Abstract
Phosphorus (P) is an essential macronutrient for plant growth and development; however, soil P available for plant absorption is often limited, putting constraints over agricultural sustainability. Understanding the physiological and molecular responses to P deficiency can help design strategies for diagnosis and mitigation of P deficiency in crop plants. The advent of the next-generation sequencing technologies has made it possible to characterize genome-wide molecular responses to P deficiency in plants. However, such research efforts are very limited for woody crops. In this study, RNA-seq was used to investigate P starvation-induced transcriptomic changes in roots of a frequently used citrus rootstock, Poncirus trifoliata (L.) Raf. A total of 1,135 genes showed differential expression in response to P deficiency. The transcriptomic data were further validated by real-time quantitative RT-PCR. Interestingly, at least one or more P-responsive cis-elements (P1BS) were found in the promoter regions of 76 differentially expressed genes. Functional annotation revealed that the predicted proteins of 117 of the differentially expressed genes were assigned to the categories of transporters, transcription factors or components involved in plant hormone signal regulation, suggesting that these genes may play important roles in response to P starvation. A comparative analysis of the citrus- and Arabidopsis-responsive transcripts under P deficiency also identified 174 commonly regulated genes, including those involved in P metabolism. Taken together, our transcriptomic data revealed changes of genome-wide gene expression in responses to P starvation in Poncirus, which should provide a solid basis for future identification and characterization of key genes involved in nutritional stress response in citrus rootstocks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is one of the most important macronutrients for plant growth and development. Because most soil P exists as insoluble forms that are unavailable to plants, P fertilizers are usually used to provide adequate soluble P to crop plants in agriculture (Ma et al. 2011). However, over supplementation of P not only increases economic costs, but also damages ecosystems (Howarth et al. 2002; Chebud et al. 2011). One effective way to solve these problems is to develop crop cultivars with enhanced P-use efficiency by genetic improvement. Understanding the molecular basis of plant acclimation to P deficiency would help identify candidate genes for use in crop breeding programs. Accumulating research suggests that plants could develop adaptive strategies to cope with P deficiency by activating the expression of a series of genes, such as those encoding secreted acid phosphatases, organic anions, and high-affinity P transporters (Vance et al. 2003; Shin et al. 2004; Plaxton and Tran 2011). Some transcription factors, such as PHR1/2, MYB62, WRKY6/75, bHLH32, PTF1 and ZAT6, appear to play essential roles in P acclimation by regulating P homeostasis and/or root system architecture (Chiou and Lin 2011). Other regulatory components such as SPX domain-containing proteins, miRNAs and plant hormones may also be involved in the plant responses to P deficiency (Secco et al. 2012; Chiou et al. 2006, 2011; Rubio et al. 2009). Recently, genome-wide investigations of gene expression by microarray or RNA-seq analysis were performed to identify differentially expressed genes in response to P deficiency (Misson et al. 2005; Nilsson et al. 2010; Woo et al. 2012; Cai et al. 2012; Lan et al. 2012; O’Rourke et al. 2013). Such studies have greatly contributed to our current understanding of the complex network of P acclimation in plants. However, most studies on this subject were conducted with model or herbaceous plants. Very little is known about the molecular mechanisms underlying P acclimation in woody plants.
Citrus is one of the most important fruit crops in the world. Grafted citrus trees are perennial evergreen plants that are often grown in hilly or marginal land where soil P deficiency is a common problem. For example, an investigation of the citrus orchards in the Three Gorges reservoir region from China discovered that the available soil P is only 44 % of the level observed in other citrus orchards without soil P deficiency (Bao et al. 2006). P deficiency can cause symptoms such as reduced flowering, bronze and smaller leaves, small fruits with reduced juice, and weak branches. Moreover, a recently study of small RNA profiling discovered that P deficiency could aggravate citrus Huanglongbing disease symptoms (Zhao et al. 2013), whereas P supplementation could alleviate the disease symptoms and increase fruit yield although it could not cure the infected trees. These observations highlight the importance of P as a macronutrient for citrus growth and perhaps immunity.
Our previous studies found that high-affinity P transporter genes in Poncirus triforniata (Pt) (a close relative of Citrus and a common rootstock of citrus) designated PtPt4 and PtPT5 were induced by P starvation (Shu et al. 2012). These P transporters might be involved in P acquisition and homeostasis in plant cells under P deficiency conditions. However, how PtPt4 and PtPT5 are regulated and what other genes might be involved in orchestrating the perception of and response to P deficiency in Pt and Citrus is not known. Next-generation sequencing technologies have enabled large-scale transcriptomic analyses to identify candidate genes from a given genome that may be associated with plant responses to P deficiency (Misson et al. 2005; Nilsson et al. 2010; Woo et al. 2012; Cai et al. 2012; Lan et al. 2012; O’Rourke et al. 2013). We thus employ this approach to investigate transcriptomic changes in roots of Pt induced by P deficiency conditions. We aim to identify candidate Pt genes and their Citrus homologs involved in acclimation to P deficiency and infer likely conserved molecular mechanisms by comparing the induced transcriptomic changes in Pt as a woody plant with those in the model herbaceous plant Arabidopsis thaliana.
