Abstract
Background and Objective
Retzius-sparing robotic-assisted radical prostatectomy (rsRARP) has gained popularity due to superior early continence outcomes compared to standard robotic prostatectomy (sRARP). We evaluate the results of a single surgeon who transitioned from sRARP to rsRARP and compare oncologic and functional outcomes.
Methods
We retrospectively reviewed all prostatectomies performed by a single surgeon between June 2018 and October 2020. Perioperative, oncologic, and functional data were collected and analyzed. Patients who underwent sRARP were compared with those who underwent rsRARP.
Results
Both groups contained 37 consecutive patients each. Preoperative patient characteristics and biopsy results were similar between the two groups. Perioperative outcomes were significant for longer operative room time and higher proportion of T3 tumors in the rsRARP group. Thirty-day complication and readmission rates were similar between groups. There was no difference in early oncologic outcomes, including positive surgical margin rate, biochemical recurrence, and need for adjuvant or salvage treatments. The time to urinary continence and immediate continence rate was superior in the rsRARP group.
Conclusions
The Retzius-sparing approach can be safely adopted by surgeons experienced in sRARP without compromising early oncologic outcomes and with the benefit of improved early continence recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The surgical treatment of prostate cancer has evolved over the years. Since the introduction of the Da Vinci robot (Intuitive, Sunnyville Ca), robotic-assisted radical prostatectomy (RARP) has become the standard treatment for localized prostate cancer. Since the first description by Abbou et al. [1], RARP has undergone numerous technical modifications. The goal of these modifications has been to optimize cancer control, continence preservation, and sexual function recovery. Functional recovery is often achieved by attempting to preserve the normal anatomy of the male pelvis. None of these technical modifications has been as successful at preserving anatomical structures as the Retzius-sparing robotic-assisted radical prostatectomy (rsRARP) approach.
In 2010, Galfano et al. published on the rsRARP technique and reported their initial results [2]. The approach involves dissection of the prostate entirely from an incision made in the pouch of Douglas; thus avoiding disruption of the Space of Retzius, including puboprostatic ligaments, detrusor apron and dorsal venous complex (DVC). The thought was that these anterior structures have an important role for continence and sparing them would result in improved continence recovery. In their initial study, Galfano et al. reported results of their first three successfully completed rsRARP cases showing that all three had immediate continence following catheter removal [2]. The approach has now become standardized and is becoming more widespread. More than 30 centers around the world have adopted the Retzius-sparing approach with over 5000 cases performed [3].
Data from randomized control trials (RCT) and a meta-analysis have consistently demonstrated superior early continence outcomes when compared to the standard RARP (sRARP) technique. The importance of this is highlighted by the Prostate Cancer Outcomes Study (PCOS), where 66% of patients considered incontinence a problem at 6 months postoperatively, with 15% rating it as moderate-to-severe [4]. Although most patients eventually regain their continence by 1 year, the delay in continence recovery can be a significant source of morbidity and patient dissatisfaction. Thus, rsRARP has the potential to improve the quality of life in patients during the initial postoperative period.
The hesitation to widespread adoption of rsRARP is based on a steep learning curve and concerns about higher positive surgical margin (PSM) rates. In this study, we evaluate the results of single surgeon experienced in sRARP who transitioned completely to rsRARP. We evaluate the early learning curve of this transition while comparing oncologic and functional outcomes.
Methods
From June 2018 through October 2020, 74 consecutive RARPs performed by a single fellowship-trained robotic surgeon were evaluated. This surgeon evaluated in this study switched from the sRARP technique to the rsRARP technique in July 2019. The surgeon had no prior training in the rsRARP technique and learned primarily from lectures and surgical videos. The surgeon had been performing standard robotic-assisted radical prostatectomies prior to transitioning to the Retzius-sparing approach for nine years following completion of training. After July 2019, all cases were done using the rsRARP technique, regardless of clinicopathologic features. Following institutional review board approval, data was collected via chart review and maintained in a password-protected database. Patients with incomplete clinical information were excluded from analysis.
Surgical technique
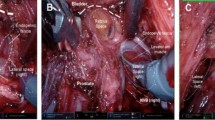
From June 2018 to July 2019, patients underwent sRARP using a 4-armed transperitoneal approach with the daVinci XI system (Intuitive, Sunnyville Ca). The modified Montsouris technique was used in which the seminal vesicles and vasa deferentia are dissected prior to dropping the bladder [5]. A nerve-sparing technique was performed for both erectile function preservation and improved postoperative continence when indicated based on the patient preoperative risk of extracapsular extension and intraoperative findings. [6] Furthermore, the degree of nerve sparing (i.e., full vs partial nerve sparing) was similarly based on preoperative risk of extracapsular extension and intraoperative findings. If partial nerve sparing was performed, it was considered utilization of a nerve-sparing technique on statistical analysis. Bladder neck sparing was also done when possible. A posterior reconstruction was performed as described by Rocco et al. [7]. Anterior urethropexy was also routinely performed.
