Abstract
Zinc oxide (ZnO) and zinc oxide-silicon dioxide (ZnO-SiO2) nanomaterials were successfully incorporated into cotton fabric yarn using a single step sonochemical method in aqueous medium. The resulting cotton fabrics were evaluated for the ultraviolet (UV) blocking properties using ultraviolet-diffused reflectance spectroscopy (UV-DRS), wetting studies measured by contact angle, and flammability test by vertical flame test (VFT) method. The hydrodynamic diameter of the incorporated nanomaterials was in the range of 30–600 nm. 1, 2, 3, 4-butanetetracarboxylic acid (BTCA) was used as a cross-linking agent between the free -OH groups of the cotton fabric yarns. The results of ZnO-SiO2 incorporated cotton fabric exhibited 8.8% enhancement in UV-B blocking, wettability of 124.5° and flame time of 9 ± 1 s as compared to control cotton fabric. The UV blocking by ZnO-SiO2 incorporated cotton fabric was retained even after 10 washing cycles. The study highlights a single step method for the simultaneous in-situ synthesis and incorporation nanomaterials in cotton fabrics sonochemically as an alternative method for enhanced multifunctionality.
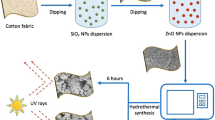
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The incorporation of particular function into textiles is presently the most important research area in both academics and industry (Park et al. 2020; Heo et al. 2018). Nowadays, several modification is being developed for fabrics with functionalities of self-cleaning (Dalawai et al. 2020), hydrophobicity (Zhou et al. 2020), and antibacterial (Qian et al. 2023). Their applications are also observed in the form of wearable sensors (Kim et al. 2022), medical treatment (Ashar et al. 2023), environmental pollution control (Mohammadipour-Nodoushan et al. 2023), and health care (Mirjalali et al. 2022). Cotton fabrics are most well-known among all textiles. Cotton has been used by humans for 4000 years ago and is produced from Gossypium genus. Cotton fabric is composed of cellulose fibres that are connected by glycosidic bonds possessing properties like breathable, non-toxic, biodegradable, comfort, softness, and hygroscopic (Sun et al. 2023; Nesa et al. 2023). Modification of pristine cotton fabrics adds to the functionalities like UV blocking, flame retardancy and superhydrophobicity making it a multifunctional cotton (Wanget al. 2022). Multifunctional cotton fabrics have two or more of these functions which are currently in demand. The incorporation ZnO nanoparticles in textiles have imparted flame retardant, UV blocking, and self-cleaning properties. Zinc oxide nanoparticles (ZnO NPs) have also been widely used in personal care products and are a bio-safe substance for human beings (Gong et al. 2023; Ma et al. 2023).
In-situ incorporation of the ZnO NPs in cotton textile using co-precipitation at different temperatures for antimicrobial and UV blocking is achieved (Belay et al. 2020). When fabric is treated with silica sol, it forms a glass-like network that exists as silanol and siloxane groups and is covalently bonded with fibres with dipolar-dipolar weak interaction, and hydrogen bond, which strengthens the coating adhesion to the fabric (Sfamen et al. 2023). Researchers use in situ sol-gel and self-assembly processes to fabricate intumescent flame retardant and hydrophobic coatings, thus enabling the production of multifunctional cotton fabrics. These coatings incorporates sodium montmorillonite (MMT), ammoniumdihydrogenphosphate (ADP), and methyltrimethoxysilane (MTMS). Zhang et al. studied that the concentration of MTMS affected the hydrophobicity, fire resistance, thermal stability and heat release behaviour of the coated fabrics. An additive can be incorporated into the textile to create a rough texture on its surface for maintaining its softness and durability, resulting in hydrophobic fabrics (Zhang et al. 2018). By coating of the nanocomposites such as zinc oxide-silicon dioxide (ZnO-SiO2) on cotton fibres using hexadecyltrimethoxysilane (HDTMS), researchers created hydrophobic textiles with a high contact angle of 150 °C (Rilda et al. 2019).
Ultrasonication or sonochemical method is a greener approach that is economical as well as ecological safe for the in-situ synthesis and incorporation of nanomaterials in cotton fabrics. Sonochemical treatment is advantageous since it speeds up the penetration of chemicals into the complex structure of yarns in a fabric and is also beneficial for the removal of natural and artificial impurities such as wax and starch (Noor et al. 2023). The sonochemical method involves formation, growth and collapsed bubbles in aqueous medium due to acoustic cavitation phenomenon. This method is used for the synthesis of various nanoparticles of varying sizes and shapes (Li et al. 2021). Sonochemical method of synthesis is a facile technique that helps to activate the surface, breaking down the agglomeration (Sharifalhoseini et al. 2018).
