Abstract
Accurate acetabular cup position remains a persistent challenge in total hip arthroplasty (THA). Studies investigating the early outcomes of robotic-assisted THA (RA-THA) systems have shown improved cup placement compared to manual THA (mTHA) approaches, however, contemporary robotic platforms are reliant on pre-operative CT imaging. The goal of this study was to analyze the accuracy of a novel, fluoroscopy-based RA-THA system compared to an unassisted mTHA approach and determine the effect of the robotic system on operative time. We performed a retrospective cohort analysis on a consecutive series of 198 patients who received mTHA and RA-THA between March 2021 and July 2022. The primary outcome of interest was the accuracy of acetabular component placement, defined by average cup inclination and anteversion. Secondary outcomes included the proportion of acetabular cups positioned within the Lewinnek safe zone, operative time, and overall room time. The RA-THA group demonstrated significantly higher accuracy of acetabular anteversion to target compared to the manual group (18.5 vs. 21.7˚; p < 0.001), and had a significantly greater proportion of acetabular cups placed within the Lewinnek safe zone (81.6 vs. 59.0%; p < 0.001). The RA-THA cohort had longer operative times compared to mTHA group (39.0 vs. 35.3 min; p = 0.003), but no difference was seen in total operating room time (101.2 vs. 101.2 min; p = 0.982). This study demonstrates that the use of a novel, fluoroscopy-based, pin-less THA robotic platform increased the accuracy of acetabular cup placement, including a 22.6% improvement in safe zone placement, compared to mTHA approach, with no increase in overall case time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Total hip arthroplasty (THA) is widely considered as one of the most effective surgeries [24]. Despite the overall success of the procedure, complications such as component malposition, impingement, and dislocation can occur, which can ultimately result in need for surgical revision [10]. One of the major factors that determines the success of THA and reduces the rates of these complications is the accuracy of acetabular component placement [4, 7, 12, 16].
To improve the accuracy of cup placement and reduce complication rates, robotic platforms have been developed and integrated into clinical practice to assist in the execution of THA [26]. Studies investigating the early outcomes of semi-autonomous robotic-assisted THA (RA-THA) published in the current literature have shown improved cup placement and decreased intraoperative complications compared to unassisted manual THA (mTHA) [5, 8, 10, 17, 28]. Likewise, several groups have demonstrated that this increased precision has translated to greater hip-specific functional outcomes in patients who have received RA-THA compared to traditional approaches [6, 22]. Several economic analyses have estimated that these recent improvements in robotic surgery have made RA-THA more cost-effective than mTHA, in part due to shorter lengths of hospital stay and lower consumption of care resources [11, 20, 23, 24].
Although these studies have illustrated the potential benefits of RA-THA workflows, a current criticism of contemporary robotic platforms is the reliance on pre-operative CT imaging and intraoperative navigation tracker pins to guide the robotic platform, which can alter intraoperative workflow, prolong operative times, introduce additional radiation associated with CT imaging, and increase overall costs of the procedure compared to unassisted techniques [16, 18, 27]. Additionally, the accuracy advantages of robotics have not been clearly demonstrated when used in conjunction with direct anterior approach THA (DAA-THA) compared with fluoroscopic guidance [25]. Novel, fluoroscopic-based robotic platforms aim to address these issues through the replacement of pre-operative CT imaging with the integration of intraoperative fluoroscopy [16]. While a cadaveric study has shown improved accuracy of acetabular cup placement in a fluoroscopy-based robotic platform compared to an unassisted approach [16], no group has examined the accuracy of this novel, pin-less fluoroscopy-based RA-THA in clinical practice. Thus, our present study sought to (1) evaluate the accuracy of a fluoroscopy-based RA-THA platform compared to manual unassisted technique, and (2) determine the effect of the robotic system on operative time. We hypothesized that use of this robotic system would increase accuracy of acetabular cup placement relative to the current unassisted standard by providing full control of the surgeon’s planned acetabular positioning while introducing no significant increase on operative times.