Materials and methods
Plant growth condition and P concentration measurement
A common rootstock, P. triforniata (Pt) (L) Raf, was used in this study. Pt seeds were sown in plastic pots filled with vermiculite as previously described (Zhou et al. 2014). Twenty days later, uniform seedlings were transplanted into sand culture as previously described (Shu et al. 2012). Pt seedlings in sand pots were grown in a chamber with a 14 h light period at 23–28 °C and a 10 h dark period at 18–20 °C. Five Pt seedlings per sand pot were irrigated with 200 ml Hoagland nutrient solution. Two-month-old seedlings were used for phosphate starvation treatment. The control samples (+P) were irrigated with Hoagland nutrient solutions containing 1 mM P, whereas P starvation samples (−P) were irrigated with Hoagland nutrient solutions containing 1 μM P (Shu et al. 2012). Root samples were collected at 0, 1, 2 or 4 weeks after (−P) irrigation treatment. Each root sample was prepared from three pots with each containing five seedlings. Three root samples/per treatment were collected for each time point from three independent experiments. P concentration was determined using the molybdate blue method as previously described (Shu et al. 2012). The one-way analysis of variance (ANOVA) was used to determine whether there are any statistically significant differences between the means of the (−P)-irrigated and (+P)-irrigated samples.
Transcriptome profiling
Pt root samples collected at 4 weeks after (−P) irrigation treatment were used for RNA extraction. Two RNA samples for (−P) irrigation treatment and two for (+P) irrigation treatment were prepared from two independent biological experiments and used for RNA-seq analysis. Total RNA was extracted using the TriZol Reagent (Invitrogen, USA) and then treated with DNaseI to eliminate DNA contamination. First-strand cDNA was synthesized using the RevertAid™ First-Strand cDNA Synthesis Kit (Fermentas, Lithuania) and used for library construction at Huada Genomics Co., Ltd., Shenzhen, China. The barcoded cDNA libraries were used for sequencing via IIIumina HiSeq™ 2000. After removing the adaptor sequences, the clean reads were used to match gene sequences deposited in a reference sweet orange genome database (Xu et al. 2013, http://citrus.hzau.edu.cn/orange/) using SOAPaligner/SOAP2 (Li et al. 2009). Mismatches no more than two bases were allowed in the alignment. The RPKM method (Mortazavi et al. 2008) was used to normalize and calculate the gene expression level according to our previous protocol (Yang et al. 2013). Differentially expressed genes were identified using the NOISeq methodology (Tarazona et al. 2011), in which q > 0.8 was selected. For Gene Ontology (GO) and pathway analysis, the differentially expressed genes were mapped to GO terms in the database (http://www.geneontology.org/) and pathway terms in KEGG database (http://www.genome.jp/kegg/), respectively.
Real-time quantitative RT-PCR
Real-time PCR analysis was performed according to our previous protocol (Pan et al. 2012). Gene-specific primers were deposited in Table S1. Primers of tested genes and the reference gene were diluted in the SYBER GREEN PCR Master Mix (PE Applied Biosystems), and 10 μl of the reaction mix was added to each well. Reactions were performed as the following: 50 °C for 2 min, 95 °C for 1 min, each reaction was then cycled at 95 °C for 15 s and 60 °C for 1 min, which was performed for 40 cycles. The output data were generated by the software Sequence Detector Version 1.3.1 (Applied Biosystems, CA, USA), and exported to Microsoft Excel for subsequent analysis. The relative mRNA abundance was calculated using the standard delta delta Ct method.
Results
P concentration in Poncirus roots under normal and P deficiency conditions
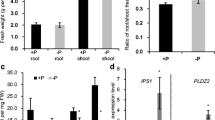
To find out when our irrigation scheme using (−P) Hoagland nutrient solution begins to impact the endogenous P in treated Pt root tissues, root P content was measured at 1, 2 or 4 weeks after the implementation of the (−P) irrigation scheme. Our data showed that the P content was not significantly affected at 1 or 2 weeks after (−P) irrigation, albeit there was a slight reduction when compared to the control [i.e. samples with (+P irrigation)] (Fig. 1). This suggests that P in the sand media was more or less adequate for Pt growth at this stage (which generally agrees with the fact that all seedlings showed similar stature at this stage; data not shown). However, at 4 weeks after (−P) irrigation, the P level in treated root samples was only 1.10 mg g−1 DW, which is significantly lower than that (2.59 mg g−1 DW) in the control sample, suggesting that 4 weeks after (−P) irrigation is a good time for checking the impact of P deficiency on gene expression.
