Abstract
Total and radical hysterectomies are the most common treatment strategies for early-stage endometrial and cervical cancers, respectively. Surgical modalities include open surgery, laparoscopy, and more recently, minimally invasive robot-assisted surgery. We searched several electronic databases for randomized controlled trials and observational studies with a comparison group, published between 2009 and 2014. Our outcomes of interest included both perioperative and morbidity outcomes. We included 35 observational studies in this review. We did not find any randomized controlled trials. The quality of evidence for all reported outcomes was very low. For women with endometrial cancer, we found that there was a reduction in estimated blood loss between the robot-assisted surgery compared to both laparoscopy and open surgery. There was a reduction in length of hospital stay between robot-assisted surgery and open surgery but not laparoscopy. There was no difference in total lymph node removal between the three modalities. There was no difference in the rate of overall complications between the robot-assisted technique and laparoscopy. For women with cervical cancer, there were no differences in estimated blood loss or removal of lymph nodes between robot-assisted and laparoscopic procedure. Compared to laparotomy, robot-assisted hysterectomy for cervical cancer showed an overall reduction in estimated blood loss. Although robot-assisted hysterectomy is clinically effective for the treatment of both endometrial and cervical cancers, methodologically rigorous studies are lacking to draw definitive conclusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer remains a significant cause of morbidity and mortality representing 7.5 % of all female cancer deaths worldwide [1]. Endometrial cancer is the fifth most common cancer among women, affecting over 300,000 women globally every year [2]. Management of women suffering from these cancers depends on the individual’s general health condition, tumor stage, and comorbidities; however, surgical removal of the uterus or hysterectomy is generally the treatment of choice for early clinical stage disease [3]. Hysterectomy in gynecologic oncology has evolved from using invasive open abdominal technique also known as laparotomy to minimally invasive procedures that provide access to the reproductive system by small incisions otherwise known as laparoscopy. Laparoscopy in the management of endometrial cancer has been shown to provide clinical benefits including shorter length of hospitalization, decreased blood loss, and reduced post-operative complications [4, 5]. Despite evidence from randomized controlled trials showing clinical benefits with laparoscopy, prior to the introduction of robot- assisted technology, the majority of cases continued to be performed via open surgery. A recent analysis of Ontario women having undergone hysterectomy reveals that although there has been an increase in the use of laparoscopy over time, only 30 % of cases in 2011 were performed in this manner [6]. The low uptake of laparoscopy is likely due to a combination of factors including inadequate training and challenges in visual-special mechanics for surgeons. Counterintuitive hand movement, an unsteady two-dimensional visual field, restricted instrument motion, ergonomic difficulty, and tremor amplification result in many surgeons having difficulty performing laparoscopy, requiring additional training [7]. Robot-assisted surgery is a relatively new minimally invasive technology that has shown some theoretical advantages compared with other surgical techniques. These advantages include improved visualization through 3D imaging, greater precision, and more accurate control of instrumentation in addition to improved ergonomics for the surgeons [8]. Since the approval of Food and Drug Administration in 2005 for the use of robot-assisted gynecological surgery, this technology has been widely adapted in the United States for conducting hysterectomy for both benign and malignant indications [9]. This systematic review aims to update previously published systematic reviews and to identify the clinical effectiveness of robot-assisted hysterectomy compared with laparoscopic and/or open hysterectomy for women diagnosed with endometrial or cervical cancer.
Methods
We conducted and reported this systematic review according to the published guidelines using a pre-specified protocol [10, 11].
Eligibility criteria
We included any randomized controlled trials (RCTs) and cohort studies with comparison group that reported outcomes for women with endometrial or cervical cancer eligible for hysterectomy. We included studies that reported at least one clinical outcome of interest comparing robot-assisted hysterectomy with laparoscopic or open hysterectomy. Our primary outcomes of interest included morbidity factors such as number of complications and length of hospitalization, perioperative factors such as operation time, amount of blood loss, and number of conversions to laparotomy. Due to the numerous challenges with interpretation of lymph node counts in the management of women with endometrial cancer and cervical cancer, number of pelvic and para-aortic lymph nodes removed was considered a secondary outcome measure. We excluded animal or in vitro studies, conference abstracts, editorials, narrative reviews, case reports, cross sectional and case–control studies. We also excluded studies that reported outcomes in pregnant women, women undergoing emergent surgeries or women undergoing hysterectomy for benign conditions.
Search strategy
We conducted a literature search with the help of a librarian. We searched the following databases from January 1, 2009 to June 24, 2014: Ovid MEDLINE, Ovid MEDLINE In-Process, and Other Non-Indexed Citations, Ovid Embase, and EBM Reviews. The search date was confined to last 5 years to provide an update to the previously published systematic reviews in this topic. The search strategy included a combination of keywords and MeSH terms and was adapted for each database to account for differences in indexing. We restricted our search to English language publications. We also searched reference lists and non-indexed journals for any additional relevant studies not identified through the search. See Online Appendix 1 for literature search strategy details.
Study selection and data abstraction
A reviewer (IN) independently screened titles and abstracts. We retrieved the full text for any article considered potentially relevant by the reviewer. Data were abstracted using a data collection form for studies considered eligible for inclusion. We abstracted the following data: (a) study characteristics such as year of publication, country where study was conducted, study design, sample size, year of study, and funding sources; (b) methodological characteristics such as definitions of population and outcomes studied, whether confounding variables were accounted for in the study, and whether the studies reported loss to follow-up; (c) patient characteristics including the number of women in each group, mean age, race, whether the control group was laparoscopy or open or both; (d) the outcomes and any adjusted measures of association. We contacted the authors of the studies included in the review for any missing data. We entered all data into Review Manager Version 5.2 [12]. We assessed the quality of the body of evidence for each outcome using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. The overall quality was determined to be high, moderate, low, or very low using a stepwise, structural methodology. Study design was the first consideration; the starting assumption was that randomized controlled trials (RCTs) are high quality, whereas observational studies start as low quality. Five additional factors—risk of bias, inconsistency, indirectness, imprecision, and publication bias—were then taken into account. Limitations in these areas resulted in downgrading the quality of evidence. Finally, we considered three main factors that may raise the quality of evidence: large magnitude of effect, dose response gradient, and accounting for all residual confounding factors [13–15].
