Abstract
Robotic surgery represents a milestone in surgical procedures, offering advantages such as less invasive methods, elimination of tremors, scaled motion, and 3D visualization. This in-depth analysis explores the complex biochemical effects of robotic methods. The use of pneumoperitoneum and steep Trendelenburg positioning can decrease pulmonary compliance and splanchnic perfusion while increasing hypercarbia. However, robotic surgery reduces surgical stress and inflammation by minimizing tissue trauma. This contributes to faster recovery but may limit immune function. Robotic procedures also limit ischemia–reperfusion injury and oxidative damage compared to open surgery. They also help preserve native antioxidant defenses and coagulation. In a clinical setting, robotic procedures reduce blood loss, pain, complications, and length of stay compared to traditional procedures. However, risks remain, including device failure, the need for conversion to open surgery and increased costs. On the oncology side, there is still debate about margins, recurrence, and long-term survival. The advent of advanced technologies, such as intraoperative biosensors, localized drug delivery systems, and the incorporation of artificial intelligence, may further improve the efficiency of robotic surgery. However, ethical dilemmas regarding patient consent, privacy, access, and regulation of this disruptive innovation need to be addressed. Overall, this review sheds light on the complex biochemical implications of robotic surgery and highlights areas that require additional mechanistic investigation. It presents a comprehensive approach to responsibly maximize the potential of robotic surgery to improve patient outcomes, integrating technical skill with careful consideration of physiological and ethical issues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of the da Vinci System in 2000 signaled new era for several surgical specialties through its innovative robotic technology [1]. This forward-thinking platform promotes tele-operational access and an enhanced 3D view of the surgical field, paving the way for less invasive surgeries [2]. Specifically, robotic techniques result in smaller incisions, minimized blood loss, and faster recovery compared to conventional open surgery. The degree of freedom and articulation offered by these robotic instruments transcends the limitations associated with traditional laparoscopy [3, 4]. As a result, robotic-assisted surgery is associated with fewer complications, a 30–50% reduction in hospital length of stay, and a faster return to routine activities [5, 6]. The capabilities of the da Vinci robotic surgical system are increasingly being utilized for a variety of procedures, including prostatectomy, hysterectomy, nephrectomy, and mitral valve repair [7]. Its advanced features, including stable 3D vision, tremor filtering, and mobile EndoWrist instruments, allow for precise dissection and less tissue damage compared to conventional surgical techniques [8, 9]. Such developments help to reduce surgical stress, inflammation, and ischemia–reperfusion injury, among other biochemical elements, which are significantly higher in conventional surgery [10, 11]. This in-depth understanding of the cellular mechanisms affected by robotic procedures can provide surgeons with critical insights, allowing them to evolve their approaches and potentially improve patient outcomes. Therefore, this detailed review attempts to explore robotic surgery from a biochemical perspective, with a particular focus on the da Vinci platform. It aims to highlight the key molecular advantages associated with robotic surgery and its role in attenuating tissue damage, surgical stress, ischemia–reperfusion injury, and inflammation. The review will also cover relevant research that links these molecular factors to improved clinical outcomes. Finally, the review will address the emerging research, emerging technologies, and prospects aimed at understanding the challenging biochemical implications of performing robotic surgery. In addition, it will provide valuable insights into how advances in the field can foster continued innovation.
The basics of robotic surgery
Robotic surgery utilizes the advanced technology of robotic systems to enable surgeons to perform minimally invasive procedures through miniature incisions using tools built into robotic arms that provide multiple points of articulation [9]. The cornerstone of this technology is a robotic surgical console that provides a high-definition, three-dimensional view of the patient’s body, as well as mechanically enhanced instruments that exceed the agility of the human hand, supplemented by computerized assistance to perform functions or prevent unwanted movements [12, 13]. This technology allows for superior precision, control, and maneuverability that surpasses conventional laparoscopic surgery. The most common and technologically sophisticated robotic system currently available is the da Vinci framework from Intuitive Surgical, Inc. The procedure for a typical robotic surgery is shown in Fig. 1 [6, 9, 14].
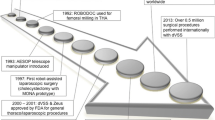
The first breakthroughs in robotic surgery date back to the 1980s, when the very first robotic mechanism, the PUMA 560, was used to perform neurosurgical biopsies [15]. However, the capabilities of this early version were limited by inadequate imaging and computer processing capabilities. A collaborative effort between surgical and engineering experts in the 1990s led to the development of the da Vinci prototype [6, 9]. Its steady improvement over the years led to its FDA clearance for general laparoscopic surgery in 2000 [16]. The following 2 decades witnessed a remarkable expansion in the capabilities and application of robotic surgery, escalating to over 1.5 million robotic procedures per year by 2018, including complex surgeries across multiple medical specialties such as urology, gynecology, colorectal surgery, cardiothoracic surgery, and others [9, 17].
In the current scenario, robotic surgery is well accepted and has become a standard practice in various medical fields. Its widespread implementation includes 80% of radical prostatectomies [18], and over 50% of partial nephrectomies [19], and over 50% of partial nephrectomies [20] performed in the United States, and its adoption continues to grow for procedures such as cystectomies, hysterectomies, etc. [21, 22]. The newer Xi model of the da Vinci system boasts adaptive tools, slimmer arms, three-dimensional fluorescent imaging, along with a host of other technological advances [23]. In addition, companies such as Medtronic and Johnson & Johnson have ventured into the development of robotic platforms, accelerating the pace of this surgical approach [24]. However, the challenges of cost, device size, haptics, and suitability for complex surgical procedures remain. The growth trajectory of robotic surgery will require a critical examination of the balance between technological advancement, clinical safety, and patient benefit.
Biochemical changes in robotic surgery
Metabolic alterations in the patient
Robotic surgery involves multiple elements, including anesthesia, pneumoperitoneum, and surgical stress, that can deep affect the patient’s metabolic state. A thorough understanding of the complex biochemical changes induced by these factors is important to improve postoperative outcomes. The biochemical processes involved in robotic surgery are outlined in Fig. 2, and the comparison of some biochemical parameters in traditional vs. robotic surgery are shown in Table 1.
Impact of anesthesia and pneumoperitoneum
Robotic procedures require the use of general anesthesia and neuromuscular blockade, which induce a hypometabolic state by reducing CO2 production and oxygen consumption [25]. Certain anesthetics, such as volatile sevoflurane and intravenous propofol, affect mitochondrial function and substrate utilization, increasing dependence on fat and glycogen stores [26]. The result is an alteration in energy metabolism due to the impairment of mitochondrial oxidation, glycolysis, and fatty acid oxidation processes [27]. However, modern short-acting anesthetics have shown a faster resumption of metabolic activity than their older counterparts [28].
Robotic surgery requires insufflation of the abdominal cavity with carbon dioxide to create the necessary space and visualization. However, pneumoperitoneum can adversely affect splanchnic perfusion, often resulting in mild hypercarbia due to CO2 absorption and changes in acid–base balance [29]. Research has documented significant reductions, greater than 30%, in hepatic perfusion and function during robotic prostatectomy [30]. The inflation of intra-abdominal pressure constricts vessels, thereby inhibiting perfusion, leading to procedural strategies such as low-pressure pneumoperitoneum and sporadic desufflation to mitigate these effects [31]. Comprehensive metabolic panel testing is used to rapidly correct any blood gas, fluid, and electrolyte abnormalities after surgery [29]. These metabolic alterations may influence outcomes such as return of bowel activity, postoperative illness, and surgical wound healing [32]. Further research is essential to develop the most effective strategies to manage anesthesia and pneumoperitoneum to reduce the likelihood of adverse biochemical changes in patients undergoing robotic surgery. Real-time monitoring of metabolic markers may be beneficial in guiding management in the operating room.
Stress response and cytokine release
Surgical stress is characterized by a variety of hormonal, metabolic, and inflammatory responses induced by tissue damage and trauma [52, 53]. These responses include hyperglycemia (36), insulin resistance [54], hyperglycemia [55], muscle protein catabolism [32], immune dysfunction [56], and impaired wound healing [54, 56]. In addition, there is increased secretion of inflammatory cytokines such as IL-6, IL-1, and TNF-α [57, 58]. Conversely, there is a decrease in the concentration of anabolic hormones such as testosterone [59]. This stress response is initiated by signals from the sympathetic nervous system, along with the release of cortisol and catecholamines [60, 61]. As several studies have shown, this neuroendocrine stress response is less pronounced with the use of robotic techniques compared to traditional open surgery. In a randomized study of 80 patients, postoperative IL-6 levels were found to be significantly lower in patients who underwent robotic hysterectomy compared to those who underwent open abdominal hysterectomy (P < 0. 05) [62]. The local release of cytokines is attenuated by reduced tissue damage due to smaller incisions, while systemic sympathetic activation is attenuated by less bowel manipulation [63]. However, the potential for some surgical stress is inevitable. Measures such as aggressive control of perioperative glucose levels with insulin infusions have been shown to reduce hyperglycemia and associated complications in surgical patients [64, 65]. Ongoing research into the complex pathways affected by surgical stress may provide additional targets for reducing the catabolic effects on the body [31, 66]. Ultimately, robotic surgical approaches significantly reduce metabolic stress compared to traditional open surgery. However, the complex changes induced by anesthetic use and tissue damage must be proactively managed and anticipated throughout the perioperative period to achieve the best patient outcomes. Clinicians can benefit from a comprehensive biochemical understanding to holistically optimize a patient's metabolic state. Further large-scale, randomized trials are needed to fully understand the impact of robotic techniques on modulating the surgical stress response.
Tissue and cellular responses
Compared to open surgery, robotic surgery offers a plethora of advantages at both the cellular and tissue levels. The complex biochemical differences inherent in these surgical procedures ultimately result in improved clinical outcomes for patients who choose robotic surgery.
Oxidative stress and antioxidant defenses
Oxidative stress is an imbalance between the production of reactive oxygen species [67] and the body's defense against antioxidants [68]. This redox homeostasis can be disrupted by surgical trauma and ischemia–reperfusion injury, phenomena that lead to overproduction of ROS and subsequent oxidative damage to proteins, lipids, and DNA [69, 70]. Major ROS include free radicals such as superoxide and hydroxyl radicals, as well as non-radicals such as hydrogen peroxide and peroxynitrite [71].
Robotic surgical techniques serve to counteract oxidative stress through precise dissection and minimization of tissue injury. A randomized trial of 80 patients demonstrated significantly lower plasma levels of malondialdehyde, indicating reduced lipid peroxidation, in individuals who underwent robotic hysterectomy compared to those who underwent open hysterectomy (2.1 vs. 3.8 μmol/L, p < 0.01) [72]. The enhanced visualization and stability provided by robotic systems allow for precise dissection, helping to avoid vascular complications and ischemia-induced injury [73, 74]. This results in reduced generation of ROS, including highly reactive radicals such as superoxide, hydroxyl, and peroxide, upon reperfusion [75]. Preservation of endogenous antioxidant systems plays a key role in neutralizing oxidative threats after surgical procedures [76]. Robotic surgery aids in this process by mitigating oxidative damage, thereby promoting faster recovery compared to open surgery. One study found that patients who underwent robotic cystectomy had a return of bowel function one day earlier than those who underwent open cystectomy [77]. In addition, these robotic techniques also help maintain antioxidant levels by reducing surgical stress and preserving liver and kidney function [78, 79].
However, some investigators have found no significant difference in markers of oxidative stress between patients who underwent robotic surgery and those who underwent open surgery [80, 81]. Therefore, additional research is warranted to further elucidate the changes in redox at the genomic, proteomic, and functional levels. A deeper understanding of these intricate biochemical changes may help to optimize the administration of antioxidants throughout the perioperative period.
In conclusion, robotic surgery offers a number of benefits in maintaining redox homeostasis. These benefits are achieved by reducing oxidative insults and maintaining the activity of endogenous antioxidants. Consequently, this contributes to the creation of an optimal biochemical environment that favors patient recovery following surgical procedures.
Inflammatory responses
Surgical trauma induces an inflammatory response that has both local effects at the site of tissue injury and broader systemic implications. However, these changes can be mitigated by robotic techniques with their precise dissection capabilities and reduced surgical trauma [82, 83]. The limited tissue disruption and smaller incisions characteristic of robotic surgery limit the local release of pro-inflammatory cytokines such as IL-6 and TNF-α [67, 82]. It also reduces leukocyte infiltration at the surgical site [84]. Evidence suggests a significant reduction in systemic levels of cytokines such as IL-6 and acute phase reactants such as CRP and PCT in patients undergoing robotic hysterectomy and prostatectomy compared to open procedures (p < 0.05) [72, 85, 86]. In addition, the attenuated neuroendocrine activation of robotic surgery also serves to suppress the production of downstream inflammatory mediators [77, 87]. The attenuation of the inflammatory response accelerates patient recovery time and helps prevent common postoperative complications such as delayed wound healing, infection, and adhesive bowel obstruction [70, 88]. The refined movements and flexibility of robotic instruments allow for gentle dissection, preserving intact anatomical planes between tissues [89]. This results in reduced postoperative adhesions, thereby reducing potential pain and organ dysfunction [89, 90]. However, it is important to recognize that some degree of inflammation is a critical component of normal surgical healing [70]. Initial cytokine activation attracts regenerative cells such as neutrophils and macrophages to the surgical site to remove debris and pave the way for repair [91]. Future studies should focus on detailing the intricate effects of robotic surgery on this complex biochemical balance. Pharmacologic therapies that could fine-tune the inflammatory response during the perioperative period may also hold promise for improving outcomes.