Gedanken and his co-workers have widely studied the ultrasonic synthesis of nanomaterials and mentioned major benefits of sonochemistry as compared to other methods. Different methods have been used for the surface functionalization of textile, including sol-gel processes, electrodeposition, layer-by-layer deposition and lithographic techniques for getting hydrophobic, flame retardant, and UV blocking properties (Perelshtein et al. 2017). However, these methods are costly, complicated, and require multiple reaction steps to complete. On the other hand, the sonochemical method is a single step, low-cost, quick, and easy technique for the synthesizing of nanomaterials. Moreover, sonochemical synthesis can obtain superior and unique morphologies of the synthesized nanomaterials because of the high rate of dissolution of the precursors, resulting in decreased particle size and high dispersion yield. (Svirinovsky et al. 2018; Noralian et al. 2021). The available literature on sonochemical methods has used ultrasonication method to functionalize cotton fabric; however, dielectric barrier discharge (DBD) and plasma treatment steps are pre-requisite of ultrasonic irradiation (Irfan et al. 2022).
Ultrasonic probe sonicator has also been used for fabricating multifunctional cotton fabrics, in a two step process i.e. first, a solution was prepared using a magnetic stirrer; then, synthesized ZnO nanoparticles were deposited on cotton using probe sonicator (Javed et al. 2021). In the present study, we have successfully in-situ synthesized and incorporated ZnO and ZnO-SiO2 nanomaterials in cotton fabric by using a laboratory bath ultrasonicator in a single step at a reaction temperature of 30 °C and reaction time of 30 min. in aqueous medium. The sonochemical method enhances the rate of diffusion of Zn2+ and Si4+ precursors in the cotton yarns due to swelling of yarns process of ultrasonic irradiation. These precursors simultaneously lead to formation of ZnO and ZnO-SiO2 nanomaterials. Upon drying the cotton fabric, the loss of water leads to the shrinkage of the yarns which provides additional physical anchoring to the ZnO and ZnO-SiO2 nanomaterials in cotton fabric. Thus, the study highlights a single step sonochemical method for simultaneous in-situ synthesis and incorporation with physical anchoring of nanomaterials in cotton fabrics.
Experimental section
Materials and methods
All the reagents and chemicals were utilized as purchased without any further purifications. Zinc acetate dehydrate Zn (CH3COO)2(H2O)2 (≥ 99.5%) was from Thomas Baker; Starch extra pure and sodium hydroxide pallets (98%) were from SRL chemicals, India, Sodium hypophosphite monohydrate (99%) was from Spectrochem Pvt. Ltd. India, and 1, 2, 3, 4-Butanetetracarboxylic acid (98%) was from Alfa Aesar, India. Methyltrimethoxysilane (MTMS) (98%) was from Sigma-Aldrich India, and absolute ethanol (99.9%) was collected from Changshu Hongsheng Fine Chemical Co., Ltd., China. All the synthesis and in-situ incorporation in cotton fabric were carried out using distilled water. DKTE’s Society’s and Engineering Institute Ichalkaranji, Maharashtra, gifted the pre-finished 100% cotton fabrics.
Incorporation of ZnO NPs in cotton fabric
In a typical in-situ synthesis and incorporation procedure, cotton fabrics of the size 2 × 2 inch. is pre-treated using soap solution, rinsed with distilled water,sonicated at 50 °C using a bath ultrasonicator to remove non-cellulose components such as wax, grease other finishing materials and finally allowed to dry at room temperature. Zinc acetate (0.16 M) dissolved in aqueous starch solution (0.5%) with continuous stirring using a magnetic stirrer. Cotton fabric were introduced in the solution after the complete dissolution of zinc acetate. Aqueous NaOH solution (0.6 M) were added dropwise into the above mixture by the wall of the beaker. After the NaOH addition, the above reaction mixture was placed in the bath ultrasonicator for 30 min. at 30 °C. The reaction mixture containing the cotton fabric was allowed to settle down. The in-situ synthesized and incorporated cotton fabric was removed and dried in a hot air oven overnight at 90 °C. Finally, the reaction mixture was centrifuged at 5000 rpm and the powder was harvested and dried at 120º C in a hot air oven for overnight.