Transcriptomic changes in Poncirus roots in response to P deficiency
Based on the above results, root samples were thus collected at 4 weeks after (−P) irrigation and used for RNA-seq analysis. Two replicate RNA samples for each treatment were used for cDNA library preparation and sequencing using IIIumina HiSeqTM2000. The four cDNA libraries, P-deficiency 1, P-deficiency 2, control 1 and control 2, produced 11910174, 11324588, 11935107 and 11583447 clean reads, respectively (Table S2). Sequence saturation analysis found that ‘genes mapped by clean reads’ stopped increasing when the number of clean reads reached over 2 million (data not shown), suggesting that the gene coverage of these four libraries was saturated and the size was enough for gene expression analysis. To profile gene expression, reads from the four libraries were mapped to gene sequences deposited in the sweet orange genome database. A total of 22,488 transcripts were detected by the reads from the four libraries, with 20543, 20850, 21071 and 21095 transcripts being identified from P-deficiency 1, P-deficiency 2, control 1 and control 2, respectively (Tables S2 and 3). The expression level of each gene was normalized and evaluated by the RPKM approach (see "Materials and methods"), and the detailed information of expression and annotation was provided in Table S4. Differentially expressed genes were defined using the NOISeq method. Specifically, 495 genes were up-regulated and 640 were down-regulated (1135 in total) in the P deficiency samples compared with the control samples (Table S5).
GO categories were assigned to the 1135 differentially expressed genes with BLASTX hit using Blast2GO. The distribution of GO terms (the 4th level) according to biological process, molecular function and cellular component was shown in Table S6. Metabolic process (232 genes, accounting for 20.4 % of the total differentially expressed genes), cellular process (170, 14.9 %), and response to stimulus (111, 9.8 %) were the major categories annotated to the biological process; cell (193, 17 %), organelle (140, 12.3 %), and membrane (107, 9.4 %) were the major categories annotated to the cellular component; catalytic activity (310, 27.3 %), binding (236, 20.8 %), and transporter activity (43, 3.8 %) were the major categories annotated to the molecular function. Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis found that 156 and 101 genes were, respectively, assigned to the metabolic pathways with no map in KEGG database and biosynthesis of secondary metabolites with no map in KEGG database (Table S7). The remaining differentially expressed genes were assigned to 97 metabolic pathways (Table S7). Notably, the pathways of plant hormone signal transduction (containing 60 genes), plant–pathogen interaction (51), stilbenoid, diarylheptanoid and gingerol biosynthesis (33) and flavonoid biosynthesis (30) are the four predicted pathways with the highest number of differentially expressed genes.
A list of selected genes with putative functions that might be important for the acclimation to P deficiency was summarized in Table S8, with a focus on genes annotated as transporters, transcription factors (TFs), and plant hormones signal regulation. Of particular note is that 43 transporter genes were differentially regulated, including transporters that transport macroelements (e.g. phosphate, nitrate, potassium, sulfate and calcium), microelements (e.g. boron, zinc, iron and copper), water (aquaporin), carbohydrate (sugar) and other organic substances (e.g. amino acid and peptide). Among them, three P transporter genes (Cs5g29860, Cs9g18560, Cs7g29450) were up-regulated, and one (Cs9g09780, PHO-like) was down-regulated in response to P deficiency. It was also noted that five out of six aquaporin genes were down-regulated, while five out of six ABC transporter genes were up-regulated in response to P deficiency. In addition, several genes encoding microelements transporters, such as transporters of zinc/iron, boron and copper, showed down-regulation patterns upon P deficiency. These results imply that, besides the direct effects on P uptake and transportation, P starvation could have a profound indirect effect on the transport of other elements and/or metabolites in Pt plants. Based on the domain characterization or putative function, the 46 differentially expressed genes encoding domain-containing proteins or TFs could be classified into the following types: SPX domain-containing, AP2/EREBP, MYB, WRKY, bHLH, Zinc finger, GRAS and others (Table S8). Interestingly, while genes encoding SPX domain-containing proteins and MYB TFs were all up-regulated, those encoding zinc finger TFs were all down-regulated. It is interesting to note that 29 differentially expressed genes were predicted to be involved in plant hormones signal regulation, including those involved in the synthesis and/or signal regulation of gibberellin, auxin, cytokinin, abscisic acid, jasmonates and brassinosteroid. Among these 29 genes, most were down-regulated. For instance, five out of seven genes implicated in gibberellin regulation, six out of seven genes in auxin regulation and all of the four genes in cytokinin regulation were down-regulated in response to P deficiency.