In summary, robotic surgery offers tangible benefits in reducing both local and systemic inflammation. However, it is important not to unduly suppress this vital biochemical process that governs surgical recovery. Further investigation of the intricate inflammatory pathways affected by minimally invasive robotic techniques will be key to optimizing patient outcomes.
Hemostasis and coagulation
Robotic surgery presents unique hurdles in successfully achieving hemostasis and preventing coagulopathy-related complications. Because the surgeon cannot directly feel or access the surgical site, innovative methods have been implemented to maintain hemostasis and normal coagulation [92].
Changes in blood clotting parameters
During surgical procedures, a variety of changes in hemorheology and hemostasis can occur, potentially leading to complications such as thromboembolism [93]. These changes can include hyperreagibility of platelets with increased aggregation and adhesion tendency, changes in protein concentrations affecting viscosity and red cell aggregation, impairment of red cell deformability, increase in clotting factors, and disturbance of fibrinolysis characterized by diminution of plasmatic plasmin and increase in antiplasmin activity [92]. Robot-assisted procedures can help maintain normal coagulation and clotting function by reducing tissue trauma and using surgical hemostats, sealants, and adhesives to rapidly achieve hemostasis [94]. By reducing tissue injury, the local procoagulant response can be suppressed, resulting in a decrease in thrombin production and fibrin formation.
In addition, maintaining blood flow and vascular integrity during surgical procedures may help maintain the anticoagulant balance of endothelial nitric oxide and prostacyclin, thereby preventing aberrant endothelial cell activation and coagulation amplification [95]. For example, one specific study found a 50% reduction in levels of the catecholamine norepinephrine after robotic-assisted prostatectomy compared to open radical prostatectomy [96]. This may be due to the minimally invasive nature of robotic surgery, which may result in less tissue damage and a more regulated surgical environment [97]. In addition, a 30–40% reduction in platelet activation has been observed after robotic surgery compared to laparoscopic or open procedures [93]. In conclusion, robotic surgery can play an important role in preserving normal coagulation function and preventing abnormal coagulation and associated complications by reducing tissue damage, skillfully using surgical hemostats, sealants, and adhesives, and maintaining the integrity of the blood flow and vasculature. These factors contribute to a regulated and balanced coagulation response, reducing the likelihood of coagulopathy-related complications.
Potential implications for thromboembolic events
Robotic surgery has several potential implications for thromboembolic events, particularly deep vein thrombosis (DVT), that warrant further investigation. First, the longer operative times associated with robotic surgery may increase venous stasis and hypercoagulability, thereby increasing the risk of DVT [98]. In a comparative study of open and robotic prostatectomy, the mean operative time was significantly longer in robotic cases (5.8 h vs. 3.6 h, p < 0.001) and the incidence of DVT was increased (8.0% vs. 1.0%, p = 0. 024)[99]. The pronounced Trendelenburg position used in robotic surgery may also exacerbate venous stasis in the lower extremities by causing blood stagnation, as evidenced by a 30–50% increase in postoperative leg swelling compared to the supine position [97].
Additionally, the pneumoperitoneum created during robotic surgery has been associated with venous endothelial injury, reductions in fibrinolytic activity by up to 30%, and temporary hypercoagulability [72]. This effect may persist during the postoperative period, as evidenced by a study noting impaired fibrinolysis for up to 2 weeks after robotic prostatectomy [99]. The prolonged analgesic effects of epidural anesthesia combined with limited mobility after surgery may provide further risk [100]. In addition, the creation of pneumoperitoneum during robotic surgery has been associated with venous endothelial damage, a decrease in fibrinolytic activity of up to 30%, and transient hypercoagulability [101]. Such effects may persist into the postoperative period, as evidenced by a study reporting impaired fibrinolysis for up to 2 weeks after robotic prostatectomy [99]. A prolonged period of analgesia from epidural anesthesia combined with limited postoperative mobility may pose another significant risk [102, 103]. Various mitigation strategies, such as intraoperative pneumatic compression devices, early mobility, and chemical thromboprophylaxis, may help to reduce the increased risk of thrombosis associated with robotic surgery [104, 105]. However, additional research is needed to elucidate optimal approaches to DVT prevention in this population. In a retrospective analysis, only 21% of patients undergoing robotic surgery received guideline-recommended thromboprophylaxis, suggesting that there is ample room for improvement [105].
In conclusion, the influence of robotic technique on hypercoagulability and thrombosis formation requires ongoing investigation across multiple surgical disciplines. Further clinical investigation is needed to formally evaluate formal thromboembolic event rates and determine appropriate preventive strategies in patients undergoing robotic surgery. Elucidation of these risks and establishment of effective DVT prevention protocols will play a pivotal role in optimizing the safety and benefits of robotic surgical approaches. The potential biochemical problems associated with robotic surgery, along with their respective countermeasures, are summarized in Table 2.
Biochemical impact on surgical outcomes
Patient outcomes
Numerous studies have shown that robotic surgery results in better patient outcomes than traditional open surgery. This improvement is likely due to differences in surgical stress and inflammatory responses [115]. Various benefits of robotic surgery over open surgery have been observed in numerous medical specialties, including less blood loss, less need for transfusions, and lower rates of complications [116,117,118].
For example, a comprehensive review of 19 different studies found that estimated blood loss from robotic prostatectomy was significantly less than that from open prostatectomy, with an average difference of approximately 305 mL [119]. The use of robotic techniques, known for their precise movements and minimal tissue disruption, facilitates careful hemostasis [120, 121]. A decrease in surgical trauma can potentially reduce the impact on coagulation cascades and fibrinolysis, resulting in less bleeding [119]. In particular, one specific study pointed to a decreased level of postoperative fibrinogen degradation in patients undergoing robotic prostatectomy, indicating reduced coagulation activity [122].
In addition, robotic procedures have been associated with a decrease in postoperative complications such as wound infection [123]. This may be due to smaller incisions and the avoidance of extensive abdominal wall retraction, which can negatively impact oxygenation and perfusion [123]. Evidence also suggests that robotic surgery results in less severe changes in cytokine levels, CRP, and other mediators of the systemic inflammatory response compared to traditional open procedures [83, 124, 125]. The resulting reduced inflammation may well contribute to improved wound healing and a reduction in infectious complications such as surgical site infections [125].
In conclusion, robotic surgery offers several advantages for patient outcomes over traditional open methods, apparently due to a reduction in surgical stress and inflammatory responses. Further investigation to quantify the influence of specific mediators on outcomes such as blood loss and wound healing may prove invaluable. Such knowledge may help to fine-tune surgical techniques and perioperative management to fully realize the benefits of robotic surgery.
Postoperative pain and analgesic requirements
There is a growing body of research that indicates a significant reduction in postoperative discomfort and the need for pain medication in patients who undergo robotic surgery rather than traditional open procedures. This result may be due to reduced tissue trauma made possible by the precision of robotic instruments and enhanced visual capabilities that allow for meticulous dissection [115, 123, 126]. In a study comparing open and robotic prostatectomy, patients who underwent robotic surgery required 48% less intravenous morphine in the first 24 h after surgery, indicating a significantly lower pain level (22 mg vs 48 mg, p < 0.001) [127]. In addition, another study concluded that postoperative pain scores at 6 and 24 h were significantly lower in patients who underwent robotic hysterectomy compared to those who underwent laparoscopic hysterectomy [128]. A reduction in inflammatory markers such as IL-6 and CRP after robotic surgery may explain the observed reduction in postoperative pain [124]. Thus, further research is recommended to delve deeper into specific biochemical pathways and molecular mechanisms involved in pain signaling, particularly those that are influenced by surgical techniques. Elucidation of these mechanisms leading to reduced pain may help to optimize protocols for pain relief, for example, a detailed assessment of changes in nociceptive neurotransmitters, opioid receptors, or pain signaling cytokines. This would essentially pave the way for novel targets for pain management in post-robotic surgery patients.
In conclusion, there is clear evidence that robotic surgery significantly reduces postoperative discomfort and analgesic requirements compared to open surgery. Expanded studies to understand the mediators behind this improvement could significantly complement recovery protocols to take full advantage of robotic techniques.
Recovery and length of hospital stay
A significant body of research indicates that patients benefit from faster recovery of gastrointestinal function, earlier ability to mobilize, and shorter hospital stays following robotic surgery compared to traditional open procedures [16, 115, 129]. For example, one specific clinical trial highlighted that robotic cystectomy led to patients regaining bowel function two days sooner (3 days versus 5 days, p < 0. 01) and resulted in a median hospital stay three days shorter than traditional open cystectomy (5 days versus 8 days, p < 0. 001) [130]. The accelerated recovery after robotic surgery may be attributed to less surgical stress and a reduction in systemic inflammation. In support of this premise, studies have shown lower postoperative cortisol levels, an accepted measure of stress response, and inflammatory markers such as IL-6 and CRP in patients who underwent robotic surgery [83, 131]. It would be beneficial for further research to focus on quantifying the effects of reducing specific biochemical mediators, as this may reveal potential targets for interventions aimed at improving and accelerating patient recovery after robotic surgery. In conclusion, the available evidence clearly signals that robotic surgical techniques offer clear advantages in terms of earlier gastrointestinal recovery, mobilization and readiness for discharge compared to traditional open methods. Elucidation of the intrinsic biochemical pathways that influence surgical recovery may open the door to refined protocols that take full advantage of minimally invasive robotic techniques.
Surgical complications
Robotic surgery, a revolutionary technique in the medical field, increases the precision and range of surgical procedures. However, as with any surgical procedure, there are inherent risks, including bleeding, infection, inadvertent damage to surrounding nerves or organs, and mechanical failure of instrument components [132, 133]. In a review of more than 2000 robotic prostatectomies, the rate of major bleeding complications requiring transfusion was 1.4% [134]. However, the rate of bleeding varies depending on the type of procedure and each patient’s unique health factors. Of concern is the potential for infection due to the complexity of the robotic system. For example, a comparative analysis found that the incidence of postoperative urinary tract infections was higher with robotic prostatectomy (8. 1%) than with traditional open prostatectomy (5.7%) [135]. Mechanical failure of robotic instrument components during surgery has been estimated to occur in 0.5–1.5% of cases [135]. The repeated use of such instruments and their torqueing can lead to these problems. Incorrect application of the robotic technique can also result in nerve injury, often caused by compression or traction. Traction-related accessory nerve injuries have been reported in 1. 3% of robotic neck dissections [136]. This type of injury is more common in procedures involving confined areas such as the pelvis. The risk of inadvertent damage to tissues or organs increases due to the limited haptic feedback offered by robotic platforms, potentially leading to unexpected postoperative complications [137]. In some cases, conversion to open surgery may be required, resulting in increased operative time and morbidity. The prevalence of such conversions ranges from 1 to 5%, depending on the type of procedure [138, 139].
The risks of robotic surgery can be mitigated by implementing measures such as rigorous patient screening, thorough training of medical teams, and continuous technological improvements. Despite these efforts, intense vigilance and further research to improve safety measures are warranted, as specific complications that inherently exceed the risks of open surgery may persist [132]. Achieving a balance between the benefits of robotics and a cognizant, proactive response to the associated risks is essential to the responsible integration of this technology into regular medical practice.
Wound healing and infection risk
Advances in robotic surgery have made significant contributions to promoting better wound healing and reducing the risk of infection compared to traditional open surgery. The key factors leading to these improvements are likely to be smaller incisions and less severe tissue damage, which in turn minimizes the body’s stress responses induced by surgery [140]. Evidence from a comparative study of conventional and robotic colectomies showed that the group that underwent robotic surgery had a relatively lower rate of wound infection. These were recorded at 4% compared to the 11% infection rate for the open surgery group, despite the longer operation time for the former group [141]. Similarly, a study comparing robotic and traditional radical cystectomies found that the former required less post-operative wound care [141]. The reduced inflammation caused by the muted release of cytokines following robotic surgery may be a factor that promotes wound healing while reducing susceptibility to infection [142]. However, further research is needed to gain a deeper understanding of the biomolecular mechanisms that influence healing and immune responses after robotic surgery.
Gastrointestinal motility and complications
Numerous studies have shown faster return of bowel function and reduced complication rates after robotic surgery compared to traditional open gastrointestinal surgery [141, 143]. As an example, a comprehensive review of patients undergoing robotic or open colorectal surgery showed an approximately 0.63 day earlier return of bowel movement in those undergoing robotic surgery [144]. Other studies of colectomy (117) and gastrectomy [145] and gastrectomy [146] demonstrated accelerated timing of flatus passage, bowel movement, and initiation of food intake with robotic procedures. These benefits may be attributed to less bowel disruption, less inflammation, and the avoidance of the need for the large abdominal incisions characteristic of open surgery [147]. In addition, robotic techniques are less likely to disrupt gastrointestinal peptides that control motility [10].