Methods
Study design
Institutional Review Board approval was obtained prior to the initiation of this study. We performed a retrospective cohort analysis on a consecutive series of patients who received mTHA and fluoroscopy-based RA-THA between March 2021 and July 2022. Inclusion criteria for this study were patients greater than 18 years of age who underwent primary unilateral DAA-THA by the primary study surgeon at a single institution. All patients received THA for a diagnosis of osteoarthritis, avascular necrosis, or rheumatoid arthritis. Exclusion criteria for this study were patients with missing or inadequate post-operative radiographs, patients who underwent THA for a femoral neck fracture, and patients less than 18 years of age. From September 2019 to August 2021, only DAA mTHA was performed with routine use of a standard intraoperative C-arm fluoroscopy for assistance. The RA-THA platform, the ROSA® Total Hip System (Zimmer Biomet, Warsaw IN, USA), was approved for clinical use by the Food and Drug Administration in September 2021, and all THA were performed after this approval utilizing the robotic system. By querying the electronic health record (EHR), we identified a consecutive series of the first 98 patients who underwent unilateral, fluoroscopy-based RA-THA at our institution, which served as the experimental group. The control group was selected from the most recent 100 patients who underwent mTHA with the same surgeon.
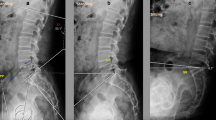
The primary outcome of interest was the accuracy of acetabular component placement, defined as average cup inclination and anteversion between the two treatment groups with respect to the preoperative goal of 40°/15° of inclination/anteversion. Secondary outcomes included the proportion of acetabular cups positioned within the Lewinnek safe zone, defined as 40 ± 10˚ of cup inclination and 15 ± 10˚ of cup anteversion [10], as well as average operative and overall operating room times. Acetabular cup orientation was determined by analyzing post-operative anteroposterior (AP) pelvic radiographs using Martell Hip Analysis Suite Software (version 8.0.4.5., Martell Hip Analysis Suite™, Chicago, IL). Radiographs used for analysis were standardized to standing AP pelvis studies obtained at routine 6-week post-operative follow-up visits. An example of the radiographic analysis using the Martell Hip Analysis Suite is presented in Fig. 1. Age at time of procedure, sex, race, body mass index (BMI), preoperative diagnosis, procedure laterality, ASA score, and operative time were recorded for each patient. Total operative time was defined as the time from incision to start of skin closure. Total operating room time was defined as the time between when the patient was transported into, then out of the operating room. Event times were calculated from intraoperative timestamps recorded in the EHR.
Surgical techniques
For the control cohort, the principal surgeon performed unassisted mTHA through a DAA on a traction table. Fluoroscopic C-arm guidance was used to assist with leveling the pelvis (i.e. symmetrical obturator foramina), bone preparation, and component positioning. For the study cohort, DAA-THA was performed with assistance of the robotic system, using the workflow previously published by Kamath et al. [16]. The principal surgeon targeted 40°/15° of inclination/anteversion for acetabular cup orientation for both manual and robotic cohorts. All patients were implanted with the G7 Acetabular System (ZimmerBiomet, Warsaw, IN) and the Avenir Complete femoral stem (ZimmerBiomet, Warsaw, IN). In both groups, the surgical technique and peri-operative management was identical apart from the use of fluoroscopic guided robotic assistance in the treatment cohort and manual fluoroscopic guidance in the control cohort.
Statistical analysis
Descriptive statistics for patient demographics and surgical data were performed. Continuous variables were reported as means and standard deviations (SD) and compared between groups using independent sample t-tests. Categorical variables were presented as frequencies and compared using Pearson’s chi-squared tests or Fisher’s exact tests when appropriate. Variances were used to define the precision of acetabular cup position and compared using F-tests.