To validate the RNA-seq results, expression levels of 28 differentially expressed genes were determined by real-time quantitative PCR (qPCR) using cDNA templates independent of those in RNA-seq experiments (Table S9). The log2 [ratios of gene expression levels of (−P) irrigation samples vs (+P) irrigation control)] from qPCR were then compared with those obtained from the RNA-seq analysis (Table S9). As expected, all the 28 genes showed substantial differences in gene expression levels measured by qPCR, which agrees well with the gene expression data from the RNA-seq analysis with a correlation coefficient of 0.774 (Fig. 2).
Prediction of P1BS elements in promoters of differentially expressed genes
The P1BS (PHR1 binding Site; GNATATNC) motif is a characterized P-responsive cis element found in the promoter regions of several P-deficiency-inducible P transporter genes (Rubio et al. 2001). To see if the P1BS motif is present in the promoter regions of these differentially expressed Pt genes (which are not available), we scanned the upstream sequences of all genes deposited in sweet oranges genome database for the presence of the P1BS motif. The search yielded 616 genes whose promoter regions (2 Kb upstream of the predicted ATG start codon) contain at least one exact P1BS motif (Table S10) and 1707 genes whose promoter regions contain at least one P1BS motif with one bp mismatch (Table S11). Among the 616 citrus genes, 76 have Pt homologs (predicted based on RNA-seq data) that were differentially expressed under P deficiency conditions (Table S12), suggesting that these 76 Pt genes are more likely to be functionally involved in Pt’s responses to P deficiency.
Conserved genes responsive to P starvation in both Poncirus and Arabidopsis
To see how conserved the mechanisms of P-deficiency response might be between Poncirus (and Citrus) and other plant species, the sweet orange homologs of the Pt genes that exhibited differential expression under P deficiency were blasted against the Arabidospsis whole genome sequences (www.plantgdb.org/XCDB/phplib/download.php?GDB=At) to search for homologs with an E value = 1 × e−5 or lower. The identified Arabidopsis genes were then examined in the published datasets from a similar study (Woo et al. 2012) to see if their expression was affected by P deficiency. This analysis identified 174 P-deficiency-responsive genes that may be conserved between Citrus and Arabidopsis with 89 being up-regulated and 85 down-regulated (Table S13). A subset (27) of the 174 genes is listed in Table 1. One group of genes with particular relevance to P-deficiency response are those that encode P metabolism-related proteins, transporters and transcription factors. Conceivably, transcriptional alteration (up-regulation in particular) of P transporters and P metabolism-related proteins (such as phosphoesterase, pyrophosphatase, and phospholipase) is a conserved mechanism important for P acquisition and utilization under starvation conditions. By contrast, transporters of oligopeptide, zinc and amino acid were down-regulated in both Citrus and Arabidopsis during P starvation. Likewise, genes encoding SPX domain or AP2/ERF domain-containing proteins, or transcription factors harboring bHLH and MYB domains were up-regulated, whereas genes encoding WRKY or zinc finger proteins were down-regulated both in Citrus and Arabidopsis under P deficiency.
Discussion
In the present study, RNA-seq was used to determine transcriptomic changes in the citrus rootstock Poncirus trifoliata (Pt) in response to P starvation. Over one thousand genes involved in multiple metabolic pathways showed altered expression in Pt seedlings grown under P-deficiency conditions compared to those with adequate supply of P, suggesting that P deficiency has a profound impact on gene transcription in Pt in general. Among these genes, 76 have been identified to contain a characterized P-responsive cis-element P1BS in the promoter regions of their citrus homologous genes. These P1BS-containing (in the promoter), P starvation-induced Pt genes may be good candidate genes for future studies. By comparing the results from this study with those from a previous transcriptomic study in Arabidopsis, we found that 174 P deficiency-responsive genes may be conserved in both Citrus (Pt) and Arabidopsis, some of which encode P transporters, or P metabolism-related phospholipases and SPX domain-containing genes. Thus, these observations suggest that 4 weeks after (−P) irrigation is indeed a good time for preparing root samples for RNA-seq analysis and that our transcriptomic data should be quite reliable. These results also support a notion that woody plants and herbaceous plants share conserved molecular mechanisms to cope with P deficiency. Because the primary purpose of this study is to identify genes involved in P acclimation in Pt (and Citrus) for future focused studies, we selected a few potentially more relevant groups of P-responsive Pt genes for discussion.