These beneficial aspects could potentially result in a lower incidence of common GI complications such as ileus following robotic surgery. One such study highlighted a significant reduction in the incidence of postoperative ileus (2.1% vs. 5.3%) associated with shorter hospital stays with robotic versus open colectomy [10, 147]. The need of the hour is to conduct additional studies to fully understand the biochemical initiators of improved gastrointestinal recovery after robotic surgery. A detailed understanding of these pathways could potentially provide critical insights for refining strategies to combat postoperative ileus, anastomotic seepage, and subsequent complications that affect surgical patients.
Oncological considerations
Robotic surgical procedures in the field of oncology have generated considerable interest as well as concern about their impact on long-term cancer outcomes [148, 149]. A thorough evaluation of such robotic approaches to tumor resection requires a holistic understanding of several factors:
-
1.
Surgical margins: While robotic instruments are adept at meticulous dissection, they unfortunately lack tactile feedback. The existence of conflicting data raises the question of whether this affects surgical margins in oncologic specimens [148, 150]. It is noteworthy that a study comparing open versus robotic mesorectal dissection for rectal tumors found an increase in positive margins in 19% of patients versus 5% [151] highlighting the need for rigorous pathologic review.
-
2.
Lymph node dissection: Robotic flexibility in complex, confined anatomic spaces such as the pelvis allows for lymph node dissection [152]. However, the efficacy of robotic lymph node dissection remains controversial for a variety of cancers [153]. It is important to note that in endometrial cancer, several studies have shown similar lymph node yields with robotic, laparoscopic, or open techniques [154].
-
3.
Recurrence patterns: Data are currently limited regarding the risk of local or distant recurrence following robotic oncologic surgery. Risks vary by cancer site, with some studies suggesting a higher recurrence rate than with laparoscopic surgery [151]. Therefore, comprehensive, and long-term research studies are urgently needed to assess the risk of recurrence over time.
-
4.
Survival: Only a few single-case analyses have evaluated the impact of robotic surgery on survival for prostate, uterine, and colorectal tumors [149, 151, 155]. Meta-analyses have not shown significant differences in survival versus laparoscopic surgery [156].
-
5.
Cost: The significant expense associated with the purchase and maintenance of robotic systems is a concern for many medical institutions. At this time, it is uncertain whether outcomes in cancer management will justify such substantial expenditures [157].
-
6.
In conclusion, while there are opportunities for robotic surgery in cancer, several uncertainties related to margins, lymph nodes, recurrence rates, and survival require further investigation through large and ongoing studies. In short, the decision-making process regarding the use of robotic surgery versus traditional laparoscopic surgery for tumor resections will be guided by future research investigations and their results.
Tumor growth, dissemination, and recurrence
Cancer progresses through multiple acquired competencies at the cellular level, including maintenance of proliferative signaling, resistance to cell death, initiation of angiogenesis, and stimulation of invasion and metastasis [158]. Understanding the molecular specificities that underlie tumor spread and recurrence is key to improving both surgical and pharmacological interventions. The initiation and progression of invasion and metastasis depend on the epithelial-mesenchymal transition (EMT), which involves the alteration of the expression of cellular adhesion molecules and transcription factors such as Twist and Snail [159, 160]. Other key elements include matrix metalloproteinases such as MMP-9 that degrade the extracellular matrix, integrins that establish interactions with matrix proteins, and cytokines that enhance cellular motility and invasion [161,162,163]. Despite entry into the circulation, metastatic colonization of distant sites remains a challenge, with a success rate of less than 0.1% for circulating tumor cells [164]. The molecular mechanisms that enable metastatic growth are the subject of ongoing research. Despite stringent treatment protocols, recurrence driven by therapy-resistant cancer stem cells continues to contribute significantly to mortality [165]. These cells have the ability to reactivate functions such as sustained proliferation, angiogenesis, EMT, and metastasis to resurrect tumors [166]. Recurrence after surgical resection is promoted by factors such as circulating tumor cells, immunosuppressive wound healing, and increased colonization [167,168,169]. Identifying the molecular vulnerabilities of recurrent tumors and stem-like cells is an important research focus.
In summary, elucidating the mechanisms that enable metastatic colonization, targeting cancer stem cells, and uncovering the dependencies of recurrent disease remain key priorities for the development of more effective anticancer therapies and the prevention of relapse.
Immune responses and cancer outcomes
The immune system has a dual nature in relation to cancer, possessing capabilities that both inhibit and promote tumor development [170]. Cells of the immune system, namely natural killer (NK) and CD8 + cytotoxic T lymphocytes (CTLs), have the ability to counteract early-stage tumors via effector molecules such as perforin, granzymes, and IFN-γ, which facilitate the demise of cancer cells [171, 172]. On the other hand, prolonged inflammation, fueled by substances such as tumor necrosis factor alpha (TNF-α), various interleukins, and chemokines, creates an environment conducive to tumor progression by providing factors necessary for their growth and survival [173, 174]. Established tumors employ a variety of strategies to evade immune retaliation, including reducing tumor antigens, secreting immunosuppressive cytokines such as TGF-β, expressing PD-L1 to restrict T cells, and recruiting regulatory T cells that suppress the body's immune response [170, 175]. The interaction between PD-L1 and PD-1 on T cells inhibits anti-tumor cytotoxicity. The balance between immune activation and suppression plays a critical role in determining patient outcomes [176].
Therapies focused on immune checkpoint inhibition, which boost the body's immunity against tumors by blocking PD-1, PD-L1 or CTLA-4, have shown exceptional efficacy against certain cancers [172, 177]. However, specific uncertainties remain regarding the determinants of the immune response. Studies aimed at characterizing immune cell infiltration and the tumor microenvironment are providing crucial information about immune factors that influence cancer progression and the success of therapies [170, 178, 179]. Key areas under investigation include increasing the efficacy of immunotherapy, determining the determinants of anti-tumor immunity, and identifying strategies to target pro-tumor inflammatory pathways.
Biochemical advances in robotic surgery
Intraoperative biochemical monitoring
Real-time biomarker assessment
Traditional methods of postoperative laboratory testing lack the ability to provide immediate, real-time feedback that could influence intraoperative decision-making [180]. This highlights the potential value of quantitative monitoring of key biochemical parameters and biomarkers during the course of surgery as a means of tailoring procedures and potentially improving postoperative outcomes [181, 182]. Notable intraoperative biomarkers currently under investigation include entities such as tumor margins, circulating tumor cells (CTCs), nucleic acids, proteins, metabolites, and microRNAs (miRNAs) [173, 183, 184]. Groundbreaking techniques such as immediate margin assessment by frozen section suggest potential methods to ensure thorough tumor removal [185]. In addition, specific miRNA patterns have been identified as potential predictors of increased likelihood of metastasis in early-stage cancers [173].
Technological advances are underway to enable intraoperative, real-time, and quantitative assessment of multiple biomarkers, allowing for more precise staging and personalized surgical strategies [186]. Devices based on mass spectrometry and Raman spectroscopy fiber-optic systems are under development. These aim to simultaneously measure proteins, metabolites, and other important molecules in situ during the surgical process [187, 188]. The integration of these technologies in conjunction with surgical robotic systems and AI algorithms could facilitate the interpretation of complex biomarker patterns and thereby enable intelligent decision making during surgical procedures [189, 190].
In summary, it is envisioned that real-time biomarker monitoring could provide essential molecular information that would enable optimization and individualization of surgical interventions for individual patients. Key potential outcomes could include reduction in positive margin rates, elimination of unnecessary lymph node dissection, reduced risk of recurrence, and avoidance of re-excision. To fully realize these benefits would require continued innovation in platforms capable of rapid, multiplex biomarker analysis during oncologic surgery.
Role of biosensors and nanotechnology
The potential lies in miniaturized biosensors and nanotechnology platforms to serve as catalysts for rapid, on-site biochemical analysis during robotic surgery [191]. Biosensors have the ability to convert biological signals into measurable outputs by using a mixture of biorecognition elements such as antibodies, aptamers, or enzymes as partners with electrochemical, optical, or mass sensitive transducers [192, 193]. The biorecognition elements provide a selective element for which the transducers generate a detectable output signal. Numerous nanomaterials possess properties suitable for their incorporation into in situ ultrasensitive biomarker detection, including quantum dots, carbon nanotubes, nanowires, and graphene, all of which possess unique electrochemical, fluorescent, and biocompatible properties [194]. For example, fluorescent semiconductor quantum dots linked to tumor-targeting ligands have demonstrated efficacy in guided tumor excision in mouse models, raising the possibility of application to direct surgery in vivo [195, 196]. Fiber optic sensors with integrated Raman spectroscopy are being developed to provide label-free, rapid biochemical fingerprinting of tissue during endoscopic procedures [197].
Previously reported intraoperative applications include electrochemical biosensors used to detect cancer biomarkers [198, 199] and lab-on-a-chip technologies implemented for proteomic, metabolomic, and nucleic acid assays [192]. Further advances in wireless, miniaturized sensors compatible with surgical instruments for direct application to tissue could improve the efficacy of in situ biochemical analysis. However, these innovations must overcome several barriers to successful applicability and implementation, including concerns about reproducibility, reliability, selectivity, interference from biological fluids, and seamless clinical integration [192, 200, 201]. By continually adapting and improving the responsiveness, functionality, and correlation to surgical outcomes of these devices, it is plausible that intraoperative biosensors and nanotechnologies will provide insightful, real-time problem-solving strategies to guide and optimize robotic surgery by providing pertinent biochemical data.
Pharmacological interventions
Novel drug delivery methods
Disadvantages of traditional chemotherapy include generalized systemic toxicity and less than ideal positioning within the tumor site, which indirectly reduces efficacy and induces side effects [202]. The enhanced visualization, instrumentation, and accessibility of robotic surgical systems provide new opportunities to explore breakthrough approaches to localized drug delivery that take advantage of these benefits [203, 204]. Targeted delivery of chemotherapy and immunotherapies to the tumor or resection margins could improve treatment outcomes while minimizing side effects [205, 206].
New innovative methods being explored include injecting therapy-loaded nanoparticles or hydrogels directly into the tumor, combining them with tiny implantable pumps for sustained local release over several weeks or months, positioning drug-eluting films along resection margins during surgery, and individualized perfusion of a limb or organ [203, 207, 208]. Nanoparticles and mini-pumps are in the early stages of preclinical testing, while perfusion is in clinical use but limited to extremities [209]. More extensive integration with real-time imaging and biosensors could provide spatiotemporal control of dosing based on factors unique to each patient [210].
Nevertheless, significant challenges remain, including demonstration of safety and efficacy, large-scale production, economic viability, and regulatory approval [210, 211]. Regardless of the hurdles, the idea of using surgical robots as multifunctional platforms for both resection and tailored local drug delivery is promising and a prospective area for ongoing research. Advances in manufacturing methods, drug formulations, and clinical trials will be paramount in translating these innovative ideas into beneficial treatments for patients.
Precision medicine approaches
Precision medicine aims to provide health care that is highly personalized to individual patients, taking into account their unique genetic, molecular, and lifestyle characteristics, as opposed to traditional one-size-fits-all treatments [212]. The advanced field of robotic surgery has expanded the ability to obtain patient-specific molecular data by profiling the genomes, transcriptomes, and proteomes of excised tumor tissues [213, 214].
The superior instrumentation of surgical robots opens the door to meticulously detailed tumor sampling. This allows us to identify specific mutations, expression patterns, and other biomarkers, enabling an unprecedented level of subtyping beyond histology that can inform personalized treatment options [215, 216]. For example, the presence of HER2 amplification indicates a more favorable response to HER2-targeted therapies such as trastuzumab [217], whereas BRCA1/2 mutations render tumors more susceptible to PARP inhibitors [218, 219]. Detailed assessment of immune cell infiltrates is useful in selecting appropriate immunotherapies [219].
The combination of precision diagnostics with robotic tumor resection and tailored pharmacotherapy has the potential to significantly improve outcomes by using treatments that target specific molecular features and are individualized for each patient [220]. However, realizing this potential faces several barriers, including effective tissue sampling, data analysis, integration into clinical practice, and reimbursement and regulatory policies. To overcome these barriers, collaborative efforts across oncology specialties are critical [220]. In summary, the merging of advanced robotic surgery with precision medicine methodologies opens new opportunities to push the boundaries of pharmacotherapy through superior molecular characterization of tumors and treatment personalization. However, the establishment of multidisciplinary partnerships, provider education, and new clinical paradigms are critical components to overcome barriers to implementation.
Personalized patient management
Precision medicine has the explicit goal of providing care that is specifically tailored to each patient's genetic makeup and health profile. These methods can be applied to robotic surgery to improve patient outcomes.
