plant 22:135–142
Ramge P, Badeck FW, Plöchl M, Kohlmaier GH (1993) Apoplastic antioxidants as decisive elimination factors within the uptake process of nitrogen dioxide into leaf tissues. New Phytol 125:771–785
Rantanen L, Palomakp V, Harrison AF, Lucas PW, Mansfield TA (1994) Interactions between combined exposure to SO2 and NO2 and nutrient status of trees: effects on nutrient content and uptake, growth, needle ultrastructure and pigments. New Phytol 128:689–701
Rennenberg H, Gessler A (1999) Consequences of N deposition to forest ecosystems-recent results and future research needs. Water Air Soil Pollut 116:47–64
Sabaratnam S, Gupta G, Mulchi C (1988) Nitrogen dioxide effects on photosynthesis in soybean. J Environ Qual 17:143–146
Schiffgens-Gruber A, Lutz C (1992) Ultrastructure of mesophyll cell chloroplasts of spruce needles exposed to O3, SO2 and NO2 alone and in combination. Environ Exp Bot 32:243–254
Schmutz P, Tarjan D, Günthardt-Goerg MS, Matyssek R, Bucher JB (1995) Nitrogen dioxide- a gaseous fertilizer of poplar trees. Phyton 35:219–232
Sharkey TD (1988) Estimating the rate of photorespiration in leaves. Physiol Plant 73:147–152
Siegwolf RTW, Matyssek R, Saurer M, Maurer S, Günthardt-Goerg MS, Schmutz P, Bucher JB (2001) Stable isotope analysis reveals differential effects of soil nitrogen and nitrogen dioxide on the water use efficiency in hybrid poplar leaves. New Phytol 149:233–246
Silim SN, Ryan N, Kubien DS (2010) Temperature responses of photosynthesis and respiration in Populus balsamifera L.: acclimation versus adaptation. Photosynth Res 104:19–30
Sparks JP (2009) Ecological ramifications of the direct foliar uptake of nitrogen. Oecologia 159:1–13
Srivastava HS, Jolliffe PA, Runeckles VC (1974a) Inhibition of gas exchange in bean leaves by NO2. Can J Bot 53:466–474
Srivastava HS, Jolliffe PA, Runeckles VC (1974b) The effects of environmental conditions on the inhibition of leaf gas exchange by NO2. Can J Bot 53:475–482
Takagi M, Gyokusen K (2004) Light and atmospheric pollution affect photosynthesis of street trees in urban environments. Urban For Urban Green 2:167–171
Takahashi M, Higaki A, Nohno M, Kamada M, Okamura U, Matsui K, Kitani S, Morikawa H (2005) Differential assimilation of nitrogen dioxide by 70 taxa of roadside trees at an urban pollution level. Chemosphere 61:633–639
Tjoelker MG, Boratynski A, Bugala W (2007) Biology and Ecology of Norway Spruce. In: Werner A (ed) Effects of Pollutants on needle and wood anatomy. Springer, Netherlands, pp 325–327. ISBN 978-83-60247-62-4
Vallano DM, Selmants PC, Zavaleta ES (2012) Simulated nitrogen deposition enhances the performance of an exotic grass relative to native serpentine grassland competitors. Plant Ecol 213:1015–1026
Van Hove LWA, Bossen ME, Mensink MGJ, Van Kooten O (1992) Physiological effects of a long term exposure to low concentrations of ammonia, nitrogen dioxide, sulfur dioxide on douglas fir (Pseudotsuga menziesii). Physiol Plant 86:559–567
Wellburn AR (1990) Why are atmospheric oxides of nitrogen usually phytotoxic and not alternative fertilizers? New Phytol 115:395–429
Yoneyama T, Sasakawa H (1979) Transformation of atmospheric NO2 absorbed in spinach leaves. Plant Cell Physiol 20:263–266
Acknowledgments
This work was supported by grants from the “Fundamental Research Funds for the Central Universities” (Grant No. 2572014CA24; DL10BB24), ‘National Natural Science Foundation’ (Grant No. 31300506), and The Major project for the Heilongjiang Province Science and Technology Program (GZ13B004). The authors gratefully acknowledged Mr. Thomas D. Dahmer and Dr. Josirley de FC Carvalho for language correction.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Z. Miszalski.
Rights and permissions
About this article
Cite this article
Hu, Y., Bellaloui, N., Tigabu, M. et al. Gaseous NO2 effects on stomatal behavior, photosynthesis and respiration of hybrid poplar leaves. Acta Physiol Plant 37, 39 (2015). https://doi.org/10.1007/s11738-014-1749-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-014-1749-8