ZnO-BTCA treated cotton fabric
Zinc acetate (0.16 M) dissolved in starch solution (0.5%) with continuous stirring using a magnetic stirrer along with the cotton fabric. NaOH solution (0.6 M) was further added dropwise in the reaction mixture by the wall of the beaker. After the NaOH addition, above reaction mixture was placed in the bath ultrasonicator for 15 min. and then added mixture of 1, 2, 3, 4-butanetetracarboxylic acid (0.09 M) as a cross-linker followed by sodium hypophosphite (0.1 M) as a catalyst with continuous stirring. The reaction mixture was further exposed to a bath ultrasonicator for another 15 min. Similar to the procedure of drying and harvesting ZnO incorporated cotton fabric and ZnO powder, the same was followed for the ZnO-BTCA treated cotton fabric and powder.
ZnO–SiO2 incorporation in the cotton fabric
As mentioned above, the procedure of the incorporation of the ZnO NPs in cotton fabric, 100 ml. of MTMS (1.4 M) in absolute ethanol were added to this reaction mixture and exposed to a bath ultrasonicator for 30 min. under optimized reaction conditions, at 30 °C. The cotton fabric was dried at 90 °C overnight. The remaining reaction mixture was centrifuged at 5000 rpm. The obtained powder was dried in the oven at 120 °C overnight.
Washing test
The wash ability tests of all nanomaterials incorporated cotton fabrics were determined using distilled water at room temperature using 1×1 cm pieces of the cotton fabrics. The washing process was carried out using an orbital shaker in a 20 ml distilled water at 120 rpm for 25 min. (5 cycles) and for 50 min (10 cycles). The samples were then dried at 90 °C overnight, furthermore, samples were tested for UV blocking ability from transmittance (%) taken by UV-DRS (El-Nahha et al. 2017).
Flammability test
The study of the combustion property of the nanomaterial incorporated in cotton fabrics was evaluated by the vertical flammability test (VFT). Cotton fabrics of the size 2 × 6 cm was used and tested for three times each. The average time to capture flame and flaming time (S) were recorded, and the average were calculated.
Characterization techniques
Dynamic light scattering (DLS) for determination of hydrodynamic diameter and zeta potential for stability using a particle size analyzer (Anton Paar, letiesizer 500) with a laser wavelength of 633 nm and power of 4.0 mW, from a solid-state He-Ne laser source at a scattering angle of 170° were used. X-ray diffraction (XRD) patterns were recorded on a D8 Advance Bruker with Cu-Kα monochromatic radiation (λ = 1.5418 Å), and XRD patterns were recorded in the range of 2θ between 5° and 80°. UV-DRS transmittance spectra of the incorporated cotton fabric were recorded on a Shimadzu UV–VIS SPECTROPHOTOMETER UV-2700, using barium sulphate as a standard. The FTIR analysis was carried out using attenuated total reflectance Fourier Transform Infrared (ATR-FTIR) Spectrophotometer from Bruker Alpha 100508 in the wavelength range from 500-4000 nm. Thermogravimetric analysis (TGA) was done using (SDT Q600 V20.9 Build 20) in the temperature range from 0 -1000 °C at heating rate of 10 °C per minute. For the harvested powder nanomaterials, 5 mg were taken in the platinum crucible and the data were recorded for a scanning time of 98 min. Surface roughness and topography were evaluated using an atomic force microscopy (AFM) (Park Systems, NX-10), in a non-contact mode with the area of 2 µm × 2 µm. Surface morphology of cotton fabric was studied by Field Emission Scanning Electron Microscopy (FESEM) (MIRA3 LMH, TESCAN, Brno, Czech Republic), with energy-dispersive spectroscopy (EDS) (Oxford Instrumentations), performed at 15 kV. Wettebility of all the nanomaterial incorporated cotton fabrics were studied using a contact angle measurement (HOLMARC Opto-mechatronics Pvt. Ltd). The sonochemical in situ synthesis and incorporation was carried out using an ultrasonic bath (Life Care Equipment Pvt. Ltd. with a capacity of 3.3 Ltr operating at 33 ± 3 kHz and100 W ). Vertical flammability test (VFT) done using flame and image data collected using a digital camera.