Genetic and epigenetic profiling
A comprehensive assessment of a patient's genomic and epigenomic landscape prior to robotic surgery can reveal genetic variations and epigenetic determinants that may influence drug metabolism, likelihood of complications, and additional surgical outcomes [221, 222]. An example of its application could be the genotyping of enzymes related to the cytochrome P450 system, such as CYP2D6 and CYP2C19, which are involved in drug metabolism and can potentially dictate operationally relevant drug dosages [223]. Specific genetic polymorphisms, including those in catechol-O-methyltransferase (COMT), may also serve as predictive markers of postoperative pain sensitivity [224]. Performing a preoperative epigenetic profile scan may reveal epigenetic dynamics that amplify surgical hazards such as infection, including miRNA regulation of immune pathways [225]. With this understanding, the surgical team can develop preemptive measures to offset genetic and epigenetic risks through personalized care approaches.
Tailored perioperative care strategies
Personalized perioperative care strategies based on genetic and epigenetic test results have several applications. Some of these include calibrating the dose of anesthesia for patients with ultra-rapid CYP2D6 metabolism to avoid adverse effects [220]; using prophylactic antibiotics for patients who genetically have a higher susceptibility to contracting infections [226]; implementing improved monitoring protocols for patients who have been identified through genetic evaluation as being at high risk for stroke [227, 228]; and selecting ideal pain control regimens determined by genetic polymorphisms that influence response to analgesics [229]. Overall, perioperative care guided by genetic knowledge could potentially reduce complications and accelerate recovery from robotic surgery by tailoring management to each patient's unique genetic and epigenetic blueprint.
Future directions and challenges
Significant progress has been made in the field of robotic surgery, but continued research and innovation are essential to fully realize its potential benefits. Future work is anticipated in the continued development of surgical biomarkers along with real-time analysis methods that could facilitate personalized and tailored procedures [230, 231]. For example, innovative technologies such as mass spectrometry and Raman spectroscopy could accelerate multi-biomarker analysis and potentially guide surgical decisions in real time [220, 232]. The integration of such technologies with surgical robotics, artificial intelligence, and molecular imaging is an exciting breakthrough in the making [233]. However, cost, workflow integration, and clinical validation remain challenges. In addition, there is a call for a deeper understanding of the intricate biochemical pathways that influence surgical stress responses, wound healing, and cancer progression [234]. This understanding could help refine pharmacological and procedural approaches, accelerate patient recovery, and improve cancer treatment. However, thorough investigation of these multifactorial pathways is a daunting task [235].
With further development, increasing the functionality of surgical robots could enable the performance of highly complex surgeries across a range of specialties [236, 237]. However, striking a balance between cost and incremental benefit remains an ongoing challenge. The solution may lie in modular, upgradeable platforms [238].
Finally, the economic and environmental concerns associated with the manufacture, use, and disposal of surgical robots cannot be overlooked [236]. Designing energy-efficient systems and recycling components could help alleviate these sustainability issues. In summary, realizing the enormous potential of robotic surgery will require a multidisciplinary approach to address the remaining challenges of integration, adaptation, mechanistic understanding, and responsible innovation. Accelerating advances in surgical procedures, patient recovery times, and outcomes, however daunting, is indeed a journey worth taking.
Conclusion
The advent of robotic surgery represents a transformative change in healthcare, offering improved surgical skills but also introducing new biochemical considerations that require attention. This analysis addresses the unique effects of pneumoperitoneum, patient positioning, ischemia–reperfusion, and systemic stress responses associated with robotic procedures. Prudent fluid management, gas exchange monitoring, tissue perfusion assessment, and organ support are the cornerstones of mitigating these effects. In addition, the impact on long-term organ functionality underscores the need for ongoing optimization of robotic techniques to avoid biochemical perturbations. Collaboration between surgeons, anesthesiologists, perfusionists, and biomedical engineers will be paramount in developing evidence-based protocols and best practices for the safe and efficient application of robotic surgery. It is also critical that processes such as standardization of training, cost–benefit analysis, consent policies, and regulatory frameworks be standardized for the ethical integration of robotics into clinical practice. Despite the remaining challenges, the biochemical implications of robotic surgery should not deter us from responsibly realizing its significant potential. The technology already offers precision, faster recovery, and superior visualization—undeniable benefits for patients and caregivers. Future improvements through continued research and innovation could lead to scarless surgery, remote telesurgery, and broader access to minimally invasive procedures. Prudent navigation of this novel territory could lead to improved patient outcomes, surgical proficiency, and quality of life as future operating rooms are realized.
Ultimately, the biochemical implications highlighted in this analysis illustrate that optimal surgical care should take a holistic perspective, encompassing the entire physiology of the patient, rather than focusing solely on technical skill. This insight provides a framework as we move into an exciting new era of surgical procedures one that should balance the promise of the future with ethical and evidence-based application.
Data availability
Data availability correspondence should be addressed to noorazarian_a@khoyums.ac.ir.
References
Bliznakova K, Kolev N, Zlatarov A, Kalinov T, Georgiev T (2023) Feasibility and safety of robotic-assisted surgery for rectal cancer: short-term outcomes of a pilot study with da Vinci Xi platform during COVID-19. Chirurgia (Bucur) 118:27–38. https://doi.org/10.21614/chirurgia.2688
Kerray F, Yule S (2021) Rise of the machines: human factors and training for robotic-assisted surgery. BMJ Surg Interv Health Technol 3:e000100. https://doi.org/10.1136/bmjsit-2021-000100
Villalobos R, Maestre Y, González Barranquero A, Olsina J (2022) V-038 inguinal tep with articulated instruments: beyond conventional laparoscopy, closer to robotic surgery. Br J Surg. https://doi.org/10.1093/bjs/znac308.290
Kumar P, Talele S, Deshpande S, Ghyar R, Rout S, Ravi B (2023) Design, analysis and experimental validation of a novel 7-degrees of freedom instrument for laparoscopic surgeries. Ann Biomed Eng 51:751–770. https://doi.org/10.1007/s10439-022-03086-w
Kayani B, Haddad FS (2019) Robotic total knee arthroplasty: clinical outcomes and directions for future research. Bone Joint Res 8:438–442. https://doi.org/10.1302/2046-3758.810.BJR-2019-0175
Marino MV, Shabat G, Gulotta G, Komorowski AL (2018) From illusion to reality: a brief history of robotic surgery. Surg Innov 25:291–296. https://doi.org/10.1177/1553350618771417
Chan F (2018) Robotic-assisted surgical procedures are the future of gynaecology in Australasia. Aust N Z J Obstet Gynaecol 58:371–374. https://doi.org/10.1111/ajo.12819
Danesh H, Rahmati J, Mahdieh M, Hemadi SM, Bahmani A (2022) Medical and chemical evaluation of robotic surgery methods; a review study. Rom J Mil Med 125:542–551. https://doi.org/10.55453/rjmm.2022.125.4.2
Rivas-Lopez R, Sandoval-Garcia-Travesi FA (2020) Robotic surgery in gynecology: review of literature. Cir Cir 88:107–116. https://doi.org/10.24875/CIRU.18000636
Krzystek-Korpacka M, Zawadzki M, Lewandowska P, Szufnarowski K, Bednarz-Misa I, Jacyna K et al (2019) Distinct chemokine dynamics in early postoperative period after open and robotic colorectal surgery. J Clin Med. https://doi.org/10.3390/jcm8060879
Mazzella A, Casiraghi M, Galetta D, Cara A, Maisonneuve P, Petrella F et al (2023) How much stress does a surgeon endure? The effects of the robotic approach on the autonomic nervous system of a surgeon in the modern era of thoracic surgery. Cancers (Basel). https://doi.org/10.3390/cancers15041207
Cepolina F, Razzoli RP (2022) An introductory review of robotically assisted surgical systems. Int J Med Robot 18:e2409. https://doi.org/10.1002/rcs.2409
Zhang QB, Zhao C, Luo XM (2011) Ergonomic analysis of the master of minimally invasive surgical robot. Adv Mater Res 418–420:2018–2023. https://doi.org/10.4028/www.scientific.net/AMR.418-420.2018
Yang Y, Song L, Huang J, Cheng X, Luo Q (2021) A uniportal right upper lobectomy by three-arm robotic-assisted thoracoscopic surgery using the da Vinci (Xi) surgical system in the treatment of early-stage lung cancer. Transl Lung Cancer Res 10:1571–1575. https://doi.org/10.21037/tlcr-21-207
Stefano GB (2017) Robotic surgery: fast forward to telemedicine. Med Sci Monit 23:1856. https://doi.org/10.12659/msm.904666
Kumar S (2016) Open versus robotic prostatectomy. Indian J Urol 32:253–254. https://doi.org/10.4103/0970-1591.191233
Brassetti A, Ragusa A, Tedesco F, Prata F, Cacciatore L, Iannuzzi A et al (2023) Robotic surgery in urology: history from PROBOT® to HUGOTM. Sensors. https://doi.org/10.3390/s23167104
Goh EZ, Ali T (2022) Robotic surgery: an evolution in practice. J Surg Prot Res Methodol. https://doi.org/10.1093/jsprm/snac003
Chen J, Xu H, Lin S, He S, Tang K, Xiao Z et al (2022) Robot-assisted pyeloplasty and laparoscopic pyeloplasty in children: a comparison of single-port-plus-one and multiport surgery. Front Pediatr 10:957790. https://doi.org/10.3389/fped.2022.957790
Yagisawa T, Takagi T, Yoshida K, Hata K, Iizuka J, Muromiya Y et al (2022) Surgical outcomes of robot-assisted laparoscopic partial nephrectomy for cystic renal cell carcinoma. J Robot Surg 16:649–654. https://doi.org/10.1007/s11701-021-01292-7
Packiam VT, Barashi NS, Shalhav AL (2018) Robot-assisted laparoscopic adrenalectomy. J Endourol 32:S82–S87. https://doi.org/10.1089/end.2017.0721
Hashizume M (2005) Robot-assisted surgery. Geka Gakkai Zasshi 106:689–93
Grimminger PP, Hadzijusufovic E, Ruurda JP, Lang H, van Hillegersberg R (2018) The da Vinci Xi robotic four-arm approach for robotic-assisted minimally invasive esophagectomy. Thorac Cardiovasc Surg 66:407–409. https://doi.org/10.1055/s-0038-1636933
Rodríguez RAC, Noguera RJS (2021) New horizons in robotic surgery: da vinci begins to compete. Revista Urol Colomb Colomb Urol J 30:e153–e154. https://doi.org/10.1055/s-0041-1737013
Pathirana S, Kam P (2018) Anaesthetic issues in robotic-assisted minimally invasive surgery. Anaesth Intensive Care 46:25–35. https://doi.org/10.1177/0310057X1804600105
Wang H, Xu Z, Wu A, Dong Y, Zhang Y, Yue Y et al (2015) 2-Deoxy-D-glucose enhances anesthetic effects in mice. Anesth Analg 120:312–319. https://doi.org/10.1213/ANE.0000000000000520
Luz AL, Lagido C, Hirschey MD, Meyer JN (2016) In vivo determination of mitochondrial function using luciferase-expressing Caenorhabditis elegans: contribution of oxidative phosphorylation, glycolysis, and fatty acid oxidation to toxicant-induced dysfunction. Curr Protoc Toxicol. https://doi.org/10.1002/cptx.10