P deficiency affects the transcription of multiple transporters
Our results showed that the transcription of 4 P transporters genes was altered due to P deficiency, 3 (Cs5g29860, Cs9g18560 and Cs7g2945) of which were up-regulated while 1 (Cs9g09780, PHO1-like) down-regulated (Table S8). The up-regulation of these three P transporters under P starvation was in line with previous observations that P starvation triggered the up-regulation of most members of the PHt1 P transporter gene family (Shu et al. 2012). It was reported that the Arabidopsis PHO1 gene may play an important role in root-to-shoot transfer of P and that reduced PHO1 expression affects not only P translocation from roots to shoots, but also the process of P acquisition (Poirier et al. 1991; Hamburger et al. 2002). Down-regulation of a PHO1-like gene in P-starved roots of Pt seedling seems a reasonable strategy to reduce P export from root to shoot, thereby alleviating Pi deficiency in root cells to temporarily accommodate a short-term P starvation.
As it is known that deficiency of one mineral will influence the uptake and accumulation of other nutrients and their corresponding transporters (Wang et al. 2002; Schachtman and Shin 2007; Cai et al. 2012), it is not surprising that expression of many other nutrient transporters was also affected in Pt seedlings by P deficiency. These included transporters involved in uptake of not only macroelements such as nitrate, potassium, sulfate and calcium, but also microelements such as boron, zinc, iron and copper. It was also proposed that P nutrition could affect sugar metabolisms via likely crosstalk between the two engaged signaling pathways (Müller et al. 2007; Park et al. 2012). Consistent with this idea, we found that the transcription of five sugar transporters was affected by P deficiency in the Pt seedlings. It is worth noting that five out of six affected ABC transporters were up-regulated under P deficiency. Similar results were reported in white lupin (O’Rourke et al. 2013). In contrast, five out of six affected aquaporins (4 PIP, 2 MIP and 1 TIP) were down-regulated under P deficiency, although the functional implication is unclear. Taken together, our results reinforce the notion that P deficiency could have a profound impact on plant growth and development by affecting not only the transport and metabolism of phosphate and other nutrients but also the balance and homeostasis of, and crosstalk between, different nutrient-regulated pathways to achieve acclimation to P deficiency in plants.
Domain-containing proteins and transcription factors
Two genes encoding SPX domain-containing proteins were up-regulated in roots of Pt seedlings by P deficiency (Table S8). Specially, the Citrus counterparts of these two Pt genes, i.e. Cs4g17870 and orange1.1t00194, are probable orthologs of AtSPX1 (At5g20150) and AtSPX3 (At2g45130), respectively. Since SPX domain-contain proteins are potential regulators in phosphate signaling and both of SPX1 and SPX3 regulate the expression of several P starvation responsive genes involved in P uptake, allocation and remobilization (Duan et al. 2008), this result add further evidence to the existence of conserved P-deficiency response mechanisms across different plant species.
Furthermore, over 40 Pt genes encoding transcription factors (TFs) falling into at least 6 groups were differentially expressed in roots of Pt seedlings under P deficiency when compared with control plants (Table S8). A similar situation can be found in Arabidopsis (Woo et al. 2012). The altered expression of these TFs may not only directly contribute to plant acclimation to a P-deficiency state via regulating expression levels of key nutrient (including P) transporters, but can also affect a large number of other genes, resulting in the extensive transcriptional changes of over one thousand genes under P deficiency. P-deficiency-induced transcriptional changes of some transcription factors, such as GRAS, NAP, CCAAT-binding and GATA TFs, were only detected in this study. This may reflect the characteristics of the transcription machinery and signaling network of Pt (and perhaps Citrus) as a woody perennial plant in response to P deficiency. In this context, it is interesting to note that the transcription of four GRASs involved in strigolactone biosynthesis and lateral root elongation (Liu et al. 2011; Battaglia et al. 2014) were affected in Pt seedlings under P deficiency. This may provide a mechanistic connection between GRASs and the earlier observation that the density and length of root hairs of Pt plants tend to increase significantly under P deficiency conditions when compared to control plants (Cao et al. 2013).