Results and discussions
Scheme 1 represents the different aspects of the sonochemical (ultrasonication) method for simultaneous in-situ synthesis and incorporation of the ZnO and ZnO-SiO2 nanoparticles into cotton fabrics. The method has advantages as: (i) It helps in the swelling of yarns of the cotton fabric resulting increase in porosity, pore size and thickness of yarn of the cotton, (ii) Zn2+ and Si4+ ions easily diffuse from a precursor in the yarn of the fabric, and (iii) ultrasonic irradiation leads to nucleation of ZnO and ZnO-SiO2 nanomaterials embedded inside the yarns which on drying provides anchoring to the ZnO and ZnO-SiO2 nanomaterials. The incorporation of zinc oxide (ZnO) nanomaterial in cotton fabric by sonochemical method follows addition of zinc acetate [Zn (CH3COO)2 2.H2O] precursor in the presence of sodium hydroxide (NaOH) that generates intermediate Zn (OH)2. Zinc ions penetrate into the matrix of the fabric resulting in the acessible hydroxyl groups of the cotton fabrics being replaced by Zn(OH2), however, some OH groups remain unreacted on the cotton fabric. 1, 2, 3, 4-butanetetracarboxylic acid (BTCA) catalysed in the presence of sodium hypophosphite (SHP) dissolved and dissociated into ions in water is cross-linked between the unreacted surface hydroxyl groups present on the cotton fabric. Thus, upon drying results in ZnO-BTCA treated cotton fabric with good potential anti-wrinkle property. BTCA is one of the best formaldehyde-free cross-linkers. On the other hand, ZnO incorporated cotton fabric was treated with methyltrimethoxysilane enhances various properties, as silane treatment decreases the particle size and increases hydrophobicity. Hydrolytically sensitive sites of cotton fabric reacts with hydroxyl groups or silanols of MTMS to form silica-modified surfaces of the cotton fabrics. In ZnO-SiO2 incorporated cotton fabric, conversion takes place between the hydrophilic to hydrophobic surfaces of the cotton fabric, some hydroxyl groups of the cotton remain unreacted. In the sonochemical method, ultrasonic waves have frequency above 20,000 oscillations per second. Ultrasonic waves promote the incorporation of newly formed active nanoparticles to the surface of cotton fabric, swelling of fibres of cotton takes place in water, and finally opens the matrix of the fabric resulting in smooth insertion or incorporation of the nanoparticles between the yarn pores of cotton fabric. The mechanism of ultrasonication also leads to the lowering the fabrics glass transition temperature, raising the partition coefficient of the fabric in ultrasonic bath, improving material transport to the fabric surface by lowering the boundary layer, breaking up micelles, and high molecular weight aggregates into uniform dispersions in the ultrasonic bath. Ultrasound energy of 20 kHz frequency is compatible to induce cavitations and oscillations to produce unstable micro-bubbles which collides each other due to momentary high temperature and pressure. The temperature of the micro-bubbles in an ultrasonic bath can reach to a maximum of 5000 °C. This active moment causes a chemical reaction between the precurssors and cotton fabric; ultrasonication reduces the agglomeration of the precurssors. Depending on the condition of the water vapour pressure in a cavitation bubble, the cavity and time, the bubble collapses, leading to sonochemical processes in aqueous solution. At this point, the water vapour is under high pressure and temperature, and the water molecules will undergo a process known as sonolysis as shown in Fig.1, producing highly reactive species of water.
After the incorporation of ZnO nanoparticles in the fabric, remaining suspension was spin down and the collected powder was dried oven at 90° C overnight. Both the hydrodynamic diameter and zeta potential of nanomaterials were studied by dynamic light scattering (DLS). ZnO, ZnO-BTCA treated and ZnO-SiO2 nanomaterial were dispersed in water. The particle size was influenced by reaction time, and precursor.. The hydrodynamic diameter of the ZnO nanoparticle was 33.64 nm. The stability of colloidal solutions was determined by zeta potential assessment based on the surface charge of particles. (Jain et al. 2020) The zeta potential correlates to the stability and charge on the particles surface in the solution. The zeta potential value of synthesized ZnO particles was −14.84 mV, indicating ZnO particles are stable. The hydrodynamic diameter of the ZnO-BTCAtreated powder was 342.1 nm, the zeta potential was −9.9 mV and hydrodynamic diameter of the ZnO-SiO2 nanoparticles was 863.5 nm with zeta potential of 0.75 mV as depicted in Figs.2 and 3 respectively. According to the study of zeta potential, the surface charge of the incorporated nanomaterials was negative and the ZnO-SiO2 nanomaterials was more stable than others because the zeta potential range was near zero.