All tests were two-tailed. P-values < 0.05 were considered statistically significant. Using β = 0.8, our study was appropriately powered to detect a 15% difference in rates of acetabular cup placement within the Lewinnek safe zone. Statistical analyses were performed using JMP Version 16.2. (SAS Institute Inc., Cary, NC, 1989–2021).
Results
The study series included 198 patients, consisting of 100 patients who received mTHA and 98 patients who received fluoroscopy-based RA-THA. Both the robotic THA and mTHA cohorts had similar baseline demographic characteristics of age, sex, BMI, and race (Table 1). Likewise, there was no significant difference in surgical side, preoperative diagnosis, or distribution of ASA scores between treatment groups.
Comparison of the accuracy of acetabular cup placement demonstrated significantly greater accuracy in the RA-THA cohort compared to the mTHA cohort. Specifically, the RA-THA group demonstrated significantly higher accuracy of acetabular anteversion to target, compared to the manual group (18.5˚ vs. 21.7˚; p < 0.001). The RA-THA group had significantly less variance in acetabular anteversion measurements compared to the mTHA group (26.0 vs. 44.5; p = 0.008). The robotic THA treatment group had a significantly greater proportion of acetabular cups placed within the Lewinnek safe zone compared to the mTHA group (81.6% vs. 59.0%; p < 0.001). While there was no difference in acetabular inclination (42.8˚ vs. 42.8˚; p = 0.976), there was significantly lower variance for the RA-THA group for this parameter (26.8 vs. 46.7; p = 0.007). Distribution of acetabular cup positions are presented as Box plots in Fig. 2. The proportion of acetabular cups placed in the Lewinnek safe zone for manual and robotic groups are graphically represented in Fig. 3.
The robotic THA cohort had significantly longer operative times compared to mTHA group (39.0 vs. 35.3 min; p = 0.003), but no difference was seen in total operating room time between groups (101.2 vs. 101.2 min; p = 0.982). Comparisons of the primary and secondary outcomes are presented in Table 2.
Discussion
This study presents the first clinical results of a novel, fluoroscopy-based, pin-less robotic-assisted platform for primary THA. The principal finding of our study is that the fluoroscopy-based RA-THA system demonstrated significantly more accurate and precise acetabular cup positioning compared to a manual approach with respect to cup placement within the Lewinnek safe zone. While the Lewinnek safe zone has come under recent scrutiny as a functional goal of procedural success [1, 9], the safe zone remains a widely used benchmark for accuracy of DAA-THA compared to a planned preoperative goal. Additionally, our study is reporting on precision of the RA-THA in achieving the surgeon’s target orientation (i.e. inclination of 40 degrees and anteversion of 15 degrees), and our findings confirm the hypothesis.
In a recent study on radiological outcomes of CT-based RA-THA compared to mTHA, Domb et al. reported that the CT-based RA-THA had significantly higher rates of cup orientation within the Lewinnek safe zone [97.0% vs. 73.8%, relative risk of placement outside safe zone, 0.11 (95% confidence interval, 0.03 to 0.46), p = 0.002], with a difference of 23.2% between cohorts, similar to that in this present study [8]. Importantly, not only do our findings further support the growing body of evidence supporting the improved accuracy of RA-THA compared to mTHA, but that the improvements in accuracy seen in this novel, fluoroscopy-based robotic platform are comparable to those observed in previous CT-based systems. Similarly, Kamara et al. found significantly smaller variance for a posterior RA-THA approach compared to a manual fluoroscopy-guided DAA THA and a manual posterior approach in both achieved inclination (14.0 vs. 24.5 vs. 37.5; p < 0.01) and anteversion (19.5 vs. 54.6 vs. 56.3; p < 0.01) [15]. Although this study was comparing differences in both robotic assistance and surgical approach across three different study cohorts, the robotic arm of this study showed significantly reduced variance of acetabular cup position similar to that in our study. In contrast, a recent study by Stewart et al. questioned whether robotics improved acetabular component precision when used for DAA THA. They compared fluoroscopic guidance versus CT-based robotics for DAA THA and found no benefit with the use haptic, CT-based robotic guidance for acetabular anteversion, safe zone placement, leg length discrepancy, or femoral and global offset [25]. One reason for this may lie in the difficulties with bony land marking and registration of the acetabulum when using DAA for THA. In comparison to this published data, the decreased variance in our RA-THA cohort demonstrates similar improvements in precision of acetabular cup placement using this novel fluoroscopy-based robotic platform compared to previous CT-based robotic approaches. Taken together, these results suggest that this system is able to achieve similar radiological results to CT-based platforms without the need of an additional pre-operative CT scan nor intra-operative guidance pins nor bony landmark registration required in CT-based RA-THA workflows. This translates to improvement in overall treatment results with RA-THA, while streamlining procedural workflows and eliminating radiation associated with CT imaging; this comes without sacrificing component placement accuracy with use of fluoroscopy-based robotic system.