Plant hormone biosynthesis and signal regulation
It is known that many hormones are implicated in P signaling processes (Chiou and Lin 2011) and that P deficiency alters transcription levels of genes involved in hormone biosynthesis or signaling (Morcuende et al. 2007; O’Rourke et al. 2013; Rubio et al. 2009; Woo et al. 2012). Consistent with this, we found that 26 differentially expressed genes may be involved in plant hormone biosynthesis or signaling (Table S8). For example, it has been reported that cytokinin signaling is repressed by P deficiency (Kuiper et al. 1988; Franco-Zorrilla et al. 2002) and that this hormone might negatively regulate P starvation response (Franco-Zorrilla et al. 2005). In accordance with this, four Pt cytokinin related genes were down-regulated under P deficiency. Auxin has been suggested to play a role in the arrest of primary root growth and induction of lateral and/or hair roots under P deprivation (Al-Ghazi et al. 2003; Jain et al. 2007). Our observation that expression of seven auxin-related genes was altered by P deficiency may help us understand the phenomenon that the initiation and elongation of Pt root hairs is activated by P deprivation (Cao et al. 2013). Although a definitive role of ABA in coordinating P stress response remains to be demonstrated (Jeschke et al. 1997; Trull et al. 1997; Ribot et al. 2008), the detection of altered expression of six ABA-related genes in our study suggests that ABA signaling might also contribute to acclimation to P starvation in the Pt seedlings. In addition, Jiang et al. (2007) found that P deficiency promoted the accumulation of a DELLA protein and caused a reduction of bioactive GA and attenuation of GA metabolism in root cells of Arabidopsis plants. Interestingly, we found that among seven GA-related genes affected by P deficiency, a DELLA-like gene (GAI1) was up-regulated, while a GA-oxidase-like gene was down-regulated in the Pt seedlings under P deficiency.
Conclusions
This study revealed that over one thousand genes showed transcriptional changes in response to P deficiency in Poncirus, a Citrus relative and primary rootstock. Of particular relevance are those genes that encode transcription factors, transporters and regulators of plant hormones because they are assumed or have been demonstrated to play important roles in plant acclimation to P deficiency. A comparative analysis between citrus and Arabidopsis identified similar patterns of P-deficiency-induced transcriptomic changes, suggesting that woody and herbaceous plants may employ similar molecular mechanisms to cope with P deficiency, though some apparent differences also exist. Our results from this study should provide a solid basis for future identification and characterization of key genes involved in P deficiency and other nutritional stress response in Poncirus and other citrus rootstocks.
Author contribution
Pan Zhiyong was responsible for the experimental design, data analysis, and manuscript writing. Bai Fuxi, Chen Chunli and An Jianyong contributed to the experimental work. Xiao Shunyuan and Deng Xiuxin contributed to the interpretation of results and coordinated the study. All authors have read and approved the final manuscript and have no conflicts of interest.
References
Al-Ghazi Y, Muller B, Pinloche S, Tranbargeri TJ, Nacry P, Rossignol M, Tardieu F, Doumas P (2003) Temporal responses of Arabidopsis root architecture to phosphate starvation: evidence for the involvement of auxin signalling. Plant Cell Environ 26:1053–1066
Bao JF, Xia RX, Peng SA, Li GH (2006) Main soil nutrient status and its effect on fruit quality of Newhall Orange in 3-gorge area. Soil Fertil Sci China 3:16–20 (in Chinese)
Battaglia M, Rípodas C, Clúa J, Baudin M, Aguilar M, Niebel A, Zanetti ME, Blanco F (2014) The NF-YC1 interacting protein SIN1, a member of the GRAS family, is required for nodule organogenesis, infection thread progression and lateral root growth. Plant Physiol. doi:10.1104/pp.113.230896
Cai HM, Xie WB, Zhu T, Lian XM (2012) Transcriptome response to phosphorus starvation in rice. Acta Physiol Plant 34:327–341
Cao X, Chen CL, Zhang DJ, Shu B, Xiao J, Xia RX (2013) Influence of nutrient deficiency on root architecture and root hair morphology of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings under sand culture. Sci Hortic 162:100–105