XRD patterns of control cotton, ZnO, ZnO-BTCA-treated, and ZnO-SiO2 incorporated cotton fabrics are shown in Fig.4. XRD patterns of control cotton fabric represented in Fig.4a where peaks observed at 17.53°, 22.68°, 25.67°, and 34.01 correspond to (111), (200), (200), and (211) XRD patterns which stated crystalline form of cellulose. The ZnO incorporated cotton fabric’s XRD peaks are observed in Fig. 4b at 17.42°, 20.4°, 22.55°, 25.11° 27.34°, 29.73°, 31.77°, 36.23°, 39.46°, 40.67°, 43.16°, 47.68°, 48.54°, 52.46°, 56.57°, 60.5°, 62.89°, 68.02°, and 69.04° which corresponds to (111), (200), (210), (211), (220), (300), (311), (222), (321), (400), (411), (411), (411), (421), (422), (430), (431), (432), and (432), XRD patterns with intense peak confirmed that incorporated ZnO nanoparticles were crystalline and of great purity. ZnO nanoparticles were of hexagonal phase compared with standard data (JCPDS No. 01-079-0206), respectively. The peaks of ZnO-BTCA treated cotton fabrics are observed at 7°, 12°, 18°, 21°, 33°, and 59° which are described to crystal planes (111), (221), (331), (431), (553), and (652) of ZnO-BTCA-treated (JCPDS card No. 00-012-0142) observed in Fig.4c. ZnO-SiO2 incorporated cotton fabric peaks associated with 2Ɵ values at 17.42°, 21.69°, 25.8°, 29.38°, 31.77°, 34.51°, 36.23°, 39.64°, 43.22°, 47.68°, 48.54°, 56.57°, 60.66°, 63.05°, 64.77°, and 68.02° which represents (111), (210), (211), (220), (310), (311), (320), (321), (322), (421), (421), (432), (441), (442), (532), and (540) crystal planes represented in Fig. 4d. Silica nanoparticles were of tetragonal shape according to standard data (JCPDS No. 00-036-1451 and 01-082-1406) (Yang et al. 2018). The particle size of the ZnO, ZnO-BTCA treated, and ZnO-SiO2 nanomaterial was determined using the Debye-Scherrer formula as given in Eq.1 which are 67.83 nm, 47.61 nm, and 51.92 nm, respectively.
UV-DRS spectroscopy is used to investigate the UV blocking property of the nanomaterials incorporated cotton fabrics in terms of transmittance. The transmittance calculated by UV-DRS, when the material has a minimum transmittance value, it shows good UV blocking properties. According to UV-DRS, control cotton fabric was higher transmittance concerning the ZnO, ZnO-BTCAtreated, and ZnO-SiO2incorporated cotton fabrics that means lower the UV blocking ability. Nanomaterials incorporated cotton fabrics were also tested after 10 washing cycles, to study washing durability as shown in Fig.5. In Table 1, transmittance value of the nanomaterial incorporated cotton fabric was described without washing and after 10 washing cycles. To study more elaborately the UV blocking performance of the fabric in each part of the UVR spectrum, the UV-A (315 to 400) and UV-B (280-315) transmittances are calculated as the arithmetic mean of all intermediate transmittance measurements in each region, by Eq.2 and Eq.3.
where \({T}_{\lambda }\) is spectral transmittance at wavelength \(\lambda\), m is the number of measurement points between 315 and 400 nm and between 280 and 315 nm, respectively, i.e. depending. ZnO-SiO2 incorporated cotton fabric performed better ability for UV blocking as low transmittance values studies by UV-DRS transmittance; in both UV-A and UV-B regions as compared to control, ZnO, ZnO-BTCA treated cotton fabrics as represented in Table1. (Louris et al. 2018). ZnO-SiO2 incorporated cotton fabric has better UV blocking retention even after 10 washing cycles according to UV-DRS transmittance which is given in Table 1.