In addition to improved accuracy, we found no prolongation in overall case time with the introduction of the robotic system during THA. While operative times for the robotic cohort were approximately 4 min longer than that of manual cases, the overall operating room time was identical between both groups. It is also unlikely that the 4 additional minutes provides clinical or resource utilization significance, despite the statistical difference [3]. While the introduction of the robotic system appears to increase the duration of the hands-on portion of the procedure, given that preparation of the patient for surgery, skin closure, and operating room turnover constitutes a significantly greater proportion of the total case time, any increase in procedural time introduced to the robot had no clinical effect on overall workflow. Importantly, this meant that the integration of the fluoroscopy-based robot had no impact on overall workflow in this clinical, real-world study inclusive of any potential learning curve. Several studies have similarly reported increased operative times for CT-based RA-THA compared to mTHA [13, 14, 19, 21]. In their case–control study investigating cup positioning of CT-based RA-THA and mTHA, Guo et al. found significantly longer operative time for the robotic cohort compared to the manual cohort (91.37 vs. 77.51 min; p < 0.001) [13]. Although it is difficult to draw direct comparisons, as our operative time was defined from incision to start of skin closure, it is worth noting that the absolute difference in operative times is much larger then that seen in the present study (39.0 vs. 35.3 min; p = 0.003). This may suggest that the fluoroscopy-based robotic workflow has reduced operative times compared to CT-based robotic workflows, but future studies would be required to directly compare potential differences in the robotic platforms standardized for operative approaches.
Our study had several limitations. Firstly, the study is subject to EHR data extraction; we utilized digitally recorded operative times to mitigate this bias. Secondly, the primary study surgeon performs a high volume of THA that could affect the generalizability of this study. The mean number of THA procedures per surgeon in the United States, as reported in the American Joint Replacement Registry, is approximately 30 THA per year, while the principal surgeon performs > 250 THA per year [2]. However, a surgeon with less experience may likely have lower accuracy with their baseline mTHA procedures. Therefore, it is possible that the significance of our findings on procedure accuracy would be strengthened if this study were repeated with lower volume surgeons. Thirdly, while cohorts were not matched a priori, the demographic analysis showed that cohorts were comparable. Finally, while standardized post-operative imaging analysis techniques (i.e., Martell analysis) were used, this modality may be subject to variations in patient positioning, such as with severe spinopelvic disease, and across time points. We sought to present a real-world scenario of using pre- and post-operative standard radiographs, which may account for the variability in safe zone and component measurements when compared to other studies in the literature. Our analysis emphasized matching pre-operative AP standing pelvis imaging to post-operative standing AP imaging to reduce potential error in measurement technique.