Chebud Y, Naja GM, Rivero R (2011) Phosphorus run-off assessment in a watershed. J Environ Monit 13:66–73
Chiou TJ, Lin SI (2011) Signaling network in sensing phosphate availability in plants. Annu Rev Plant Biol 62:185–206
Chiou TJ, Aung K, Lin SI, Wu CC, Chiang SF, Su CL (2006) Regulation of phosphate homeostasis by MicroRNA in Arabidopsis. Plant Cell 18:412–421
Duan K, Yi K, Dang L, Huang H, Wu W, Wu P (2008) Characterization of a sub-family of Arabidopsis genes with the SPX domain reveals their diverse functions in plant tolerance to phosphorus starvation. Plant J 54:965–975
Franco-Zorrilla JM, Martin AC, Solano R, Rubio V, Leyva A, Paz-Ares J (2002) Mutations at CRE1 impair cytokinin-induced repression of phosphate starvation responses in Arabidopsis. Plant J 32:353–360
Franco-Zorrilla JM, Martin AC, Leyva A, Paz-Ares J (2005) Interaction between phosphatestarvation, sugar, and cytokinin signaling in Arabidopsis and the roles of cytokinin receptors CRE1/AHK4 and AHK3. Plant Physiol 138:847–857
Hamburger D, Rezzonico E, MacDonald-Comber Petétot J, Somerville C, Poirier Y (2002) Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell 14:889–902
Howarth R, Sharpley A, Walker D (2002) Sources of nutrient pollution to coastal waters in the United States: implications for achieving coastal water quality goals. Estuaries Coasts 25:656–676
Jain A, Poling MD, Karthikeyan AS, Blakeslee JJ, Peer WA, Titapiwatanakun B, Murphy AS, Raghothama KG (2007) Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiol 144:232–247
Jeschke WD, Peuke AD, Pate JS, Hartung W (1997) Transport, synthesis and catabolism of abscisic acid (ABA) in intact plants of castor bean (Ricinus communis L.) under phosphate deficiency and moderate salinity. J Exp Bot 48:1737–1747
Jiang C, Gao X, Liao L, Harberd NP, Fu X (2007) Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis. Plant Physiol 145:1460–1470
Kuiper D, Schuit J, Kuiper P (1988) Effects of internal and external cytokinin concentrations on root growth and shoot to root ratio of Plantago major ssp. pleiosperma at different nutrient conditions. Plant Soil 111:231–236
Lan P, Li W, Schmidt W (2012) Complementary proteome and transcriptome profiling in phosphate-deficient Arabidopsis roots reveals multiple levels of gene regulation. Mol Cell Proteomics 11:1156–1166
Li R, Yu C, Li Y, Lam TW, Yiu SM, Kristiansen K, Wang J (2009) SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics 25:1966–1967
Liu W, Kohlen W, Lillo A, Op den Camp R, Ivanov S, Hartog M, Limpens E, Jamil M, Smaczniak C, Kaufmann K, Yang WC, Hooiveld GJ, Charnikhova T, Bouwmeester HJ, Bisseling T, Geurts R (2011) Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-Type transcription factors NSP1 and NSP2. Plant Cell 23:3853–3865
Ma W, Ma L, Li J, Wang F, Sisak I, Zhang F (2011) Phosphorus flows and use efficiencies in production and consumption of wheat, rice, and maize in China. Chemosphere 84:814–821
Misson J, Raghothama KG, Jain A, Jouhet J, Block MA, Bligny R, Ortet P, Creff A, Somerville S, Rolland N, Doumas P, Nacry P, Herrerra-Estrella L, Nussaume L, Thibaud MC (2005) A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc Natl Acad Sci USA 102:11934–11939
Morcuende R, Bari R, Gibon Y, Zheng W, Pant BD, Bläsing O, Usadel B, Czechowski T (2007) Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ 30:85–112
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5:621–628
Müller R, Morant M, Jarmer H, Nilsson L, Nielsen TH (2007) Genome-wide analysis of the Arabidopsis leaf transcriptome reveals interaction of phosphate and sugar metabolism. Plant Physiol 143:156–171
Nilsson L, Müller R, Nielsen TH (2010) Dissecting the plant transcriptome and the regulatory responses to phosphate deprivation. Physiol Plant 139:129–143
O’Rourke JA, Yang SS, Miller SS, Bucciarelli B, Liu J, Rydeen A, Bozsoki Z, Uhde-Stone C, Tu ZJ, Allan D, Gronwald JW, Vance CP (2013) An RNA-Seq transcriptome analysis of orthophosphate-deficient white lupin reveals novel insights into phosphorus acclimation in plants. Plant Physiol 161:705–724
Pan ZY, An JY, Zeng WF, Xiao SY, Deng XX (2012) Array-comparative genome hybridization reveals genome variations between a citrus bud mutant and its parental cultivar. Tree Genet Genomes 8:1379–1387