From July 2019 to October 2020, patients underwent rsRARP using a 4-armed transperitoneal approach with the daVinci XI system. The technique used was similar to that described by Galfano et al. [2]. Again, nerve sparing was performed based on clinicopathologic characteristics and intraoperative findings.
Variables
Data were collected and divided into preoperative, perioperative, and functional variables. Preoperative data included patient age, BMI, prior abdominal surgeries, PSA, biopsy results, NCCN risk group, prostate size, international prostate symptom score (IPSS) and sexual health inventory for men (SHIM) scores. Intraoperative variables included estimated blood loss (EBL), operating room (OR) time, nerve spare status, final pathology, pathologic stage, number of lymph nodes removed, margin status, extracapsular extension (ECE), seminal vesical invasion (SVI), lymphovascular invasion (LVI), perineural invasion (PNI), length of stay (LOS), urethral catheter duration, complications, and re-admissions within 30 days. Oncologic outcomes assessed included biochemical recurrence (BCR), clinical recurrence, and adjuvant or salvage treatments. The functional outcomes evaluated included time to continence, IPSS and SHIM score at 3 and 6 months postoperatively. Urinary continence was defined as the use of one safety pad per day or less. Immediate continence was defined as continence within 1 week of urethral catheter removal.
Statistical analysis
Statistical analysis was performed using JMP statistical software (SAS Institute, Cary, NC). Continuous data were presented as means and standard deviation (SD) or medians and interquartile ranges (IQR). Categorical data were presented as percentages. The Student t test or Mann–Whitney U test was used to compare continuous variables while Chi-square test was used for categorical variables. p values were calculated and considered statistically significant if p < 0.05.
Results
A total of 37 patients underwent sRARP followed by 37 who underwent rsRARP. Preoperative patient and tumor characteristics were similar between groups, except rsRARP patients had higher preoperative SHIM scores (Table 1). There was one conversion from rsRARP to sRARP during the anastomosis portion of the procedure.
Perioperative findings were significant for a longer OR time in the rsRARP group (285 vs 252 min, p < 0.001). There were also more adverse pathologic features in the rsRARP group. 75% were pT3 in the rsRARP group compared to 54% in sRARP group. Foley catheter duration was slightly longer in the rsRARP group. Complications and readmission rates within 30 days were low and similar between groups. The mean duration of follow-up was 18.3 months in the sRARP group, compared to 8.8 months in the rsRARP group, given the temporal difference in the use of each technique (Table 2).
Oncologic outcomes were similar between the two groups. The PSM rate was 29% for sRARP and 35% rsRARP (p = 0.62). There was a low rate of adjuvant or salvage treatments as well as BCR in both groups (Table 3).
Immediate continence was achieved in 40.5% in rsRARP group compared to 8.1% in sRARP group (p < 0.001). The rsRARP group also had a shorter time to continence compared to sRARP at 40.8 days versus 102.2 days respectively (p = 0.003). Urinary function evaluated with IPSS scores was similar at baseline and 3 months postop. There was a slightly lower IPSS score at 6 months postoperatively in the rsRARP group (6 vs 9.3; p = 0.023) (Table 4). Sexual function outcomes were not evaluated due to the low number of patients with preoperative erectile function.
Discussion
We found that 40% of patients undergoing a Retzius-sparing approach had immediate continence after catheter removal compared to 8% in the standard group in our series. These findings have been corroborated by other groups such as Nyarangi-Dix et al., who reported 38% continence at one week after rsRARP [8]. The continence rate also improved as more experience in the Retzius-sparing technique was gained. These superior continence results are typically attributed to the preservation of structures anterior to the prostate [2]. In a recent systemic review, Checucci et al. showed that continence was superior in the rsRARP at all time points up to 1 year postoperatively when compared to sRARP [9]. These results would suggest that there may not be just a benefit in faster time to continence but also improved long-term continence. Incontinence is a major source of morbidity and dissatisfaction in prostatectomy patients and a more rapid return of continence will lead to improved quality of life.