Conclusion
The findings of this study suggest that the use of a novel, fluoroscopy-based, pin-less THA robotic platform increased the accuracy and precision of acetabular cup placement compared to fluoroscopy-guided, manual THA. Additionally, there was no difference in overall case time between robotic and manual cohorts. Relative to CT-based robotic workflows presented in the current literature, use of fluoroscopy-based RA-THA demonstrated similar benefits of increased accuracy of cup positioning with a notably smaller increase in operative time. Therefore, given the improved accuracy with the fluoroscopy-based robotic technique used in this study, our findings can be used to justify the further investigation of fluoroscopy-based THA robotic assistance systems in clinical practice.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Abdel MP, von Roth P, Jennings MT, Hanssen AD, Pagnano MW (2016) What safe zone? The vast majority of dislocated THAs are within the Lewinnek safe zone for acetabular component position. Clin Orthop Relat Res 474:386–391
Anon. American Joint Replacement Registry (AJRR): 2020 Annual Report. Rosemont, IL; 2020.
Beyer F, Lützner C, Stalp M, Köster G, Lützner J (2022) Does the use of patient-specific instrumentation improve resource use in the operating room and outcome after total knee arthroplasty? A multicenter study. PLoS ONE 17:e0277464
Biedermann R, Tonin A, Krismer M, Eibl G, Stöckl B (2005) Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br 87:762–769
Chen X, Xiong J, Wang P, Zhu S, Qi W, Peng H, Yu L, Qian W (2018) Robotic-assisted compared with conventional total hip arthroplasty: systematic review and meta-analysis. Postgrad Med J 94:335
Clement ND, Gaston P, Bell A, Simpson P, Macpherson G, Hamilton DF, Patton JT (2021) Robotic arm-assisted versus manual total hip arthroplasty: a propensity score matched cohort study. Bone Joint Res 10:22
Danoff JR, Bobman JT, Cunn G, Murtaugh T, Gorroochurn P, Geller JA, Macaulay W (2016) Redefining the acetabular component safe zone for posterior approach total hip arthroplasty. J Arthroplasty 31:506–511
Domb BG, Chen JW, Lall AC, Perets I, Maldonado DR (2020) Minimum 5-year outcomes of robotic-assisted primary total hip arthroplasty with a nested comparison against manual primary total hip arthroplasty: a propensity score-matched study. J Am Acad Orthop Surg 28:847–856
Dorr LD, Callaghan JJ (2019) Death of the Lewinnek “Safe Zone.” J Arthroplasty 34:1–2
Emara AK, Samuel LT, Acuña AJ, Kuo A, Khlopas A, Kamath AF (2021) Robotic-arm assisted versus manual total hip arthroplasty: systematic review and meta-analysis of radiographic accuracy. Int J Med Robot 17:e2332
Emara AK, Zhou G, Klika AK, Koroukian SM, Schiltz NK, Higuera-Rueda CA, Molloy RM, Piuzzi NS (2021) Is there increased value in robotic arm-assisted total hip arthroplasty? A nationwide outcomes, trends, and projections analysis of 4,699,894 cases. Bone Joint J 103-B:1488–1496
Grammatopoulos G, Thomas GER, Pandit H, Beard DJ, Murray DW, Gill HS (2015) The effect of orientation of the acetabular component on outcome following total hip arthroplasty with small diameter hard-on-soft bearings. Bone Joint J 97-B:164–172
Guo D, Li X, Ma S, Zhao Y, Qi C, Xue Y (2022) Total hip arthroplasty with robotic arm assistance for precise cup positioning: a case-control study. Orthop Surg 14:1498–1505
Honl M, Dierk O, Gauck C, Carrero V, Lampe F, Dries S, Quante M, Schwieger K, Hille E, Morlock MM (2003) Comparison of robotic-assisted and manual implantation of a primary total hip replacement. A prospective study. J Bone Joint Surg Am 85:1470–1478
Kamara E, Robinson J, Bas MA, Rodriguez JA, Hepinstall MS (2017) Adoption of robotic vs fluoroscopic guidance in total hip arthroplasty: is acetabular positioning improved in the learning curve? J Arthroplasty 32:125–130