Results
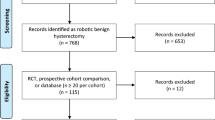
We screened and evaluated 1236 citations published between January 1, 2009 and June 24, 2014. We obtained 42 full text articles for further assessment. Figure 1 shows the flow diagram of studies identified, included, and excluded from the review. Thirty-five observational studies met the inclusion criteria, including 23 for endometrial cancer [16–36] and 12 for cervical cancer [37–48] (Table 1). List of studies not included in the review, detailed description of the intervention and comparator of each of the included study, study characteristics of included studies, the evidence GRADE profile, and the PRISMA checklist are presented in Online Appendices 2, 3, 4, 5, 6, and 7, respectively. Figures 2 and 3 show forest plots with individual point estimates derived from the included studies comparing robot-assisted hysterectomy with laparoscopic hysterectomy and open hysterectomy, respectively. Figures 2 and 3 have data from individual studies included for endometrial and cervical cancer within each of the individual outcomes. Due to a high level of both clinical and statistical heterogeneity observed in the included studies, the pooled point estimates are not reported.
Depicts a forest plot with different study estimates for the included studies comparing robotic hysterectomy with laparoscopic hysterectomy; a shows the outcome of mean-estimated blood loss among women who underwent hysterectomy for cervical and endometrial cancer separately, b shows the outcome of mean number of hospital stay in days post-op for both the cervical and endometrial cancer cohort, c shows the outcome of mean pelvic lymph nodes removed, d shows complication rates including perioperative for both endometrial and cervical cancer, intraoperative for endometrial cancer (as data were not available for cervical cancer from the included studies), minor and major complications for women who underwent hysterectomy for endometrial and cervical cancer, e shows the mean operative time for both endometrial and cervical cancer, f shows mean number of para-aortic lymph nodes removed in both the cohorts, g shows the number of total lymph nodes removed among the endometrial cancer cohort, h shows the mean number of conversions from laparoscopy and robotic hysterectomy to open hysterectomy
Depicts a forest plot with different study estimates for the included studies comparing robotic hysterectomy with open hysterectomy/laparotomy; a shows the outcome of mean estimated blood loss among women who underwent hysterectomy for cervical and endometrial cancer separately as subgroups, b shows the outcome of mean number of hospital stay in days post-op for both the cervical and endometrial cancer cohort, c shows the outcome of mean pelvic lymph nodes removed, d shows complication rates including perioperative and intraoperative for both endometrial and cervical cancer, minor and major complications for women who underwent hysterectomy for endometrial and cervical cancer, e shows the mean operative time for both endometrial and cervical cancer, f shows mean number of para-aortic lymph nodes removed in the endometrial cancer cohort, g shows the number of total lymph nodes removed among both cohorts
Endometrial cancer
Among the 23 studies that examined outcomes in women with endometrial cancer, 11 studies compared robot assisted with laparoscopic hysterectomy [16–18, 21–24, 26, 27, 30, 33], 8 studies compared robot-assisted with open hysterectomy [20, 28, 29, 31, 32, 35, 49, 50], and 4 studies compared robot-assisted with laparoscopic and open hysterectomy [19, 25, 34, 36]. Of the 23 studies, 6 studies were prospective and had historic control group [24–26, 30, 34, 49] and 17 studies were retrospective cohort studies [16–23, 27–29, 31–33, 35, 36, 50]. Eighteen studies were from USA [16–18, 20, 21, 23–27, 29–32, 34–36, 50], followed by one study each from Italy [23], Finland [33], Canada [49], Spain [19], and Singapore [28]. The number of participants in the included studies ranged from 30 to 377. Length of follow-up ranged from 30 days to 5 years post-op. Length of follow-up was not reported for all the comparison groups.
Robot-assisted compared with laparoscopic hysterectomy for endometrial cancer
When compared with laparoscopic hysterectomy, robot-assisted hysterectomy was associated with lower mean volume of blood loss in all included studies (109 ± 83 vs. 187 ± 187) [17]; (89 ± 45 vs. 209 ± 92) [25]; (81 ± 46 vs. 207 ± 109) [26]; (99 ± 75 vs. 190 ± 120) [19]; (108 ± 94 vs. 194 ± 110) [34]; (110 ± 83 vs. 187 ± 169) [18] (Fig. 2a). Mean length of hospital stay was shown in two studies to be reduced with robot-assisted hysterectomy (1.6 ± 0.7 vs. 2.6 ± 0.9) [25]; (1.5 ± 0.9 vs. 3.2 ± 2.3) [26], while a trend in reduction of mean length of stay in favor of robot-assisted surgery was demonstrated in three other studies (1.8 ± 1.6 vs. 2.3 ± 2.2) [17]; (3.5 ± 3.6 vs. 4.6 ± 4) [19]; (2 ± 2 vs. 2.5 ± 2.1) [18] (Fig. 2b). Four studies demonstrated increase in the mean number of pelvic lymph nodes removed among the laparoscopy group (19 ± 8 vs. 24 ± 12) [25]; (13 ± 7 vs. 16 ± 9) [17]; (19 ± 9 vs. 25 ± 12) [26]; (13 ± 6 vs. 15 ± 8) [18], while two other studies showed no difference (16 ± 8 vs. 18 ± 8) [19]; (15 ± 8 vs. 16 ± 7) [33]. One of the included studies demonstrated more pelvic lymph nodes removed by robotic surgery (14 ± 7 vs. 12 ± 5) [27] (Fig. 2c). When looking at perioperative complication rates, we observed that the included studies did not show a difference between robot assisted and laparoscopic hysterectomy [22, 30, 33, 34]. However, intraoperative events were shown in two studies to be reduced with robot-assisted hysterectomy (0/56 vs. 7/56) [25]; (1/122 vs. 7/122) [26] (Fig. 2d).