Sheridan RL, Prelack K, Szyfelbein SK (1997) Neuromuscular blockade does not decrease oxygen consumption or energy expenditure beyond sedation in the mechanically ventilated child. J Intensive Care Med 12:321–323. https://doi.org/10.1177/088506669701200606
Nair AS, Christopher A, Kotthapalli KK, Mantha SP (2021) Laparoscopic surgeries and carbon dioxide pneumoperitoneum during COVID-19 pandemic: problems and solutions. Med Gas Res 11:46. https://doi.org/10.4103/2045-9912.310060
Badani KK, Shapiro EY, Berg WT, Kaufman S, Bergman A, Wambi C et al (2013) A pilot study of laparoscopic Doppler ultrasound probe to map arterial vascular flow within the neurovascular bundle during robot-assisted radical prostatectomy. Prostate Cancer 2013:810715. https://doi.org/10.1155/2013/810715
Aditianingsih D, Mochtar CA, Lydia A, Siregar NC, Margyaningsih NI, Madjid AS et al (2020) Effects of low versus standard pressure pneumoperitoneum on renal syndecan-1 shedding and VEGF receptor-2 expression in living-donor nephrectomy: a randomized controlled study. BMC Anesthesiol 20:37. https://doi.org/10.1186/s12871-020-0956-7
Johnson DJ, Brooks DC, Pressler VM, Hulton NR, Colpoys MF, Smith RJ et al (1986) Hypothermic anesthesia attenuates postoperative proteolysis. Ann Surg 204:419–429. https://doi.org/10.1097/00000658-198610000-00010
Slavin M, Goldstein A, Raguan B, Rudnicki Y, Avital S, White I (2022) Postoperative CRP levels can rule out anastomotic leaks in Crohn’s disease patients. J Pers Med. https://doi.org/10.3390/jpm12010054
Preisser F, Nazzani S, Mazzone E, Marchioni M, Bandini M, Tian Z et al (2019) Comparison of open versus robotically assisted cytoreductive radical prostatectomy for metastatic prostate cancer. Clin Genitourin Cancer 17:e939–e945. https://doi.org/10.1016/j.clgc.2019.05.022
Fan S, Zhong JL, Chen WX, Chen WL, Li QX, Wang YY et al (2017) Postoperative immune response and surgical stress in selective neck dissection: comparison between endoscopically assisted dissection and open techniques in cT1-2N0 oral squamous cell carcinoma. J Craniomaxillofac Surg 45:1112–1116. https://doi.org/10.1016/j.jcms.2016.11.021
He L, Qing F, Li M, Lan D (2021) Effects of laparoscopic and traditional open surgery on the levels of IL-6, TNF-alpha, and Gal-3 in patients with thyroid cancer. Gland Surg 10:1085–1092. https://doi.org/10.21037/gs-21-60
Mueller AA, Kalak N, Schwenzer-Zimmerer K, Holsboer-Trachsler E, Brand S (2014) Cortisol levels and sleep patterns in infants with orofacial clefts undergoing surgery. Neuropsychiatr Dis Treat 10:1965–1972. https://doi.org/10.2147/NDT.S71785
Fleszar MG, Fortuna P, Zawadzki M, Hodurek P, Bednarz-Misa I, Witkiewicz W et al (2021) Sex, type of surgery, and surgical site infections are associated with perioperative cortisol in colorectal cancer patients. J Clin Med. https://doi.org/10.3390/jcm10040589
Landesberg G, London MJ (2016) The enigma of postoperative troponin elevation. Anesth Analg 123:5–7. https://doi.org/10.1213/ANE.0000000000001336
Sjodahl R, Davidson T, Aldman A, Lennmarken C, Kammerlind AS, Gustavsson E, et al. (2022) [Robotic-assisted pelvic and renal surgery—an overview]. Lakartidningen. p119
Neidert MC, Losa M, Regli L, Sarnthein J (2015) Elevated serum creatine kinase after neurosurgeries in lateral position with intraoperative neurophysiological monitoring is associated with OP duration. BMI and age Clin Neurophysiol 126:2026–2032. https://doi.org/10.1016/j.clinph.2014.12.019
Tsuchiya Y, Munakata S, Tsukamoto R, Okazawa Y, Mizukoshi K, Sugimoto K et al (2020) Creatine kinase elevation after robotic surgery for rectal cancer due to a prolonged lithotomy position. BMC Surg 20:136. https://doi.org/10.1186/s12893-020-00771-2
Sahutoglu C, Yasar A, Kocabas S, Askar FZ, Ayik MF, Atay Y (2018) Correlation between serum lactate levels and outcome in pediatric patients undergoing congenital heart surgery. Turk Gogus Kalp Damar Cerrahisi Derg 26:375–385. https://doi.org/10.5606/tgkdc.dergisi.2018.15791
Kumar L, Kumar K, Sandhya S, Koshy DM, Ramamurthi KP, Rajan S (2020) Effect of liberal versus restrictive fluid therapy on intraoperative lactate levels in robot- assisted colorectal surgery. Indian J Anaesth 64:599–604. https://doi.org/10.4103/ija.IJA_401_20
Soltanizadeh S, Jensen KK, Nordklint AK, Jorgensen HL, Jorgensen LN (2023) Even minor alteration of plasma creatinine after open abdominal surgery is associated with 30-day mortality: A single-centre cohort study. J Visc Surg 160:19–26. https://doi.org/10.1016/j.jviscsurg.2021.10.008
Windisch OL, Matter M, Pascual M, Sun P, Benamran D, Buhler L et al (2022) Robotic versus hand-assisted laparoscopic living donor nephrectomy: comparison of two minimally invasive techniques in kidney transplantation. J Robot Surg 16:1471–1481. https://doi.org/10.1007/s11701-022-01393-x
Minami E, Ito S, Sugiura T, Fujita Y, Sasano H, Sobue K (2014) Markedly elevated procalcitonin in early postoperative period in pediatric open heart surgery: a prospective cohort study. J Intensive Care 2:38. https://doi.org/10.1186/2052-0492-2-38
Cave J, Clarke S (2018) Paediatric robotic surgery. Ann R Coll Surg Engl 100:18–21. https://doi.org/10.1308/rcsann.supp2.18
Djordjevic A, Kotnik P, Horvat D, Knez Z, Antonic M (2020) Pharmacodynamics of malondialdehyde as indirect oxidative stress marker after arrested-heart cardiopulmonary bypass surgery. Biomed Pharmacother 132:110877. https://doi.org/10.1016/j.biopha.2020.110877
Kozlik J, Przybylowska J, Mikrut K, Zukiewicz-Sobczak WA, Zwolinski J, Piatek J et al (2015) Selected oxidative stress markers in gynecological laparoscopy. Wideochir Inne Tech Maloinwazyjne 10:92–100. https://doi.org/10.5114/wiitm.2014.47449
Koźlik J, Przybyłowska J, Mikrut K, Żukiewicz-Sobczak WA, Zwoliński J, Piątek J et al (2015) Selected oxidative stress markers in gynecological laparoscopy. Videosurg Other Miniinvasive Tech 1:92–100. https://doi.org/10.5114/wiitm.2014.47449
Burton D, Nicholson G, Hall G (2004) Endocrine and metabolic response to surgery. Cont Educ Anaesth Crit Care Pain 4:144–147. https://doi.org/10.1093/bjaceaccp/mkh040
Dronkers J, Witteman B, van Meeteren N (2016) Surgery and functional mobility: doing the right thing at the right time. Tech Coloproctol 20:339–341. https://doi.org/10.1007/s10151-016-1487-6
Narra GR, Aparna SM, Miraz M, Santhosh S (2015) Comparison of surgical stress response under general anaesthesia in open laparotomy vs. laparoscopic abdominal surgeries. J Evol Med Dental Sci. 4:15125–15133. https://doi.org/10.14260/jemds/2015/2148
Azemati S, Savai M, Khosravi MB, Allahyari E, Jahanmiri F (2013) Combination of remifentanil with isoflurane or propofol: effect on the surgical stress response. Acta Anaesthesiol Belg 64:25–31
Hernández-Avalos I, Flores-Gasca E, Mota-Rojas D, Casas-Alvarado A, Miranda-Cortés AE, Domínguez-Oliva A (2021) Neurobiology of anesthetic-surgical stress and induced behavioral changes in dogs and cats: a review. Veterinary World 14:393–404. https://doi.org/10.14202/vetworld.2021.393-404
Kaneko N, Kurata M, Yamamoto T, Morikawa S, Masumoto J (2019) The role of interleukin-1 in general pathology. Inflamm Regen 39:12. https://doi.org/10.1186/s41232-019-0101-5
Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, Tarhriz V, Farjami A, Ghasemian Sorbeni F et al (2022) COVID-19 infection: an overview on cytokine storm and related interventions. Virol J 19:92. https://doi.org/10.1186/s12985-022-01814-1
Anderson HL, Stryjewska B, Boyanton BL, Schwartz MR (1981) Review of the literature. Acta Anaesthesiol Scand 25:8–16. https://doi.org/10.1111/j.1399-6576.1981.tb01707.x
Funahashi H, Mizuno S (1985) Pituitary-adrenocortical response to surgical stress in male patients. Nihon Geka Gakkai Zasshi. 86:240–250
Hirokawa K, Miwa M, Taniguchi T, Tsuchiya M, Kawakami N (2016) Moderating effects of salivary testosterone levels on associations between job demand and psychological stress response in Japanese medical workers. Ind Health 54:194–203. https://doi.org/10.2486/indhealth.2015-0113
Pucheril D, Fletcher SA, Chen X, Friedlander DF, Cole AP, Krimphove MJ et al (2021) Workplace absenteeism amongst patients undergoing open vs. robotic radical prostatectomy, hysterectomy, and partial colectomy. Surg Endosc 35:1644–1650. https://doi.org/10.1007/s00464-020-07547-y
Vlot J (2019) Invited brief commentary on IUVS -2017-0216. J Invest Surg 32:61–62. https://doi.org/10.1080/08941939.2017.1383537
Todd LA, Vigersky RA (2021) Evaluating perioperative glycemic control of non-cardiac surgical patients with diabetes. Mil Med 186:e867–e872. https://doi.org/10.1093/milmed/usaa467
Pick A, Baralli JD, Sandhu H, Khorzad R (2020) 2231-PUB: perioperative glucose control protocol: hip and knee arthroplasty. Diabetes. https://doi.org/10.2337/db20-2231-PUB
Behrenbruch C, Shembrey C, Paquet-Fifield S, Molck C, Cho HJ, Michael M et al (2018) Surgical stress response and promotion of metastasis in colorectal cancer: a complex and heterogeneous process. Clin Exp Metastasis 35:333–345. https://doi.org/10.1007/s10585-018-9873-2
Rossano F (2019) Complications of robotic surgery in oncological gynecology: the experience of the Brazilian National Institute of Cancer. J Gynecol Res Obstetr. https://doi.org/10.17352/jgro.000065
Sies H (2020) Oxidative stress: concept and some practical aspects. Antioxidants (Basel). https://doi.org/10.3390/antiox9090852
Ou W, Liang Y, Qin Y, Wu W, Xie M, Zhang Y et al (2021) Hypoxic acclimation improves cardiac redox homeostasis and protects heart against ischemia-reperfusion injury through upregulation of O-GlcNAcylation. Redox Biol 43:101994. https://doi.org/10.1016/j.redox.2021.101994
Sugita J, Fujiu K (2018) Systemic inflammatory stress response during cardiac surgery. Int Heart J 59:457–459. https://doi.org/10.1536/ihj.18-210
Rutkowski R, Pancewicz SA, Rutkowski K, Rutkowska J (2007) Reactive oxygen and nitrogen species in inflammatory process. Pol Merkur Lekarski 23:131–136
Sodha S, Nazarian S, Adshead JM, Vasdev N, Mohan SG (2016) Effect of pneumoperitoneum on renal function and physiology in patients undergoing robotic renal surgery. Curr Urol 9:1–4. https://doi.org/10.1159/000442842
Gielen CE, Pignez Y, Swaelens C (2023) Two cases of vascular complications after urologic robotic surgery. Acta Chir Belg 123:333–336. https://doi.org/10.1080/00015458.2021.2021357
Gokmen Karasu AF, Kiran G, Sanlikan F (2022) Intraoperative complications and conversion to laparatomy in gynecologic robotic surgery. J Invest Surg 35:912–915. https://doi.org/10.1080/08941939.2021.1949411
Bastopcu M, Senay S, Gullu AU, Kocyigit M, Alhan C (2022) Percutaneous cannulation for cardiopulmonary bypass in robotic mitral valve surgery with zero groin complications. J Card Surg 37:280–284. https://doi.org/10.1111/jocs.16090
Pechan I, Danova K, Olejarova I, Halcak L, Rendekova V, Fabian J (2003) Oxidative stress and antioxidant defense systems in patients after heart transplantation. Wien Klin Wochenschr 115:648–651. https://doi.org/10.1007/BF03040470
Tokusoglu O (2019) Robotic approaches help maintain higher levels of key antioxidants like glutathione, superoxide dismutase, and catalase compared to open procedures. Food Health Technol Innov. 2:193–196
Hubner M, Mantziari S, Demartines N, Pralong F, Coti-Bertrand P, Schafer M (2016) Postoperative albumin drop is a marker for surgical stress and a predictor for clinical outcome: a pilot study. Gastroenterol Res Pract 2016:8743187. https://doi.org/10.1155/2016/8743187
Breuer JP, Heymann CV, Spies C (2009) Perioperative Ernährung—metabolische Konditionierung. Aktuelle Ernährungsmedizin 34:107–113. https://doi.org/10.1055/s-0028-1090143
Mila-Kierzenkowska C, Wozniak A, Drewa T, Wozniak B, Szpinda M, Krzyzynska-Malinowska E et al (2013) Effects of open versus laparoscopic nephrectomy techniques on oxidative stress markers in patients with renal cell carcinoma. Oxid Med Cell Longev 2013:438321. https://doi.org/10.1155/2013/438321