Kamath AF, Durbhakula SM, Pickering T, Cafferky NL, Murray TG, Wind MA, Méthot S (2021) Improved accuracy and fewer outliers with a novel CT-free robotic THA system in matched-pair analysis with manual THA. J Robot Surg. https://doi.org/10.1007/s11701-021-01315-3
Kort N, Stirling P, Pilot P, Müller JH (2021) Clinical and surgical outcomes of robot-assisted versus conventional total hip arthroplasty: a systematic overview of meta-analyses. EFORT Open Rev 6:1157
Kunze KN, Bovonratwet P, Polce EM, Paul K, Sculco PK (2022) Comparison of surgical time, short-term adverse events, and implant placement accuracy between manual, robotic-assisted, and computer-navigated total hip arthroplasty: a network meta-analysis of randomized controlled trials. J Am Acad Orthop Surg Glob Res Rev. https://doi.org/10.5435/JAAOSGlobal-D-21-00200
Lim S-J, Ko K-R, Park C-W, Moon Y-W, Park Y-S (2015) Robot-assisted primary cementless total hip arthroplasty with a short femoral stem: a prospective randomized short-term outcome study. Comput Aided Surg 20:41–46
Maldonado DR, Go CC, Kyin C, Rosinsky PJ, Shapira J, Lall AC, Domb BG (2021) Robotic arm-assisted total hip arthroplasty is more cost-effective than manual total hip arthroplasty: a Markov model analysis. J Am Acad Orthop Surg 29:e168–e177
Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H (2010) A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop Relat Res 468:1072
Ng N, Gaston P, Simpson PM, Macpherson GJ, Patton JT, Clement ND (2021) Robotic arm-assisted versus manual total hip arthroplasty : a systematic review and meta-analysis. Bone Joint J. 103-B:1009–1020
Pierce J, Needham K, Adams C, Coppolecchia A, Lavernia C (2021) Robotic-assisted total hip arthroplasty: an economic analysis. J Comp Eff Res 10:1225–1234
Remily EA, Nabet A, Sax OC, Douglas SJ, Pervaiz SS, Delanois RE (2021) Impact of robotic assisted surgery on outcomes in total hip arthroplasty. Arthroplast Today 9:46–49
Stewart NJ, Stewart JL, Brisbin A (2022) A comparison of component positioning between fluoroscopy-assisted and robotic-assisted total hip arthroplasty. J Arthroplasty 37:1602-1605.e3
Sugano N (2013) Computer-assisted orthopaedic surgery and robotic surgery in total hip arthroplasty. Clin Orthop Surg 5:1
Sweet MC, Borrelli GJ, Manawar SS, Miladore N (2021) Comparison of outcomes after robotic-assisted or conventional total hip arthroplasty at a minimum 2-year follow-up: a systematic review. JBJS Rev. https://doi.org/10.2106/JBJS.RVW.20.00144
Tompkins GS, Sypher KS, Li HF, Griffin TM, Duwelius PJ (2021) Robotic vs manual total knee arthroplasty in high volume surgeons: a comparison of cost and quality metrics. J Arthroplasty. https://doi.org/10.1016/j.arth.2021.12.018
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by GBJB and CJH. The first draft of the manuscript was written by GBJB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
GBJB and CJH declare they have no competing interests. DK serves on the speakers’ bureau and receives royalties from Zimmer Biomet. DL serves on the speakers’ bureau, is a paid consultant, and receives royalties and research support from Zimmer Biomet. LM is a paid consultant and receives research support from Zimmer Biomet. AFK serves on the speakers’ bureau, is a paid consultant, and owns stock or stock options in Zimmer Biomet.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Cleveland Clinic Foundation Institutional Review Board (June 3rd, 2022/ No. 22-528).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Buchan, G.B.J., Hecht, C.J., Liu, D. et al. Improved accuracy of a novel fluoroscopy-based robotically assisted THA system compared to manual THA. J Robotic Surg 17, 2073–2079 (2023). https://doi.org/10.1007/s11701-023-01623-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-023-01623-w