While there is strong evidence supporting superior continence outcomes following rsRARP, there is a paucity of data evaluating postoperative erectile function. In a RCT comparing rsRARP to sRARP, Menon et al. noticed no difference in the return of erectile function in men who were potent preoperatively [10]. Bocciardi’s group reported 70% potency rate in men < 65 years old who had bilateral nerve sparing [11]. We were unable to properly assess postoperative erectile function due to the limited number of patients who were potent preoperatively. This study, like many previous ones, only evaluates early outcomes, but the true effect on erectile function may not be adequately known until longer follow-up data is obtained.
With regard to margin positivity, concern has been raised that the Retzius-sparing approach increases risk of PSM, especially anteriorly, but previous studies have demonstrated mixed findings. In a recent systematic review comparing rsRARP to sRARP, a higher PSM rate was seen with the rsRARP approach, which was predominantly in anteriorly located tumors [9]. On the contrary, another meta-analysis showed no difference in PSM rate between approaches [12]. These differences may be explained by the learning curve associated with rsRARP, as Lim et al. showed an improvement in the PSM rate when comparing their initial 25 patients undergoing rsRARP to the subsequent 25 patients [13]. Our results did not demonstrate a significantly increased risk of PSM with rsRARP, with rates of 29% for sRARP and 35% with rsRARP. While this is higher overall PSM rate than that reported in other studies comparing sRARP to rsRARP, it may be explained by the higher rate of T3 disease in our cohort. When stratified by pathologic stage, our PSM was 7.7% in T2 and 45.8% in T3 tumors (Table 3). This is more congruent with existing literature, such as the 50% PSM rate reported by Harty et al. in a cohort of primarily T3 patients [14].
Although some studies have reported higher PSM rate with the Retzius-sparing approach, none have shown an increased risk of biochemical recurrence (BCR). A study by Chang et al. demonstrated no difference in BCR between sRARP and rsRARP at one year follow-up (16.7% sRARP vs 13.3% rsRARP) [15]. In a RCT comparing rsRARP with sRARP, Menon et al. also showed a similar biochemical recurrence-free survival of 0.84 and 0.93, respectively [10]. We did not find an increased risk of BCR with rsRARP in the short-term follow-up period either. It is known that a PSM does not always correlate with BCR and that small focal margins have questionable prognostic significance. Long-term data are still needed to determine whether oncologic outcomes are jeopardized by the Retzius-sparing approach.
Despite well-documented improvement in functional outcomes, one of the main barriers to widespread adoption of rsRARP is the perspective of a steep learning curve. Fortunately, evidence in literature suggests that the learning curve may be more favorable than generally believed. In a study comparing outcomes of surgeons experienced in rsRARP to those just beginning to perform rsRARP, Bocciardi et al. found no difference in complications, PSM rate, BCR rate, and early continence [16]. We also did not note any significant difference in complications or re-admissions, while still demonstrating improved functional outcomes, during our initial experience with rsRARP. This may suggest that experienced robotic surgeons can achieve satisfactory oncologic and functional outcomes quite early in their transition from sRARP to rsRARP. It should be noted that conversion from rsRARP to sRARP always remains an option for surgeons more familiar with the latter approach, and this may be necessary early in the learning curve. This was required once in our experience due to difficulty with completing the anastomosis. Before fully switching to rsRARP, we traditionally performed a posterior approach to radical prostatectomy with dissecting out the seminal vesicles and performing posterior dissection prior to dropping the bladder. We gradually started to dissect out the prostate more laterally and began dividing the vascular pedicles and releasing neurovascular bundles prior to dropping the bladder in preparation for converting to the Retzius-sparing approach. This hybrid approach gave us more confidence when we eventually transitioned to a complete Retzius-sparing technique and a possible pathway to consider for surgeons transitioning to rsRARP.
There are several notable limitations of this study. First, this is a single-center, retrospective, non-randomized study and thus at risk for sampling bias and unmeasured confounding variables. We also had a small sample size for comparisons as we only recently adopted the Retzius-sparing approach. The follow-up in the rsRARP group was significantly shorter than the sRARP group and, therefore, we did not evaluate any long-term oncologic or functional outcomes. Our findings about continence relied primarily on what patients reported, creating a potential for recall bias. Lastly, this was a single surgeon’s experience at a tertiary care center, which limits it generalizability. Large, prospective, multi-centered RCT with long-term follow-up may further elucidate more reliable oncologic and functional outcomes data in the Retzius-sparing approach.
Conclusions
The Retzius-sparing technique can be safely adopted by robotic surgeons experienced in the standard approach with the benefit of improved early continence outcomes. Based on our results, there does not appear to be any compromise in early oncologic outcomes. Longer follow-up data on functional and oncologic outcomes are needed to better understand the long-term effects of this approach.
Data availability
The data is available upon request.