Comparison of the mean length of operative time between robot assisted and laparoscopic hysterectomy was inconclusive, with shorter mean length of operative time favoring robot-assisted hysterectomy observed in four of the included studies (242 ± 53 vs. 287 ± 55) [30]; (163 ± 53 vs. 192 ± 56) [25]; (147 ± 48 vs. 187 ± 60) [26]; (189 ± 35 vs. 218 ± 54) [19]. In contrast, four other studies demonstrated shorter mean length of operative time with laparoscopic hysterectomy (237 ± 57 vs. 178 ± 59) [17]; (109 ± 38 vs. 218 ± 54) [34]; (210 ± 66 vs. 120 ± 41) [33]; (218 ± 59 vs. 161 ± 59) [18] (Fig. 2e).
Of the six studies comparing the mean number of para-aortic lymph nodes removed by robot-assisted and laparoscopic hysterectomy, two studies demonstrated more para-aortic nodes removed by laparoscopic surgery (13 ± 8 vs. 21 ± 12) [25]; (6 ± 8 vs. 18 ± 10) [26], while the results of one study showed more nodes removed in the robot-assisted surgery (9 ± 6 vs. 7 ± 6) [17]. The other three studies demonstrated no difference between the two procedures (Fig. 2f). When comparing the mean number of total lymph nodes removed, one study demonstrated more nodes removed by laparoscopy compared with robot-assisted hysterectomy (25 ± 13 vs. 43 ± 18) [26], while the rest of the included studies showed no difference between the two procedures (Fig. 2g).
Two of the 11 included studies demonstrated a reduction in conversion to open surgery with robot-assisted surgery compared to laparoscopy (13/05 vs. 20/76) [30]; (1/187 vs. 10/245) [18], while the rest of the studies showed no statistical difference (Fig. 2h).
Robot-assisted compared with laparotomy (open hysterectomy) for endometrial cancer
When robot-assisted hysterectomy was compared with laparotomy, all of the eight included studies showed significant reduction in the mean estimated blood loss in robot-assisted procedure (89 ± 45 vs. 266 ± 145) [25]; (232 ± 48 vs. 308 ± 34) [50]; (96 ± 109 vs. 409 ± 290) [31]; (119 ± 45 vs. 185 ± 304) [20]; (108 ± 94 vs. 412 ± 312) [34]; (111 ± 25 vs. 250 ± 84) [28]; (99 ± 75 vs. 232 ± 10) [19]; (160 ± 150 vs. 292 ± 226) [32] (Fig. 3a). All eight included studies also demonstrated a reduction in the mean length of hospital stay, favouring robot-assisted hysterectomy (1.6 ± 0.7 vs. 4.9 ± 1.9) [25]; (2.73 ± 1.84 vs. 5.07 ± 2.54) [31]; (1.28 ± 0.87 vs. 3.26 ± 0.64) [50]; (1.5 ± 2 vs. 4 ± 3) [20]; (3.5 ± 3.4 vs. 8.1 ± 4.8) [19]; (1.9 ± 1.5 vs. 4.1 ± 2.3) [34]; (1.5 ± 1 vs. 4.1 ± 2.2) [32]; (2.06 ± 1.1 vs. 6.02 ± 4.53) [28] (Fig. 3b). Two of the three included studies reported increase in the number of pelvic lymph nodes removed in the open hysterectomy group (19 ± 8 vs. 31 ± 14) [25]; (15 ± 6.3 vs. 25.6 ± 12.9) [28] (Fig. 3c).
When comparing the perioperative complication rates between robot-assisted and open hysterectomy, two out of two included studies demonstrated a better outcome with robot-assisted procedure (2/66 vs. 10/43) [35]; (11/102 vs. 20/78) [34], while no difference in intraoperative complications was demonstrated between the two procedures. Among studies comparing the minor and major complications in robot-assisted and open hysterectomy, all but one showed no significant difference (Fig. 3d). Mean length of operative time was consistently longer for the robot-assisted group across the eight included studies, with one study demonstrating a shorter mean time of operation with robot-assisted procedure (Fig. 3e).
Of the three included studies examining the mean number of para-aortic lymph nodes removal, two studies reported lower mean number of para-aortic lymph nodes removed in the robot-assisted group compared with the open hysterectomy group (13 ± 8 vs. 25 ± 14) [25]; (1.9 ± 0.4 vs. 3.5 ± 0.7) [50] (Fig. 3f). Of the four included studies, comparing the mean total number of lymph nodes removed, two studies showed no difference [20, 31], whereas two studies reported higher number of total lymph nodes removed among the robot-assisted hysterectomy group (16 ± 8 vs. 11 ± 9) [34]; (16 ± 10 vs. 11 ± 11) [32] (Fig. 3g).