Arsalani-Zadeh R, Ullah S, Khan S, MacFie J (2011) Oxidative stress in laparoscopic versus open abdominal surgery: a systematic review. J Surg Res 169:e59-68. https://doi.org/10.1016/j.jss.2011.01.038
Jaradeh M, Curran B, Poulikidis K, Rodrigues A, Jeske W, Abdelsattar ZM et al (2022) Inflammatory cytokines in robot-assisted thoracic surgery versus video-assisted thoracic surgery. J Thorac Dis 14:2000–2010. https://doi.org/10.21037/jtd-21-1820
Pilka R, Marek R, Adam T, Kudela M, Ondrova D, Neubert D et al (2016) Systemic inflammatory response after open, laparoscopic and robotic surgery in endometrial cancer patients. Anticancer Res 36:2909–2922
LeBlanc K, M. Sweitzer S, (2015) Systematic review of clinical evidence for local anesthetic wound infiltration in reduction of post-surgical pain. Internal Med Open Access. https://doi.org/10.4172/2165-8048.1000207
Martinschek A, Stumm L, Ritter M, Heinrich E, Bolenz C, Trojan L (2017) Prospective, controlled study of invasiveness and post-aggression metabolism in patients undergoing robotic-assisted radical prostatectomy. Urol Int 99:201–206. https://doi.org/10.1159/000478027
Pathak S, Goel N, Chowdhury I, Bhageria V (2016) Anaesthetic management of a patient with glucose-6-phosphate dehydrogenase deficiency undergoing robotic-assisted laparoscopic radical prostatectomy. J Soc Anesthesiol Nepal 3:93–95. https://doi.org/10.3126/jsan.v3i2.15621
Xu Q, Zhao B, Ye Y, Li Y, Zhang Y, Xiong X et al (2021) Relevant mediators involved in and therapies targeting the inflammatory response induced by activation of the NLRP3 inflammasome in ischemic stroke. J Neuroinflammation 18:123. https://doi.org/10.1186/s12974-021-02137-8
Bailey EH, Glasgow SC (2015) Challenges in the medical and surgical management of chronic inflammatory bowel disease. Surg Clin North Am 95(1233–44):vii. https://doi.org/10.1016/j.suc.2015.08.003
Cohen BD, Marshall MB (2020) Robotic-assisted tracheobronchial surgery. J Thorac Dis 12:6173–6178. https://doi.org/10.21037/jtd.2020.03.05
Pelizzo G, Nakib G, Romano P, Avolio L, Mencherini S, Zambaiti E et al (2015) Five millimetre-instruments in paediatric robotic surgery: advantages and shortcomings. Minim Invasive Ther Allied Technol 24:148–153. https://doi.org/10.3109/13645706.2014.975135
Máca J, Peteja M (2007) Alarmins and surgical injury. Rozhl Chir 96:105–113
Spotnitz WD (2012) Getting to hemostasis: general and thoracic surgical challenges. Tex Heart Inst J 39:868–870
Muller R, Musikic P (1987) Hemorheology in surgery–a review. Angiology 38:581–592. https://doi.org/10.1177/000331978703800802
Wareing A (2017) Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Int J Nurs Pract. https://doi.org/10.1111/ijn.12552
Puca AA, Carrizzo A, Ferrario A, Villa F, Vecchione C (2012) Endothelial nitric oxide synthase, vascular integrity and human exceptional longevity. Immun Ageing 9:26. https://doi.org/10.1186/1742-4933-9-26
Pessoa RR, Maroni P, Kukreja J, Kim SP (2021) Comparative effectiveness of robotic and open radical prostatectomy. Transl Androl Urol 10:2158–2170. https://doi.org/10.21037/tau.2019.12.01
Wikiel KJ, Robinson TN, Jones EL (2021) Energy in robotic surgery. Ann Laparosc Endosc Surg. 6:9. https://doi.org/10.21037/ales.2020.03.06
Shakir N, Wong D, Cadeddu J, Roehrborn C (2019) Pd40–12 impact of single dose perioperative enoxaparin on rate of thromboembolic events following robotic assisted prostatectomy: a hospital system-wide analysis. J Urol. https://doi.org/10.1097/01.Ju.0000556544.63234.67
Waigankar SS, Yuvaraja TB, Dev P, Agarwal V, Pednekar AP, Kulkarni B (2021) Robotic Freyer’s prostatectomy: operative technique and single-center experience. Indian J Urol 37:247–253. https://doi.org/10.4103/iju.IJU_78_21
Pogatzki-Zahn E (2010) Prevention and therapy of prolonged, chronic pain after surgery. Anasth Intensivmed Notfallmed Schmerzther 45:496–503. https://doi.org/10.1055/s-0030-1262479. (Quiz 4)
Meghana N, Bharadwaj M, Goel N, Shukla S (2022) Endotracheal tube cuff pressure changes with pneumoperitoneum and steep head down position in patients undergoing robotic urogynecological surgeries—a prospective observational study. J Indian College Anaesthesiol. https://doi.org/10.4103/jica.jica_15_22
Faas CL, Acosta FJ, Campbell MD, O’Hagan CE, Newton SE, Zagalaniczny K (2002) The effects of spinal anesthesia vs epidural anesthesia on 3 potential postoperative complications: pain, urinary retention, and mobility following inguinal herniorrhaphy. AANA J 70:441–447
Watrowski R, Kostov S, Alkatout I (2021) Complications in laparoscopic and robotic-assisted surgery: definitions, classifications, incidence and risk factors - an up-to-date review. Wideochir Inne Tech Maloinwazyjne 16:501–525. https://doi.org/10.5114/wiitm.2021.108800
Saily VM, Petas A, Joutsi-Korhonen L, Taari K, Lassila R, Rannikko AS (2014) Dabigatran for thromboprophylaxis after robotic assisted laparoscopic prostatectomy: retrospective analysis of safety profile and effect on blood coagulation. Scand J Urol 48:153–159. https://doi.org/10.3109/21681805.2013.817482
Naghi I, Seader K, Hashemi E, Sommers G, Anderson PS (2016) The role of robotic surgery versus laparotomy on the incidence of thromboembolism in uterine cancer [21P]. Obstet Gynecol 127:136S-S137. https://doi.org/10.1097/01.AOG.0000483550.73369.fc
Kapoor I, Rath G (2018) Robotized surgical assistant in neurosurgery: anaesthetic implications! J Neuroanaesthesiol Crit Care 03:151–152. https://doi.org/10.4103/2348-0548.182328
Zemmar A, Lozano AM, Nelson BJ (2020) The rise of robots in surgical environments during COVID-19. Nat Mach Intell 2:566–572. https://doi.org/10.1038/s42256-020-00238-2
Orsolya M, Daniela BS, Rodica R, Cipriana C, Attila MZ, Loan C (2012) Renal function monitoring in urogenital robot-assisted laparoscopic surgery performed in general anaesthesia. Med Con 23:13–18. https://doi.org/10.33311/medcon.2012.23.3.3
Martini A, Sfakianos JP, Paulucci DJ, Abaza R, Eun DD, Bhandari A et al (2019) Predicting acute kidney injury after robot-assisted partial nephrectomy: implications for patient selection and postoperative management. Urol Oncol 37:445–451. https://doi.org/10.1016/j.urolonc.2019.04.018
Bogdanov RR, Nurimanshin AF, Husaenova AA, Khasanov AR (2023) Anesthetic aspects of robot-assisted surgery (a review). Pacific Medical Journal. https://doi.org/10.34215/1609-1175-2023-1-11-18
Di Benedetto F, Petrowsky H, Magistri P, Halazun KJ (2020) Robotic liver resection: hurdles and beyond. Int J Surg 82S:155–162. https://doi.org/10.1016/j.ijsu.2020.05.070
Mori C, Iwasaki H, Sato I, Takahoko K, Inaba Y, Kawasaki Y et al (2023) Impact of intraoperative fluid restriction on renal outcomes in patients undergoing robotic-assisted laparoscopic prostatectomy. J Robot Surg. https://doi.org/10.1007/s11701-023-01610-1
Nomine-Criqui C, Demarquet L, Schweitzer ML, Klein M, Brunaud L, Bihain F (2020) Robotic adrenalectomy: when and how? Gland Surg 9:S166–S172. https://doi.org/10.21037/gs.2019.12.11
Ortenzi M, Ghiselli R, Baldarelli M, Cardinali L, Guerrieri M (2018) Is the bipolar vessel sealer device an effective tool in robotic surgery? A retrospective analysis of our experience and a meta-analysis of the literature about different robotic procedures by investigating operative data and post-operative course. Minim Invasive Ther Allied Technol 27:113–118. https://doi.org/10.1080/13645706.2017.1329212
Muaddi H, Hafid ME, Choi WJ, Lillie E, de Mestral C, Nathens A et al (2021) Clinical outcomes of robotic surgery compared to conventional surgical approaches (laparoscopic or open): a systematic overview of reviews. Ann Surg 273:467–473. https://doi.org/10.1097/SLA.0000000000003915
Lindfors A, Akesson A, Staf C, Sjoli P, Sundfeldt K, Dahm-Kahler P (2018) Robotic vs open surgery for endometrial cancer in elderly patients: surgical outcome, survival, and cost analysis. Int J Gynecol Cancer 28:692–699. https://doi.org/10.1097/IGC.0000000000001240
Yamamoto S (2020) Comparison of the perioperative outcomes of laparoscopic surgery, robotic surgery, open surgery, and transanal total mesorectal excision for rectal cancer: an overview of systematic reviews. Ann Gastroenterol Surg 4:628–634. https://doi.org/10.1002/ags3.12385
Khan H, Dhillon K, Mahapatra P, Popat R, Zakieh O, Kim WJ et al (2021) Blood loss and transfusion risk in robotic-assisted knee arthroplasty: a retrospective analysis. Int J Med Robot 17:e2308. https://doi.org/10.1002/rcs.2308
Salciccia S, Rosati D, Viscuso P, Canale V, Scarrone E, Frisenda M et al (2021) Influence of operative time and blood loss on surgical margins and functional outcomes for laparoscopic versus robotic-assisted radical prostatectomy: a prospective analysis. Cent European J Urol 74:503–515. https://doi.org/10.5173/ceju.2021.0177
Hockstein NG, Weinstein GS, O’Malley BW Jr (2005) Maintenance of hemostasis in transoral robotic surgery. ORL J Otorhinolaryngol Relat Spec 67:220–4. https://doi.org/10.1159/000088012
Becker F, Morgul H, Katou S, Juratli M, Holzen JP, Pascher A et al (2021) Robotic liver surgery—current standards and future perspectives. Z Gastroenterol 59:56–62. https://doi.org/10.1055/a-1329-3067
Ziegler S, Ortu A, Reale C, Proietti R, Mondello E, Tufano R et al (2008) Fibrinolysis or hypercoagulation during radical prostatectomy? An evaluation of thrombelastographic parameters and standard laboratory tests. Eur J Anaesthesiol 25:538–543. https://doi.org/10.1017/S0265021508003852
Ye L, Childers CP, de Virgilio M, Shenoy R, Mederos MA, Mak SS et al (2021) Clinical outcomes and cost of robotic ventral hernia repair: systematic review. BJS Open. https://doi.org/10.1093/bjsopen/zrab098
Fontalis A, Kayani B, Asokan A, Haddad IC, Tahmassebi J, Konan S et al (2022) Inflammatory response in robotic-arm-assisted versus conventional jig-based TKA and the correlation with early functional outcomes: results of a prospective randomized controlled trial. J Bone Joint Surg Am 104:1905–1914. https://doi.org/10.2106/JBJS.22.00167
Kayani B, Tahmassebi J, Ayuob A, Konan S, Oussedik S, Haddad FS (2021) A prospective randomized controlled trial comparing the systemic inflammatory response in conventional jig-based total knee arthroplasty versus robotic-arm assisted total knee arthroplasty. Bone Joint J 103-B:113–22. https://doi.org/10.1302/0301-620X.103B1.BJJ-2020-0602.R2
Colon KC, Seligsohn D, Salame G (2021) Transversus abdominis plane block in robotic gynecologic oncology surgery. Austin J Anesth Analg. https://doi.org/10.26420/austinjanesthesiaandanalgesia.2021.1097
Webster TM, Herrell SD, Chang SS, Cookson MS, Baumgartner RG, Anderson LW et al (2005) Robotic assisted laparoscopic radical prostatectomy versus retropubic radical prostatectomy: a prospective assessment of postoperative pain. J Urol 174:912–4. https://doi.org/10.1097/01.ju.0000169455.25510.ff
Akdemir A, Yildirim N, Zeybek B, Karaman S, Sendag F (2015) Single incision trans-umbilical total hysterectomy: robotic or laparoscopic? Gynecol Obstet Invest 80:93–98. https://doi.org/10.1159/000370000
Tan YG, Allen JC, Tay KJ, Huang HH, Lee LS (2020) Benefits of robotic cystectomy compared with open cystectomy in an enhanced recovery after surgery program: a propensity-matched analysis. Int J Urol 27:783–788. https://doi.org/10.1111/iju.14300
Gul ZG, Katims AB, Winoker JS, Wiklund P, Waingankar N, Mehrazin R (2021) Robotic assisted radical cystectomy versus open radical cystectomy: a review of what we do and don’t know. Transl Androl Urol 10:2209–2215. https://doi.org/10.21037/tau.2019.11.32
Zhao H, Sun W (2022) Effect of enhanced recovery after surgery with integrated traditional chinese and western medicine on postoperative stress response of patients with gastrointestinal tumors. Comput Math Methods Med 2022:3663246. https://doi.org/10.1155/2022/3663246