Park MR, Baek SH, de Los Reyes BG, Yun SJ, Hasenstein KH (2012) Transcriptome profiling characterizes phosphate deficiency effects on carbohydrate metabolism in rice leaves. J Plant Physiol 169:193–205
Plaxton WC, Tran HT (2011) Metabolic Adaptations of Phosphate-Starved Plants. Plant Physiol 156:1006–1015
Poirier Y, Thoma S, Somerville C, Schiefelbein J (1991) Mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiol 97:1087–1093
Ribot C, Wang Y, Poirier Y (2008) Expression analyses of three members of the AtPHO1 family reveal differential interactions between signaling pathways involved in phosphate deficiency and the responses to auxin, cytokinin, and abscisic acid. Planta 227:1025–1036
Rubio V, Linhares F, Solano R, Martín AC, Iglesias J, Leyva A, Paz-Ares J (2001) A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev 15:2122–2133
Rubio V, Bustos R, Irigoyen M, Cardona-L′ opez X, Rojas-Triana M, Paz-Ares J (2009) Plant hormones and nutrient signaling. Plant Mol Biol 69:361–373
Schachtman DP, Shin R (2007) Nutrient sensing and signaling: nPKS. Annu Rev Plant Biol 58:47–69
Secco D, Wang C, Arpat BA, Wang Z, Poirier Y, Tyerman SD, Wu P, Shou H, Whelan J (2012) The emerging importance of the SPX domain-containing proteins in phosphate homeostasis. New Phytol 193:842–851
Shin H, Shin HS, Dewbre GR, Harrison MJ (2004) Phosphate transport in Arabidopsis: pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J 39:629–642
Shu B, Xia RX, Wang P (2012) Differential regulation of Pht1 phosphate transporters from trifoliate orange (Poncirus trifoliata L. Raf) seedlings. Sci Hortic 146:115–123
Tarazona S, Garcı´a-Alcalde F, Dopazo J, Ferrer A, Conesa A (2011) Differential expression in RNA-seq: a matter of depth. Genome Res 21:2213–2223
Trull MC, Guiltinan MJ, Lynch JP, Deikman J (1997) The responses of wild-type and ABA mutant Arabidopsis thaliana plants to phosphorus starvation. Plant Cell Environ 20:85–92
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable source. New Phytol 157:423–447
Wang YH, Garvin DF, Kochian LV (2002) Rapid induction of regulatory and transporter genes in response to phosphorus, potassium, and iron deficiencies in tomato roots. Evidence for cross talk and root/rhizosphere-mediated signals. Plant Physiol 130:1361–1370
Woo J, MacPherson CR, Liu J, Wang H, Kiba T, Hannah MA, Wang XJ, Bajic VB, Chua NH (2012) The response and recovery of the Arabidopsis thaliana transcriptome to phosphate starvation. BMC Plant Biol 12:62
Xu Q, Chen LL, Ruan XA, Chen DJ, Zhu AD, Chen CL, Bertrand D, Jiao WB, Hao BH, Lyon MP, Chen JJ, Gao S, Xing F, Lan H, Chang JW, Ge XH, Lei Y, Hu Q, Miao Y, Wang L, Xiao SX, Biswas MK, Zeng WF, Guo F, Cao HB, Yang XM, Xu XW, Cheng YJ, Xu J, Liu JH, Luo OJ, Tang ZH, Guo WW, Kuang HH, Zhang HY, Roose ML, Nagarajan N, Deng Xiu-Xin, Ruan Yijun (2013) The draft genome of sweet orange (Citrus sinensis). Nat Genet 45:59–66
Yang CQ, Liu YZ, An JC, Li S, Jin LF, Zhou GF, Wei QJ, Yan HQ, Wang NN, Fu LN, Liu X, Hu XM, Yan TS, Peng SA (2013) Digital gene expression analysis of corky split vein caused by boron deficiency in ‘Newhall’ navel orange (Citrus sinensis Osbeck) for selecting differentially expressed genes related to vascular hypertrophy. PLoS One 8:e65737
Zhao H, Sun R, Albrecht U, Padmanabhan C, Wang A, Coffey MD, Girke T, Wang Z, Close TJ, Roose M, Yokomi RK, Folimonova S, Vidalakis G, Rouse R, Bowman KD, Jin H (2013) Small RNA profiling reveals phosphorus deficiency as a contributing factor in symptom expression for citrus huanglongbing disease. Mol Plant 6:301–310
Zhou GF, Peng SA, Liu YZ, Wei QJ, Han J, Islam MZ (2014) The physiological and nutritional responses of seven different citrus rootstock seedlings to boron deficiency. Trees 28:295–307
Acknowledgments
This work was supported by National Natural Science Foundation of China (Nos. 31201612, 31328018, 31221062), Ministry of Education of China (20120146120032), and the Fundamental Research Funds for the Central Universities (52902-0900202123).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Y. Wang.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bai, F., Chen, C., An, J. et al. Transcriptome responses to phosphate deficiency in Poncirus trifoliata (L.) Raf. Acta Physiol Plant 36, 3207–3215 (2014). https://doi.org/10.1007/s11738-014-1687-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-014-1687-5