Cervical cancer
Among the 12 studies that examined the outcomes in women with cervical cancer, 2 studies compared robot assisted with laparoscopic hysterectomy [38, 47], 7 studies compared robot assisted with open procedure [37, 39–44], and 3 studies compared all the 3 techniques [45, 46, 48]. Of the 12 studies, 5 studies were prospective cohort studies with a historic control [38, 41–43, 45], 1 was a prospective cohort study [40], and 6 studies were retrospective cohort studies [37, 39, 44, 46–48]. Five studies were from USA [37, 39, 46–48], two from Korea [38], one study each from Italy [42], Netherlands [44], Turkey [40], Canada [41], and Norway [45]. Sample size ranged from 7 to 1610 among the 12 included studies. Only three studies reported the length of follow-up which ranged from 2 days to 15 months post-op [43, 45, 47].
Robot assisted compared with laparoscopic hysterectomy for cervical cancer
When robot-assisted hysterectomy was compared with laparoscopic hysterectomy for cervical cancer, we found one study favoring the robot-assisted procedure (55 ± 32 vs. 202 ± 148) [38], and one study showing less blood loss with laparoscopic procedures (157 ± 7 vs. 95 ± 5) [47] (Fig. 2a).
Mean length of hospital stay was shorter among the robot-assisted group in two of the three studies that reported this outcome [45, 47] (Fig. 2b). Only one of the included studies reported complication rates comparing robot assisted with laparoscopic hysterectomy, demonstrating fewer perioperative complication events in robot-assisted group (7/50 vs. 13/50) [38] (Fig. 2d).
Comparing the mean operative time between the two procedures, one study reported that laparoscopic surgery was less time consuming compared to the robot assisted (323 ± 30 vs. 255 ± 25) [47], whereas the other study reported otherwise (211 ± 47 vs. 230 ± 36) [38] (Fig. 2e). The number of pelvic (Fig. 2c) and para-aortic lymph nodes (Fig. 2f) removed did not differ among the comparison groups [45, 47]. One study that reported conversion to open surgery did not find a difference between the two groups (Fig. 2h) [38].
Robot-assisted compared with open hysterectomy for cervical cancer
When robot-assisted hysterectomy was compared with laparotomy in women with cervical cancer, there was an overall decrease in mean estimated blood loss in robot-assisted group as demonstrated in all four included studies (78 ± 95 vs. 222 ± 132) [42]; (106 ± 113 vs. 546 ± 570) [41]; (221 ± 135 vs. 532 ± 436) [43]; (82 ± 74 vs. 595 ± 285) [45] (Fig. 3a).
Mean length of hospital stay was also consistently shown in all three included studies to be reduced by robot-assisted surgery (3.7 ± 1.2 vs. 5 ± 2.4) [42]; (1.9 ± 0.9 vs. 7.2 ± 5.3) [41]; (3.8 ± 0.9 vs. 9.2 ± 2) [45] (Fig. 3b). Less number of pelvic lymph nodes were removed in the robot-assisted procedure compared to laparotomy in one study that reported this outcome (20 ± 7 vs. 26 ± 7) [45] (Fig. 3c). Mean operative time was lower in the laparotomy group compared with robot-assisted hysterectomy [41, 42, 45] (Fig. 3e). One study showed decreased number of mean total lymph nodes removed in robot-assisted group (20 ± 7 vs. 26 ± 12) [42], while two other studies showed no difference between the two groups [41, 43] (Fig. 3g).
GRADE evidence profile
The quality of evidence for all reported outcomes was considered as very low quality using the GRADE evidence profile. This was primarily based on risk of bias, inconsistency, indirectness, imprecision, and publication bias (Online Appendix Tables 5 and 6). Since all included studies were observational, they started as low quality and further downgraded for serious risk of bias. In majority of studies, inadequate reporting of patient selection process and/or inadequate adjusting in the analysis for the level of surgeon experience were the reasons for downgrading. In addition, these confounding factors contributed to high degree of inconsistency across the included studies. Indirectness of the reported outcomes also contributed as a serious limitation in the quality of evidence. Examples of these include complication rates not being adequately defined in studies and the number of removed lymph nodes being reported as a surrogate outcome for cancer staging.
Discussion
Our systematic review found that for endometrial cancer, robot-assisted hysterectomy compared to both laparoscopy and laparotomy showed reduced mean estimated blood loss and length of hospital stay although compared to laparoscopy it was not statistically significant. There was no difference in complications and although not significant, there was a trend toward more conversions to open surgery with laparoscopy compared to robot-assisted surgery. Secondary outcome measures of lymph node count did not favor one modality over another although studies did show an increase in pelvic lymph node count removal with laparoscopy compared to robotic surgery but the clinical significance is unclear. Furthermore, the variation in histologic lymph node counting, location of lymph nodes and operator bias make this difficult to interpret. The data from the current analysis can be considered an early snapshot in the adoption of robotic-assisted technology form the management of endometrial and cervical cancers as the data represent the initial work in the field. Our results are consistent with a recently published population-based registry study of women with newly diagnosed endometrial cancer. Women who underwent robot-assisted hysterectomy had reduced days to normal activity of daily living, return to work, blood loss, and length of hospital stay compared to abdominal hysterectomy [51]. In other recently published studies, robotic hysterectomy was found to be superior to laparoscopy in terms of intra- and post-operative complications, conversion rates, length of hospital stay as well as better health-related quality of life score after surgery [52, 53]. Similar results were obtained in morbidly obese women with endometrial cancer. Minimally invasive robotic or laparoscopic surgeries were associated with fewer complications, less days of hospitalization relative to open surgery and found to be safe and feasible in this population [54, 55].