Maerz DA, Beck LN, Sim AJ, Gainsburg DM (2017) Complications of robotic-assisted laparoscopic surgery distant from the surgical site. Br J Anaesth 118:492–503. https://doi.org/10.1093/bja/aex003
Campos J, Ueda K (2014) Update on anesthetic complications of robotic thoracic surgery. Minerva Anestesiol 80:83–88
El-Hakim A, Leung RA, Tewari A (2006) Robotic prostatectomy: a pooled analysis of published literature. Expert Rev Anticancer Ther 6:11–20. https://doi.org/10.1586/14737140.6.1.11
Zhang R, Li T, Ye L, Lin L, Wei Y (2022) The evidence behind robot-assisted abdominopelvic surgery. Ann Intern Med 175:W22. https://doi.org/10.7326/L21-0781
Oblak T (2021) The incidence of peripheral nerve injuries related to patient positioning during robotic assisted surgery: an evidence summary. J Perioper Nurs 34:49–53. https://doi.org/10.26550/2209-1092.1166
El Rassi I, El Rassi JM (2020) A review of haptic feedback in tele-operated robotic surgery. J Med Eng Technol 44:247–254. https://doi.org/10.1080/03091902.2020.1772391
Fotiou A, Iavazzo C (2022) Gynecologic robotic surgery: intraoperative complication and conversion rates. J Invest Surg 35:916–917. https://doi.org/10.1080/08941939.2021.1962440
Ackerman SJ, Daniel S, Baik R, Liu E, Mehendale S, Tackett S et al (2018) Comparison of complication and conversion rates between robotic-assisted and laparoscopic rectal resection for rectal cancer: which patients and providers could benefit most from robotic-assisted surgery? J Med Econ 21:254–261. https://doi.org/10.1080/13696998.2017.1396994
Gibber M, Lehr EJ, Kon ZN, Wehman PB, Griffith BP, Bonatti J (2014) Is there a role for robotic totally endoscopic coronary artery bypass in patients with a colostomy? Innovations (Phila) 9:448–450. https://doi.org/10.1177/155698451400900610
De la Harb Rosa A, Garcia-Castaneda J, Hsu CH, Zeng J, Batai K, Lee BR et al (2020) Perioperative outcomes of open vs. robotic radical cystectomy: a nationwide comparative analysis (2009-2014). Cent European J Urol. 73:427–31. https://doi.org/10.5173/ceju.2020.0230
Schlager JG, Hartmann D, Wallmichrath J, RuizSanJose V, Patzer K, French LE et al (2022) Patient-dependent risk factors for wound infection after skin surgery: a systematic review and meta-analysis. Int Wound J 19:1748–1757. https://doi.org/10.1111/iwj.13780
Simhal RK, Wang KR, Shah Y, Simon DP, Mark JR, Shah MS et al (2023) Risk analysis of open vs. robotic assisted radical cystectomy. J Clin Oncol 41:576. https://doi.org/10.1200/JCO.2023.41.6_suppl.576
Wang J, Johnson NW, Casey L, Carne PWG, Bell S, Chin M et al (2023) Robotic colon surgery in obese patients: a systematic review and meta-analysis. ANZ J Surg 93:35–41. https://doi.org/10.1111/ans.17749
Zarak A, Castillo A, Kichler K, de la Cruz L, Tamariz L, Kaza S (2015) Robotic versus laparoscopic surgery for colonic disease: a meta-analysis of postoperative variables. Surg Endosc 29:1341–1347. https://doi.org/10.1007/s00464-015-4197-7
Karategos A, Yassin N (2022) P557 Robotic surgery significantly improves outcomes for patients with inflammatory bowel disease. J Crohns Colitis 16:i502-i. https://doi.org/10.1093/ecco-jcc/jjab232.683
Ravendran K, Abiola E, Balagumar K, Raja AZ, Flaih M, Vaja SP et al (2023) A review of robotic surgery in colorectal surgery. Cureus 15:e37337. https://doi.org/10.7759/cureus.37337
Ito H, Moritake T, Isaka K (2022) Does the use of a uterine manipulator in robotic surgery for early-stage endometrial cancer affect oncological outcomes? Int J Med Robot 18:e2443. https://doi.org/10.1002/rcs.2443
Hori S, Nakai Y, Tomizawa M, Morizawa Y, Gotoh D, Miyake M et al (2022) Trends in primary treatment for localized prostate cancer according to the availability of treatment modalities and the impact of introducing robotic surgery. Int J Urol 29:1371–9. https://doi.org/10.1111/iju.15003
Gagnon LO, Goldenberg SL, Lynch K, Hurtado A, Gleave ME (2014) Comparison of open and robotic-assisted prostatectomy: the University of British Columbia experience. Can Urol Assoc J 8:92–7. https://doi.org/10.5489/cuaj.1707
Jimenez-Rodriguez RM, Flynn J, Patil S, Widmar M, Quezada-Diaz F, Lynn P et al (2021) Comparing outcomes of robotic versus open mesorectal excision for rectal cancer. BJS Open. https://doi.org/10.1093/bjsopen/zrab135
(2022) Scientific Impact Paper No. 71: Robotic surgery in gynaecology. The Obstetr Gynaecol. 24:298. https://doi.org/10.1111/tog.12842
Ornellas AA (2013) Phase 1 prospective evaluation of the oncological adequacy of robotic assisted video-endoscopic inguinal lymphadenectomy in patients with penile carcinoma. BJU Int 111:1010. https://doi.org/10.1111/j.1464-410X.2013.11733.x
Casarin J, Multinu F, Tortorella L, Cappuccio S, Weaver AL, Ghezzi F et al (2020) Sentinel lymph node biopsy for robotic-assisted endometrial cancer staging: further improvement of perioperative outcomes. Int J Gynecol Cancer 30:41–7. https://doi.org/10.1136/ijgc-2019-000672
Asanoma K, Yahata H, Okugawa K, Ohgami T, Yasunaga M, Kodama K et al (2022) Impact of obesity on robotic-assisted surgery in patients with stage IA endometrial cancer and a low risk of recurrence: an institutional study. J Obstet Gynaecol Res 48:3226–32. https://doi.org/10.1111/jog.15434
Martinez-Perez A, Carra MC, Brunetti F, de Angelis N (2017) Pathologic outcomes of laparoscopic vs open mesorectal excision for rectal cancer: a systematic review and meta-analysis. JAMA Surg 152:165665. https://doi.org/10.1001/jamasurg.2016.5665
Attaluri V, McLemore EC (2016) The cost of robotic surgery. Semin Colon Rectal Surg 27:134–5. https://doi.org/10.1053/j.scrs.2016.04.004
Oshimori N, Guo Y, Taniguchi S (2021) An emerging role for cellular crosstalk in the cancer stem cell niche. J Pathol 254:384–94. https://doi.org/10.1002/path.5655
Brzozowa M, Michalski M, Wyrobiec G, Piecuch A, Dittfeld A, Harabin-Slowinska M et al (2015) The role of Snail1 transcription factor in colorectal cancer progression and metastasis. Contemp Oncol (Pozn) 19:265–70. https://doi.org/10.5114/wo.2014.42173
Baldini E, Tuccilli C, Pironi D, Catania A, Tartaglia F, Di Matteo FM et al (2021) Expression and clinical utility of transcription factors involved in epithelial-mesenchymal transition during thyroid cancer progression. J Clin Med. https://doi.org/10.3390/jcm10184076
Niland S, Riscanevo AX, Eble JA (2021) Matrix metalloproteinases shape the tumor microenvironment in cancer progression. Int J Mol Sci. https://doi.org/10.3390/ijms23010146
Umamaheswari A, Katari S, Pasala C, Nalamolu R, Vankadoth U, Alexander S et al (2019) Pathophysiology of matrix metalloproteinases in breast cancer progression. J Clin Sci Res. https://doi.org/10.4103/jcsr.Jcsr_67_19
Sidorkiewicz I, Piskor B, Dabrowska E, Guzinska-Ustymowicz K, Pryczynicz A, Zbucka-Kretowska M et al (2019) Plasma levels and tissue expression of selected cytokines, metalloproteinases and tissue inhibitors in patients with cervical cancer. Anticancer Res 39:6403–12. https://doi.org/10.21873/anticanres.13854
Jin B, Zhang YY, Pan JX (2023) The role and significance of hepatic environmental cells in tumor metastatic colonization to liver. Sichuan Da Xue Xue Bao Yi Xue Ban 54:469–74. https://doi.org/10.12182/20230560301
Dahn ML, Marcato P (2020) Targeting the roots of recurrence: new strategies for eliminating therapy-resistant breast cancer stem cells. Cancers (Basel). https://doi.org/10.3390/cancers13010054
Colak S, Medema JP (2014) Cancer stem cells–important players in tumor therapy resistance. FEBS J 281:4779–91. https://doi.org/10.1111/febs.13023
Savelieva OE, Tashireva LA, Kaigorodova EV, Buzenkova AV, Mukhamedzhanov RK, Grigoryeva ES et al (2020) Heterogeneity of stemlike circulating tumor cells in invasive breast cancer. Int J Mol Sci. https://doi.org/10.3390/ijms21082780
Feldheiser A, Aziz O, Baldini G, Cox BP, Fearon KC, Feldman LS (2016) Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand 60(3):289–334. https://doi.org/10.1111/aas.12651
Tang F, Tie Y, Lan TX, Yang JY, Hong WQ, Chen SY et al (2023) Surgical treatment of osteosarcoma induced distant pre-metastatic niche in lung to facilitate the colonization of circulating tumor cells. Adv Sci (Weinh). https://doi.org/10.1002/advs.202207518
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331:1565–70. https://doi.org/10.1126/science.1203486
Del Vecchio F, Martinez-Rodriguez V, Schukking M, Cocks A, Broseghini E, Fabbri M (2021) Professional killers: the role of extracellular vesicles in the reciprocal interactions between natural killer, CD8+ cytotoxic T-cells and tumour cells. J Extracell Vesicles 10:e12075. https://doi.org/10.1002/jev2.12075
Farhood B, Najafi M, Mortezaee K (2019) CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol 234:8509–21. https://doi.org/10.1002/jcp.27782
Khan S, Jain M, Mathur V, Feroz SM (2016) Chronic inflammation and cancer: paradigm on tumor progression, metastasis and therapeutic intervention. Gulf J Oncolog 1:86–93
Do HTT, Lee CH, Cho J (2020) Chemokines and their receptors: multifaceted roles in cancer progression and potential value as cancer prognostic markers. Cancers (Basel). https://doi.org/10.3390/cancers12020287
Schmitt TM, Stromnes IM, Chapuis AG, Greenberg PD (2015) New strategies in engineering T-cell receptor gene-modified T cells to more effectively target malignancies. Clin Cancer Res 21:5191–7. https://doi.org/10.1158/1078-0432.CCR-15-0860
Pu Y, Ji Q (2022) Tumor-associated macrophages regulate PD-1/PD-L1 immunosuppression. Front Immunol. https://doi.org/10.3389/fimmu.2022.874589
Kaptein P, Jacoberger-Foissac C, Dimitriadis P, Voabil P, de Bruijn M, Brokamp S et al (2022) Addition of interleukin-2 overcomes resistance to neoadjuvant CTLA4 and PD1 blockade in ex vivo patient tumors. Sci Transl Med 14:eabj9779. https://doi.org/10.1126/scitranslmed.abj9779
Gonzalez H, Hagerling C, Werb Z (2018) Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 32:1267–84. https://doi.org/10.1101/gad.314617.118
Berraondo P, Minute L, Ajona D, Corrales L, Melero I, Pio R (2016) Innate immune mediators in cancer: between defense and resistance. Immunol Rev 274:290–306. https://doi.org/10.1111/imr.12464
Wang CS, Kozlow JR, Troost JP, Corcoran JF (2022) Reliability of a case-based oral examination by case and competency for evaluation of plastic surgery residents. Plastic Reconstr Surg Global Open. 10:8–9. https://doi.org/10.1097/01.Gox.0000834952.14979.78
Holoman M, Zahorec R, Kusy J, Pechan I (1995) Biochemical monitoring in patients during revascularization surgery. Vasa 24:23–8
Pechan I, Halcak L, Rendekova V, Barta E, Cornak V, Zahorec R et al (1993) Biochemical parameters in the blood of patients during open-heart surgery. I. Monitoring changes in energy metabolism. Bratisl Lek Listy 94:349–53
Rijs Z, Shifai AN, Bosma SE, Kuppen PJK, Vahrmeijer AL, Keereweer S et al (2021) Candidate biomarkers for specific intraoperative near-infrared imaging of soft tissue sarcomas: a systematic review. Cancers (Basel). https://doi.org/10.3390/cancers13030557
Jiang P (2018) Abstract B24: digital signatures of T cell dysfunction predict immunotherapy response. Cancer Immunol Res 6:B24. https://doi.org/10.1158/2326-6074.Tumimm17-b24
Algadi HH, Abou-Bakr AA, Jamali OM, Fathy LM (2020) Toluidine blue versus frozen section for assessment of mucosal tumor margins in oral squamous cell carcinoma. BMC Cancer 20:1147. https://doi.org/10.1186/s12885-020-07644-0
Schaefer JM, Barth CW, Davis SC, Gibbs SL (2019) Diagnostic performance of receptor-specific surgical specimen staining correlates with receptor expression level. J Biomed Opt 24:1–9. https://doi.org/10.1117/1.JBO.24.2.026002