References
Abbou CC, Hoznek A, Salomon L et al (2001) Laparoscopic radical prostatectomy with a remote controlled robot. J Urol 165(6 Pt 1):1964–1966. https://doi.org/10.1097/00005392-200106000-00027
Galfano A, Ascione A, Grimaldi S, Petralia G, Strada E, Bocciardi AM (2010) A new anatomic approach for robot-assisted laparoscopic prostatectomy: a feasibility study for completely intrafascial surgery. Eur Urol 58(3):457–461. https://doi.org/10.1016/j.eururo.2010.06.008
Galfano A, Secco S, Bocciardi AM, Mottrie A (2020) Retzius-sparing robot-assisted laparoscopic radical prostatectomy: an international survey on surgical details and worldwide diffusion. Eur Urol Focus 6(5):1021–2023. https://doi.org/10.1016/j.euf.2019.02.002
Stanford JL, Feng Z, Hamilton AS et al (2000) Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA 283(3):354–360. https://doi.org/10.1001/jama.283.3.354
Guillonneau B, Vallancien G (2000) Laparoscopic radical prostatectomy: the Montsouris technique. J Urol 163(6):1643–1649. https://doi.org/10.1016/s0022-5347(05)67512-x
Nandipati KC, Raina R, Agarwal A et al (2007) Nerve-sparing surgery significantly affects long-term continence after radical prostatectomy. Urology 70(6):1127–1130. https://doi.org/10.1016/j.urology.2007.07.042
Rocco F, Carmignani L, Acquati P et al (2006) Restoration of posterior aspect of rhabdosphincter shortens continence time after radical retropubic prostatectomy. J Urol 175(6):2201–2206. https://doi.org/10.1016/S0022-5347(06)00262-X
Nyarangi-Dix JN, Görtz M, Gradinarov G et al (2019) Retzius-sparing robot-assisted laparoscopic radical prostatectomy: functional and early oncologic results in aggressive and locally advanced prostate cancer. BMC Urol 19(1):113. https://doi.org/10.1186/s12894-019-0550-9. (Published 2019 Nov 12)
Checcucci E, Veccia A, Fiori C et al (2020) Retzius-sparing robot-assisted radical prostatectomy vs the standard approach: a systematic review and analysis of comparative outcomes. BJU Int 125(1):8–16. https://doi.org/10.1111/bju.14887
Menon M, Dalela D, Jamil M et al (2018) Functional recovery, oncologic outcomes and postoperative complications after robot-assisted radical prostatectomy: an evidence-based analysis comparing the Retzius sparing and standard approaches. J Urol 199(5):1210–1217. https://doi.org/10.1016/j.juro.2017.11.115
Galfano A, Secco S, Bocciardi AM (2017) Will Retzius-sparing prostatectomy be the future of prostate cancer surgery? Eur Urol 72(5):686–688. https://doi.org/10.1016/j.eururo.2017.06.023
Jiang YL, Zheng GF, Jiang ZP et al (2020) Comparison of Retzius-sparing robot-assisted laparoscopic radical prostatectomy vs standard robot-assisted radical prostatectomy: a meta-analysis. BMC Urol 20(1):114. https://doi.org/10.1186/s12894-020-00685-4. (Published 2020 Aug 3)
Lim SK, Kim KH, Shin TY et al (2014) Retzius-sparing robot-assisted laparoscopic radical prostatectomy: combining the best of retropubic and perineal approaches. BJU Int 114(2):236–244. https://doi.org/10.1111/bju.12705
Harty NJ, Kozinn SI, Canes D, Sorcini A, Moinzadeh A (2013) Comparison of positive surgical margin rates in high risk prostate cancer: open versus minimally invasive radical prostatectomy. Int Braz J Urol 39(5):639–648. https://doi.org/10.1590/S1677-5538.IBJU.2013.05.05
Chang LW, Hung SC, Hu JC, Chiu KY (2018) Retzius-sparing robotic-assisted radical prostatectomy associated with less bladder neck descent and better early continence outcome. Anticancer Res 38(1):345–351. https://doi.org/10.21873/anticanres.12228
Olivero A, Galfano A, Piccinelli M et al (2021) Retzius-sparing robotic radical prostatectomy for surgeons in the learning curve: a propensity score-matching analysis. Eur Urol Focus 7(4):772–778. https://doi.org/10.1016/j.euf.2020.03.002
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, data collection, analysis, and manuscript creation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elliott, N., Pahouja, G., Felice, M. et al. Transition from standard robotic prostatectomy to Retzius-sparing prostatectomy: feasibility and early outcomes. J Robotic Surg 17, 2035–2040 (2023). https://doi.org/10.1007/s11701-023-01596-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-023-01596-w