For cervical cancer, robot-assisted hysterectomy demonstrated a reduction in estimated blood loss compared to open surgery but not compared to laparoscopy. Length of hospital stay was also consistently reduced among the robot-assisted group compared with both laparoscopy and laparotomy. Few of the recently published studies concluded that robot-assisted hysterectomy was safe, reliable, and feasible in women undergoing hysterectomy for cervical cancer [56–58]. Similarly, a few of the published systematic reviews and meta-analyses suggest robot-assisted hysterectomy to be superior to open hysterectomy with shorter hospital stay, reduced blood loss, and fewer wound related complications [59–61]. In another study, 5 year disease free and overall survival outcomes did not differ much among women with cervical cancer, irrespective of operative approach [62]. Overall, our results were consistent with the recently published literature on this topic. There is also an ongoing randomized control trial comparing minimally invasive radical hysterectomy and open surgery for women with cervical cancer. This study includes laparoscopy and robot-assisted surgeries in the minimally invasive arm and will hopefully provide some important insight into the respective benefits.
Of note, these data are derived from small observational studies with overall low methodological quality. While there is a clinical consensus that it is safe for women to undergo robot-assisted hysterectomy compared with other techniques for hysterectomy, the magnitude of the benefit is unclear. In addition, there is no clear evidence as to the degree of risk involved at various stages of cancer, which is an important information needed to make an informed decision. The results from our systematic review warrant a need for future large multicenter randomized controlled trials to better quantify the benefits and risks associated with robot-assisted hysterectomy. Unfortunately, for endometrial cancer, this is unlikely ever to happen in large part because randomized controlled data already exist favoring laparoscopy to open surgery [63] and as mentioned above, the only randomized trial in cervix cancer is combining robot assisted and laparoscopy in a minimally invasive arm.
Our systematic review has a number of strengths. It is one of the most comprehensive reviews that complement other recent reviews on this topic [59–61, 64–66]. It also presents an “early” representation of the data which allows people to compare differences over time. We did a comprehensive search to identify relevant literature in accordance with published guidelines and a pre-specified protocol. During our protocol phase, we had discussions with other methodologists as to what type of study designs should be included in our review. We determined a priori that randomized controlled studies, prospective cohorts with comparison groups and retrospective cohorts with comparison groups should be included to obtain reasonable valid effect estimates. Our literature search was comprehensive and reproducible. One recent study was not captured in our search because it was indexed (July 2014) after our search was completed (June 2014), this study compared robot-assisted hysterectomy with open hysterectomy [67]. The authors of this study found elderly women with endometrial cancer who underwent robot-assisted surgery had a significantly lower rate of minor complications, less operative blood loss, and shorter hospitalization [67]. Other studies published after our search date have been included in “Discussion”. We do not expect any publication bias, as review of gray literature for unpublished studies did not yield any results.
The quality of studies included in this review inherently limits the conclusions that can be drawn. Thus, this review serves efficiently to summarize past studies but is not definitive as to what benefits or risks can be quoted to women who opt to have robot-assisted hysterectomy. Confounding factors such as surgeon’s experience, tumor staging, women’s age at the time of surgery, Body Mass Index, uterine weight, parity, comorbidities at the time of surgery etc. may distort the association between the exposure and outcome [68, 69]. In many of the studies, these confounding factors were not adequately addressed in the study design or in the statistical analysis. Studies were either retrospective relying on administrative database or prospective with a historic control where there was a possibility of differential misclassification, selection bias, and ascertainment bias [70]. For example, there were baseline differences between the comparison groups in many of the included studies; time period effects were of concern in most of the included studies where a group was compared with a historic control. Furthermore, a dose-response relationship between tumor staging and clinical risks was not considered in many of the included studies.
Given the rapid diffusion of robot-assisted hysterectomy world-wide, women opting for robot-assisted hysterectomy may assume that the technology is safe and effective compared to laparoscopic or open hysterectomy. The data from this report is consistent with other published literature (including randomized data) showing that minimally invasive surgery is superior to open surgery for endometrial cancer and likely for cervical cancer management but differences between robot assisted and laparoscopy are difficult to interpret. Nonetheless, appropriate counseling can reduce anxiety and avoid bias in patient preference. This review summarizes key published information on the overall clinical effectiveness of robot-assisted surgery compared to laparoscopic and/or open hysterectomy. This review provides surgeons with evidence and underscores the limitations of the current published literature. Surgeons and other healthcare professionals can integrate this information with their surgical expertise when they counsel women before hysterectomy.
Conclusions
This systematic review provides important information for decision-makers and policy-makers to make recommendations based on clinical effectiveness of robot-assisted hysterectomy. This systematic review of observational studies also highlights the need for future methodologically rigorous studies to estimate the magnitude of benefits or risks associated with robot-assisted hysterectomy. The clinical benefits of robotic surgery compared to laparoscopy are less clear. Until such data become available, health care professionals can use currently available evidence, along with their clinical expertise and patient preferences to guide decisions on robot-assisted hysterectomy.