Marochkov AV (2012) Control of laboratory parameters level as a component of anesthesia monitoring in patients undergoing abdominal surgery. Health and Ecology Issues. https://doi.org/10.51523/2708-6011.2012-9-3-18
Bay E, Dean-Ben XL, Pang GA, Douplik A, Razansky D (2015) Real-time monitoring of incision profile during laser surgery using shock wave detection. J Biophotonics 8:102–11. https://doi.org/10.1002/jbio.201300151
Ding J (2006) Realtime control algorithm for teleoperated surgery robot. Chin J Mech Eng. https://doi.org/10.3901/jme.2006.12.163
Andrew BY, Andrew EY, Cherry AD, Hauck JN, Nicoara A, Pieper CF et al (2018) Intraoperative renal resistive index as an acute kidney injury biomarker: development and validation of an automated analysis algorithm. J Cardiothorac Vasc Anesth 32:2203–9. https://doi.org/10.1053/j.jvca.2018.04.014
Matsubara TJ, Fujiu K, Shimizu Y, Oshima T, Matsuda J, Matsunaga H et al (2020) Fluoroless and contrast-free catheter ablation without a lead apron in routine clinical practice. Sci Rep 10:17096. https://doi.org/10.1038/s41598-020-74165-y
Connolly P (2004) The potential for biosensors in cardiac surgery. Perfusion 19:247–9. https://doi.org/10.1191/0267659104pf747oa
Ding D, Li J, Bai D, Fang H, Lin J, Zhang D (2020) Biosensor-based monitoring of the central metabolic pathway metabolites. Biosens Bioelectron 167:112456. https://doi.org/10.1016/j.bios.2020.112456
Perdomo SA, Marmolejo-Tejada JM, Jaramillo-Botero A (2021) Review—bio-nanosensors: fundamentals and recent applications. J Electrochem Soc. https://doi.org/10.1149/1945-7111/ac2972
Smith BR, Cheng Z, De A, Rosenberg J, Gambhir SS (2010) Dynamic visualization of RGD-quantum dot binding to tumor neovasculature and extravasation in multiple living mouse models using intravital microscopy. Small 6:2222–9. https://doi.org/10.1002/smll.201001022
Liu X, Braun GB, Zhong H, Hall DJ, Han W, Qin M et al (2016) Tumor-targeted multimodal optical imaging with versatile cadmium-free quantum dots. Adv Funct Mater 26:267–76. https://doi.org/10.1002/adfm.201503453
Bergholt MS, Lin K, Wang J, Zheng W, Xu H, Huang Q et al (2016) Simultaneous fingerprint and high-wavenumber fiber-optic Raman spectroscopy enhances real-time in vivo diagnosis of adenomatous polyps during colonoscopy. J Biophotonics 9:333–42. https://doi.org/10.1002/jbio.201400141
Khan H, Shah MR, Barek J, Malik MI (2023) Cancer biomarkers and their biosensors: a comprehensive review. TrAC Trends Anal Chem. https://doi.org/10.1016/j.trac.2022.116813
Ravalli A, Marrazza G (2015) Gold and magnetic nanoparticles-based electrochemical biosensors for cancer biomarker determination. J Nanosci Nanotechnol 15:3307–19. https://doi.org/10.1166/jnn.2015.10038
Lalvani SB (2019) Electrochemical biosensors for detection of cancer biomarkers. Int J Biosens Bioelectron. https://doi.org/10.15406/ijbsbe.2019.05.00170
Asuvaran A, Elatharasan G (2021) Design of two-dimensional photonic crystal based biosensors for abnormal tissues analysis. Silicon 14(12):1–8. https://doi.org/10.21203/rs.3.rs-628354/v1
Yin B, Wang X, Yuan F, Li Y, Lu P (2022) Research progress on the effect of gut and tumor microbiota on antitumor efficacy and adverse effects of chemotherapy drugs. Front Microbiol 13:899111. https://doi.org/10.3389/fmicb.2022.899111
Askari E, Seyfoori A, Amereh M, Gharaie SS, Ghazali HS, Ghazali ZS et al (2020) Stimuli-responsive hydrogels for local post-surgical drug delivery. Gels. https://doi.org/10.3390/gels6020014
Hakim ML, Nahar N, Saha M, Islam MS, Reza HM, Sharker SM (2020) Local drug delivery from surgical thread for area-specific anesthesia. Biomed Phys Eng Express 6:015028. https://doi.org/10.1088/2057-1976/ab6a1e
Park CG, Hartl CA, Schmid D, Carmona EM, Kim HJ, Goldberg MS (2018) Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aar1916
Das KP (2022) Nanoparticles and convergence of artificial intelligence for targeted drug delivery for cancer therapy: current progress and challenges. Front Med Technol. 4:1067144. https://doi.org/10.3389/fmedt.2022.1067144
Bu LL, Yan J, Wang Z, Ruan H, Chen Q, Gunadhi V et al (2019) Advances in drug delivery for post-surgical cancer treatment. Biomaterials 219:119182. https://doi.org/10.1016/j.biomaterials.2019.04.027
Wolinsky JB, Colson YL, Grinstaff MW (2012) Local drug delivery strategies for cancer treatment: gels, nanoparticles, polymeric films, rods, and wafers. J Control Release 159:14–26. https://doi.org/10.1016/j.jconrel.2011.11.031
Yokoyama M (2005) Drug targeting with nano-sized carrier systems. J Artif Organs 8:77–84. https://doi.org/10.1007/s10047-005-0285-0
Dupoiron D, Douillard T, Carvajal G (2020) Usefulness of imaging for intrathecal drug delivery systems: an update. Med Res Arch. https://doi.org/10.18103/mra.v8i7.2175
Zerla PA, Canelli A, Cerne L, Caravella G, Gilardini A, De Luca G et al (2017) Evaluating safety, efficacy, and cost-effectiveness of PICC securement by subcutaneously anchored stabilization device. J Vasc Access 18:238–42. https://doi.org/10.5301/jva.5000655
Wang L, Wang H, Li HZ (2023) Real-time customized precision combination therapies based on potential actionability and coalterations to provide therapeutic opportunities for hyperprogressive disease after immune checkpoint inhibitor therapy. J Clin Oncol 41:14688. https://doi.org/10.1200/JCO.2023.41.16_suppl.e14688
Park SH, Kim KY, Kim YM, Hyung WJ (2023) Patient-specific virtual three-dimensional surgical navigation for gastric cancer surgery: a prospective study for preoperative planning and intraoperative guidance. Front Oncol 13:1140175. https://doi.org/10.3389/fonc.2023.1140175
Franklin WA, Carbone DP (2003) Molecular staging and pharmacogenomics. Clinical implications: from lab to patients and back. Lung Cancer 41(Suppl 1):147–54. https://doi.org/10.1016/s0169-5002(03)00158-2
Fuji L, Louw D (2007) Surgical robotics for patient safety in the perioperative environment: realizing the promise. Surg Innov 14:77–82. https://doi.org/10.1177/1553350607303880
Pilie PG, LoRusso PM, Yap TA (2017) Precision medicine: progress, pitfalls, and promises. Mol Cancer Ther 16:2641–4. https://doi.org/10.1158/1535-7163.MCT-17-0904
Mercogliano MF, Bruni S, Mauro FL, Schillaci R (2023) Emerging targeted therapies for HER2-positive breast cancer. Cancers (Basel). https://doi.org/10.3390/cancers15071987
Stukan AI, Goryainova AY, Riger NA, Sharov SV, Shatokhina AS, Chukhray OY et al (2021) Germinal <i>BRCA</i>-mutation significance in the tumor microenvironment formation Efficacy of PARP inhibition in late-line therapy of metastatic castration-resistant prostate cancer. Cancer Urol 17:85–94. https://doi.org/10.17650/1726-9776-2021-17-3-85-94
Bound NT, Vandenberg CJ, Kartikasari AER, Plebanski M, Scott CL (2022) Improving PARP inhibitor efficacy in high-grade serous ovarian carcinoma: a focus on the immune system. Front Genet 13:886170. https://doi.org/10.3389/fgene.2022.886170
Pacanowski M, Liu Q (2020) Precision medicine 2030. Clin Pharmacol Ther 107:62–4. https://doi.org/10.1002/cpt.1675
Roukos DH (2011) Cancer genome explosion and systems biology: impact on surgical oncology? Ann Surg Oncol 18:12–5. https://doi.org/10.1245/s10434-010-1355-y
Key BA, Mumba M (2019) Using precision medicine to individualize healthcare. Nursing 49:43–5. https://doi.org/10.1097/01.NURSE.0000569760.88666.61
Tobin AA, Little C, Schneider RJ, Thompson VM, Jannetto PJ, Wong SH et al (2008) Pharmacogenomic evaluation of a pediatric dextromethorphan fatality following cold medication therapy: the role of cytochrome P450 2D6 polymorphisms. The FASEB J. https://doi.org/10.1096/fasebj.22.1_supplement.1134.6
Matic M, de Hoogd S, de Wildt SN, Tibboel D, Knibbe CA, van Schaik RH (2020) OPRM1 and COMT polymorphisms: implications on postoperative acute, chronic and experimental pain after cardiac surgery. Pharmacogenomics 21:181–93. https://doi.org/10.2217/pgs-2019-0141
Silva CA, Ribeiro-Dos-Santos A, Goncalves WG, Pinto P, Pantoja RP, Vinasco-Sandoval T et al (2021) Can miRNA indicate risk of illness after continuous exposure to M. tuberculosis? Int J Mol Sci. https://doi.org/10.3390/ijms22073674
Deghmane AE, Taha MK (2021) Invasive bacterial infections in subjects with genetic and acquired susceptibility and impacts on recommendations for vaccination: a narrative review. Microorganisms. https://doi.org/10.3390/microorganisms9030467
Ercisli M, Lechun G, Azeez S, Hamasalih R, Song S, Aziziaram Z (2021) Relevance of genetic polymorphisms of the human cytochrome P450 3A4 in rivaroxaban-treated patients. Cell, Mol Biomed Rep 1:33–41. https://doi.org/10.55705/cmbr.2021.138880.1003
Chenoweth MJ, Giacomini KM, Pirmohamed M, Hill SL, van Schaik RHN, Schwab M et al (2020) Global pharmacogenomics within precision medicine: challenges and opportunities. Clin Pharmacol Ther 107:57–61. https://doi.org/10.1002/cpt.1664
Soares FFC, Ferreira D, Raimundini AA, Dionisio TJ, Dos Santos CF, Conti PCR et al (2023) Influence of genetic polymorphisms on mechanical pain sensitivity and endogenous pain modulation of trigeminal and spinal areas. J Oral Rehabil 50:39–53. https://doi.org/10.1111/joor.13384
Su H, Kwok KW, Cleary K, Iordachita I, Cavusoglu MC, Desai JP et al (2022) State of the art and future opportunities in mri-guided robot-assisted surgery and interventions. Proc IEEE Inst Electr Electron Eng 110:968–92. https://doi.org/10.1109/jproc.2022.3169146
Pellegrini G, Rasperini G, Pagni G, Giannobile WV, Milani S, Musto F et al (2017) Local wound healing biomarkers for real-time assessment of periodontal regeneration: pilot study. J Periodontal Res 52:388–96. https://doi.org/10.1111/jre.12403
Hall OM, Peer CJ, Figg WD (2018) Tissue preservation with mass spectroscopic analysis: implications for cancer diagnostics. Cancer Biol Ther 19:953–5. https://doi.org/10.1080/15384047.2018.1456610
Sikdar S, Ganguly K, Guha S, Bag S, Barman H (2020) Molecular imaging technology: a promising frontier of interventional radiology. SSRN Electron J. https://doi.org/10.2139/ssrn.3515891
Shurin MR, Baraldi JH, Shurin GV (2021) Neuroimmune regulation of surgery-associated metastases. Cells. https://doi.org/10.3390/cells10020454
Kranke P, Redel A, Schuster F, Muellenbach R, Eberhart LH (2008) Pharmacological interventions and concepts of fast-track perioperative medical care for enhanced recovery programs. Expert Opin Pharmacother 9:1541–64. https://doi.org/10.1517/14656566.9.9.1541
Haidegger T, Speidel S, Stoyanov D, Satava RM (2022) Robot-assisted minimally invasive surgery—surgical robotics in the data age. Proc IEEE 110:835–46. https://doi.org/10.1109/jproc.2022.3180350
Brodie A, Vasdev N (2018) The future of robotic surgery. Ann R Coll Surg Engl 100:4–13. https://doi.org/10.1308/rcsann.supp2.4
Black C, Voigts J, Agrawal U, Ladow M, Santoyo J, Moore C et al (2017) Open ephys electroencephalography (Open Ephys + EEG): a modular, low-cost, open-source solution to human neural recording. J Neural Eng 14:035002. https://doi.org/10.1088/1741-2552/aa651f
Funding
There was no financial support for this study.
Author information
Authors and Affiliations
Contributions
LM and FH: participated in article writing, AR: involved in article writing and final revision, FH and LM: contributed to drawing the manuscript tables and figures, and AN: consented to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Research involving human and animal participants
This article contains no studies with human participants or animals performed by the authors.
Consent for publication
This manuscript has been approved for publication by all the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mokhtari, L., Hosseinzadeh, F. & Nourazarian, A. Biochemical implications of robotic surgery: a new frontier in the operating room. J Robotic Surg 18, 91 (2024). https://doi.org/10.1007/s11701-024-01861-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11701-024-01861-6