Abbreviations
- RCT:
-
Randomized controlled trials
- GRADE:
-
Grading of recommendations assessment, development, and evaluation
- USA:
-
United States of America
- MeSH:
-
Medical subject headings
- SD:
-
Standard deviation
- OP:
-
Open
- RB:
-
Robotic
- LP:
-
Laparoscopic
References
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49:1374–1403
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA: Cancer J Clin 65:87–108. doi:10.3322/caac.21262
Galaal K, Bryant A, Fisher AD, Al-Khaduri M, Kew F, Lopes AD (2012) Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst Rev 9:CD006655
Janda M, Gebski V, Brand A, Hogg R, Jobling TW, Land R, Manolitsas T, McCartney A, Nascimento M, Neesham D, Nicklin JL, Oehler MK, Otton G, Perrin L, Salfinger S, Hammond I, Leung Y, Walsh T, Sykes P, Ngan H, Garrett A, Laney M, Ng TY, Tam K, Chan K, Wrede CD, Pather S, Simcock B, Farrell R, Obermair A (2010) Quality of life after total laparoscopic hysterectomy versus total abdominal hysterectomy for stage I endometrial cancer (LACE): a randomised trial. Lancet Oncol 11:772–780
Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, Spiegel G, Barakat R, Pearl ML, Sharma SK (2009) Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol 27:5331–5336
Kroft J, Li Q, Saskin R, Elit L, Bernardini MQ, Gien LT (2015) Trends over time in the use of laparoscopic hysterectomy for the treatment of endometrial cancer. Gynecol Oncol 138:536–541
Peplinski R (2006) Past, present and future of the Da Vinci robot, vol 2. UK Robotic Urology Course, Guy’s Hospital, London
Herron DM, Marohn M, SAGES-MIRA Robotic Surgery Consensus Group (2008) A consensus document on robotic surgery. Surg Endosc 22(2):313–325 (discussion 311–312)
Mendivil A, Holloway RW, Boggess JF (2009) Emergence of robotic assisted surgery in gynecologic oncology: American perspective. Gynecol Oncol 114:S24–S31
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 3:e123–e130
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012
Review Manager (RevMan) [computer program] (2014) Version 5 3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration
Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64:401–406
Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A (2011) GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 64:380–382
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926
Boruta DM, Fagotti A, Bradford LS, Escobar PF, Scambia G, Kushnir CL, Michener CM, Fader AN (2014) Laparoendoscopic single-site radical hysterectomy with pelvic lymphadenectomy: initial multi-institutional experience for treatment of invasive cervical cancer. J minim Invasive Gynecol 21:394–398
Cardenas-Goicoechea J, Adams S, Bhat SB, Randall TC (2010) Surgical outcomes of robotic-assisted surgical staging for endometrial cancer are equivalent to traditional laparoscopic staging at a minimally invasive surgical center. Gynecol Oncol 117:224–228
Cardenas-Goicoechea J, Soto E, Chuang L, Gretz H, Randall TC (2013) Integration of robotics into two established programs of minimally invasive surgery for endometrial cancer appears to decrease surgical complications. J Gynecol Oncol 24:21–28
Coronado PJ, Herraiz MA, Magrina JF, Fasero M, Vidart JA (2012) Comparison of perioperative outcomes and cost of robotic-assisted laparoscopy, laparoscopy and laparotomy for endometrial cancer. Eur J Obstet Gynecol Reprod Biol 165:289–294
ElSahwi KS, Hooper C, De Leon MC, Gallo TN, Ratner E, Silasi DA, Santin AD, Schwartz PE, Rutherford TJ, Azodi M (2012) Comparison between 155 cases of robotic vs. 150 cases of open surgical staging for endometrial cancer. Gynecol Oncol 124:260–264
Escobar PF, Frumovitz M, Soliman PT, Frasure HE, Fader AN, Schmeler KM, Ramirez PT (2012) Comparison of single-port laparoscopy, standard laparoscopy, and robotic surgery in patients with endometrial cancer. Ann Surg Oncol 19:1583–1588
Fagotti A, Corrado G, Fanfani F, Mancini M, Paglia A, Vizzielli G, Sindico S, Scambia G, Vizza E (2013) Robotic single-site hysterectomy (RSS-H) vs. laparoendoscopic single-site hysterectomy (LESS-H) in early endometrial cancer: a double-institution case-control study. Gynecol Oncol 130:219–223
Fagotti A, Gagliardi ML, Fanfani F, Salerno MG, Ercoli A, D’Asta M, Tortorella L, Turco LC, Escobar P, Scambia G (2012) Perioperative outcomes of total laparoendoscopic single-site hysterectomy versus total robotic hysterectomy in endometrial cancer patients: a multicentre study. Gynecol Oncol 125:552–555
Leitao MM Jr, Briscoe G, Santos K, Winder A, Jewell EL, Hoskins WJ, Chi DS, Abu-Rustum NR, Sonoda Y, Brown CL, Levine DA, Barakat RR, Gardner GJ (2012) Introduction of a computer-based surgical platform in the surgical care of patients with newly diagnosed uterine cancer: outcomes and impact on approach. Gynecol Oncol 125:394–399
Lim PC, Kang E, Park do H (2010) Learning curve and surgical outcome for robotic-assisted hysterectomy with lymphadenectomy: case-matched controlled comparison with laparoscopy and laparotomy for treatment of endometrial cancer. J Minim Invasive Gynecol 17:739–748
Lim PC, Kang E, Park do H (2011) A comparative detail analysis of the learning curve and surgical outcome for robotic hysterectomy with lymphadenectomy versus laparoscopic hysterectomy with lymphadenectomy in treatment of endometrial cancer: a case-matched controlled study of the first one hundred twenty two patients. Gynecol Oncol 120:413–418
Martino MA, Shubella J, Thomas MB, Morcrette RM, Schindler J, Williams S, Boulay R (2011) A cost analysis of postoperative management in endometrial cancer patients treated by robotics versus laparoscopic approach. Gynecol Oncol 123:528–531
Mok ZW, Yong EL, Low JJ, Ng JS (2012) Clinical outcomes in endometrial cancer care when the standard of care shifts from open surgery to robotics. Int J Gynecol Cancer 22:819–825
Paley PJ, Veljovich DS, Shah CA, Everett EN, Bondurant AE, Drescher CW, Peters WA 3rd (2011) Surgical outcomes in gynecologic oncology in the era of robotics: analysis of first 1000 cases. Am J Obstet Gynecol 204(551):e551–e559
Seamon LG, Cohn DE, Henretta MS, Kim KH, Carlson MJ, Phillips GS, Fowler JM (2009) Minimally invasive comprehensive surgical staging for endometrial cancer: robotics or laparoscopy? Gynecol Oncol 113:36–41
Subramaniam A, Kim KH, Bryant SA, Zhang B, Sikes C, Kimball KJ, Kilgore LC, Huh WK, Straughn JM Jr, Alvarez RD (2011) A cohort study evaluating robotic versus laparotomy surgical outcomes of obese women with endometrial carcinoma. Gynecol Oncol 122:604–607
Tang KY, Gardiner SK, Gould C, Osmundsen B, Collins M, Winter WE 3rd (2012) Robotic surgical staging for obese patients with endometrial cancer. Am J Obstet Gynecol 206(513):e511–e516
Turunen H, Pakarinen P, Sjoberg J, Loukovaara M (2013) Laparoscopic vs robotic-assisted surgery for endometrial carcinoma in a centre with long laparoscopic experience. J Obstet Gynaecol 33:720–724
Estape R, Lambrou N, Estape E, Vega O, Ojea T (2012) Robotic-assisted total laparoscopic hysterectomy and staging for the treatment of endometrial cancer: a comparison with conventional laparoscopy and abdominal approaches. J Robot Surg 6:199–205
Nevadunsky N, Clark R, Ghosh S, Muto M, Berkowitz R, Vitonis A, Feltmate C (2010) Comparison of robot-assisted total laparoscopic hysterectomy and total abdominal hysterectomy for treatment of endometrial cancer in obese and morbidly obese patients. J Robot Surg 4:247–252
Nicole N, Rachel C, Michael M, Ross B, Sue G, Allison V, Colleen F (2012) Robotic assisted, total laparoscopic, and total abdominal hysterectomy for management of uterine cancer. J Cancer Ther 3:162–166
Cantrell LA, Mendivil A, Gehrig PA, Boggess JF (2010) Survival outcomes for women undergoing type III robotic radical hysterectomy for cervical cancer: a 3-year experience. Gynecol Oncol 117:260–265
Chong GO, Lee YH, Hong DG, Cho YL, Park IS, Lee YS (2013) Robot versus laparoscopic nerve-sparing radical hysterectomy for cervical cancer: a comparison of the intraoperative and perioperative results of a single surgeon’s initial experience. Int J Gynecol Cancer 23:1145–1149
Geisler JP, Orr CJ, Khurshid N, Phibbs G, Manahan KJ (2010) Robotically assisted laparoscopic radical hysterectomy compared with open radical hysterectomy. Int J Gynecol Cancer 20:438–442
Göçmen A, Şanlıkan F, Uçar MG (2010) Comparison of outcomes between laparotomy and robotic technique for cervical cancer. J Robot Surg 4:123–128
Halliday DLS, Vaknin Z, Deland C, Levental M, McNamara E, Gotlieb R, Kaufer R, How J, Cohen E, Gotlieb W (2010) Robotic radical hysterectomy: comparison of outcomes and cost. J Robot Surg 4:211–216
Maggioni A, Minig L, Zanagnolo V, Peiretti M, Sanguineti F, Bocciolone L, Colombo N, Landoni F, Roviglione G, Velez JI (2009) Robotic approach for cervical cancer: comparison with laparotomy: a case control study. Gynecol Oncol 115:60–64
Nam EJ, Kim SW, Kim S, Kim JH, Jung YW, Paek JH, Lee SH, Kim JW, Kim YT (2010) A case-control study of robotic radical hysterectomy and pelvic lymphadenectomy using 3 robotic arms compared with abdominal radical hysterectomy in cervical cancer. Int J Gynecol Cancer 20:1284–1289
Schreuder HW, Zweemer RP, van Baal WM, van de Lande J, Dijkstra JC, Verheijen RH (2010) From open radical hysterectomy to robot-assisted laparoscopic radical hysterectomy for early stage cervical cancer: aspects of a single institution learning curve. Gynecol Surg 7:253–258
Sert MB, Abeler V (2011) Robot-assisted laparoscopic radical hysterectomy: comparison with total laparoscopic hysterectomy and abdominal radical hysterectomy; one surgeon’s experience at the Norwegian Radium Hospital. Gynecol Oncol 121:600–604
Soliman PT, Frumovitz M, Sun CC, Dos Reis R, Schmeler KM, Nick AM, Westin SN, Brown J, Levenback CF, Ramirez PT (2011) Radical hysterectomy: a comparison of surgical approaches after adoption of robotic surgery in gynecologic oncology. Gynecol Oncol 123:333–336
Tinelli R, Malzoni M, Cosentino F, Perone C, Fusco A, Cicinelli E, Nezhat F (2011) Robotics versus laparoscopic radical hysterectomy with lymphadenectomy in patients with early cervical cancer: a multicenter study. Ann Surg Oncol 18:2622–2628
Wright JD, Herzog TJ, Neugut AI, Burke WM, Lu YS, Lewin SN, Hershman DL (2012) Comparative effectiveness of minimally invasive and abdominal radical hysterectomy for cervical cancer. Gynecol Oncol 127:11–17
Bernardini MQ, Gien LT, Tipping H, Murphy J, Rosen BP (2012) Surgical outcome of robotic surgery in morbidly obese patient with endometrial cancer compared to laparotomy. Int J Gynecol Cancer 22:76–81
Goel MZW, Moore D (2011) Surgical staging of endometrial cancer: robotic versus open technique outcomes in a contemporary single surgeon series. J Robot Surg 5:109–114
Borgfeldt C, Kalapotharakos G, Asciutto KC, Lofgren M, Hogberg T (2016) A population-based registry study evaluating surgery in newly diagnosed uterine cancer. Acta Obstet Gynecol Scand 95(8):901–911. doi:10.1111/aogs.12918
Corrado G, Cutillo G, Pomati G, Mancini E, Sperduti I, Patrizi L, Saltari M, Vincenzoni C, Baiocco E, Vizza E (2015) Surgical and oncological outcome of robotic surgery compared to laparoscopic and abdominal surgery in the management of endometrial cancer. Eur J Surg Oncol 41:1074–1081
Herling SF, Moller AM, Palle C, Thomsen T (2016) Health-related quality of life after robotic-assisted laparoscopic hysterectomy for women with endometrial cancer—a prospective cohort study. Gynecol Oncol 140:107–113
Chan JK, Gardner AB, Taylor K, Thompson CA, Blansit K, Yu X, Kapp DS (2015) Robotic versus laparoscopic versus open surgery in morbidly obese endometrial cancer patients—a comparative analysis of total charges and complication rates. Gynecol Oncol 139:300–305
Corrado G, Chiantera V, Fanfani F, Cutillo G, Lucidi A, Mancini E, Pedone Anchora L, Scambia G, Vizza E (2016) Robotic hysterectomy in severely obese patients with endometrial cancer: a multicenter study. J Minim Invasive Gynecol 23:94–100
Corrado G, Cutillo G, Saltari M, Mancini E, Sindico S, Vici P, Sergi D, Sperduti I, Patrizi L, Pomati G, Baiocco E, Vizza E (2016) Surgical and oncological outcome of robotic surgery compared with laparoscopic and abdominal surgery in the management of locally advanced cervical cancer after neoadjuvant chemotherapy. Int J Gynecol Cancer 26:539–546
Sert BM, Boggess JF, Ahmad S, Jackson AL, Stavitzski NM, Dahl AA, Holloway RW (2016) Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Eur J Surg Oncol 42:513–522
Zanagnolo V, Minig L, Rollo D, Tomaselli T, Aletti G, Bocciolone L, Landoni F, Cardenas Rebollo JM, Maggioni A (2016) Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer 26:568–574
Hao X, Han S, Wang Y (2015) Comparison of conventional laparoscopy and robotic radical hysterectomy for early-stage cervical cancer: a meta-analysis. J Cancer Res Ther 11(Suppl):C258–c264
Shazly SA, Murad MH, Dowdy SC, Gostout BS, Famuyide AO (2015) Robotic radical hysterectomy in early stage cervical cancer: a systematic review and meta-analysis. Gynecol Oncol 138:457–471
Zhou J, Xiong BH, Ma L, Cheng Y, Huang W, Zhao L (2016) Robotic vs laparoscopic radical hysterectomy for cervical cancer: a meta-analysis. Int J Med Robot 12:145–154
Mendivil AA, Rettenmaier MA, Abaid LN, Brown JV 3rd, Micha JP, Lopez KL, Goldstein BH (2016) Survival rate comparisons amongst cervical cancer patients treated with an open, robotic-assisted or laparoscopic radical hysterectomy: a five year experience. Surg Oncol 25:66–71
He H, Zeng D, Ou H, Tang Y, Li J, Zhong H (2013) Laparoscopic treatment of endometrial cancer: systematic review. J Minim Invasive Gynecol 20:413–423
Gala RB, Margulies R, Steinberg A, Murphy M, Lukban J, Jeppson P, Aschkenazi S, Olivera C, South M, Lowenstein L, Schaffer J, Balk EM, Sung V, Society of Gynecologic Surgeons Systematic Review Group (2014) Systematic review of robotic surgery in gynecology: robotic techniques compared with laparoscopy and laparotomy. J Minim Invasive Gynecol 21(3):353–361
Tapper AM, Hannola M, Zeitlin R, Isojarvi J, Sintonen H, Ikonen TS (2014) A systematic review and cost analysis of robot-assisted hysterectomy in malignant and benign conditions. Eur J Obstet Gynecol Reprod Biol 177:1–10
Weinberg L, Rao S, Escobar PF (2011) Robotic surgery in gynecology: an updated systematic review. Obstet Gynecol Int 2011:852061
Lavoue V, Zeng X, Lau S, Press JZ, Abitbol J, Gotlieb R, How J, Wang Y, Gotlieb WH (2014) Impact of robotics on the outcome of elderly patients with endometrial cancer. Gynecol Oncol 133:556–562
Di Pierro GB, Wirth JG, Ferrari M, Danuser H, Mattei A (2014) Impact of a single-surgeon learning curve on complications, positioning injuries, and renal function in patients undergoing robot-assisted radical prostatectomy and extended pelvic lymph node dissection. Urology 84:1106–1111
Zeng XZ, Lavoue V, Lau S, Press JZ, Abitbol J, Gotlieb R, How J, Wang Y, Gotlieb WH (2015) Outcome of robotic surgery for endometrial cancer as a function of patient age. Int J Gynecol Cancer 25(4):637–644. doi:10.1097/IGC.0000000000000411
Hammer GP, du Prel JB, Blettner M (2009) Avoiding bias in observational studies: part 8 in a series of articles on evaluation of scientific publications. Dtsch Arztebl Int 106:664–668
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors did not receive any external funding to conduct this review.
Conflict of interest
Authors IN, BV, CH, ID, DU, and MB declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nevis, I.F., Vali, B., Higgins, C. et al. Robot-assisted hysterectomy for endometrial and cervical cancers: a systematic review. J Robotic Surg 11, 1–16 (2017). https://doi.org/10.1007/s11701-016-0621-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-016-0621-9