FTIR absorption bands of all fabrics associated with ZnO, ZnO-BTCA treated, and ZnO-SiO2 incorporated cotton fabrics are represented in Fig.6. Functional groups of the cotton fabrics of control and incorporated cotton fabrics were examined by the attenuated total reflectance fourier transform infrared (ATR-FTIR). From Fig.6, the ATR-FTIR spectra of ZnO incorporated cotton fabric compared with control cotton fabric and measured in the range of 500-4000 cm−1 presented in Fig.5. Peaks are observed in Fig.6a at 1150 cm−1, 1314 cm−1, 1718 cm−1, and 2906 cm−1 that represents cellulose band which shows O–H stretching. The peak 1150 cm−1 attributed to strong peaks belongs to the cellulosic structure. The peak at 1314 cm−1 was attributed to the C-H asymmetric deforming. The peak at 1718 cm−1 was attributed to the bending vibration of C–O bonds in control cotton fabric. The new peak appeared in ZnO incorporated cotton fabric in Fig. 6b at 461 cm−1 was attributed to the Zn–O vibration (Yazhini et al. 2015). The peak at 1718 cm−1 corresponds to the formation of ester and carboxylate in the cross-linked cellulose. In Fig.6c, this peak was shifted in ZnO-BTCA treated cotton fabric at 1327 cm−1 and 1714 cm−1 that were attributed to the presence of carbonyl group which shows stretching vibrations of (COO–), and the formation of ester linkage was confirmed. The band at 995 cm−1 was attributed to the CO bond, and the peak at 705 cm−1 was attributed to C–O–C stretching of the cyclic anhydride in ZnO-BTCA treated cotton fabric (Aslam et al. 2019). Peak 477 cm−1 was attributed to Zn–O vibrations, and a band in the range of 721 cm−1 was associated with the Si–O–Si stretching vibrations bonds represented in Fig.6d for ZnO-SiO2 incorporated cotton fabric. (Seeharaj et al. 2018; Saravanan et al. 2020).
Thermal stability of the control cotton, ZnO, ZnO-BTCA treated, and ZnO-SiO2 incorporated cotton fabrics examined by thermogravimetric analysis (TGA) as depicted in Fig.7. Thermal degradation of control cotton fabric performed quick weight loss, and it stared from 268.71 °C of 19.71% after 369.39 °C major decomposition started of the control cotton and 50.71% weight loss observed after it slowly decomposed carbon residue at 504.97 °C and residue remained 14.31%. Cellulose decomposition temperature has been reported to be 300–380 °C (Rilda et al. 2020). TGA of ZnO incorporated cotton fabric, initially minimum weight loss of 4.56% observed at 99.34o C, major weight loss of 63.97%, started at 213.15 °C finally residue remained at 980.10 °C of 14.86% because the presence of ZnO content in cotton fabric. When ZnO-BTCA treated cotton fabrics were studied for thermogravimetric analysis where initially showed weight loss of 18.71% at 98.70 °C then, the second weight loss of 43.66% started at 359.02 °C and the final 6.54% residue remained. ZnO-SiO2 incorporated cotton fabric quickly decomposed but the final residue remained at 17.61% at 981 °C due to the effect of ZnO-SiO2 nanocomposites incorporation in cotton. In addition, for a small change to the decomposition with increased residual percentage, therefore it confirmed that the incorporation of the nanoparticles had no effect on the thermal stability of the cotton fabrics.
ZnO, ZnO-BTCA treated, and ZnO-SiO2 nanomaterials were sucessfully incorporated in cotton fabrics and confirmed by FESEM and EDX. The morphology of the control, ZnO, ZnO-BTCA treated, and ZnO-SiO2 incorporated cotton fabrics as well as their incorporation in the cotton fabrics was studied using a field emission scanning electron microscopy embedded with energy-dispersive spectroscopy used for the elemental analysis and mapping as depicted in Figs.8, 9, 10 and 11. Fig.8a represented the FESEM morphology of the yarn of the original control cotton fabrics another Fig. 8b ZnO incorporated cotton fabric having a rod-like structure with less densely occupied in the surface of the cotton fabric (Wang et al. 2018). Figure 8c depicted random incorporation of the ZnO-BTCA treated cotton fabric. FESEM morphology of the ZnO-SiO2 incorporated more densely into yarns of the cotton fabric as depicted in Fig.8d.
Figure 12 describes AFM analysis of the control cotton, ZnO, ZnO-BTCA treated, and ZnO-SiO2 incorporated cotton fabrics representing the surface topography of the control cotton and nanomaterial incorporated cotton fabrics. Surface roughness of the ZnO incorporated cotton fabrics having larger roughness value with respective control cotton, ZnO-BTCA treated, and ZnO SiO2 incorporated cotton fabrics which indicated high ZnO incorporation in cotton fabrics (Arputharaj et al. 2017). Table 2 represents the surface roughness values of the control and ZnO, ZnO-BTCA treated, and ZnO SiO2 cotton fabrics.
The wetting behaviour of the cotton fabrics was studied using contact angle (WCA) measurements as depicted in Fig.13. Original control cotton was hydrophilic according to results as revealed in Fig.13a. The average contact angle between water and the surface of the ZnO incorporated cotton fabric was 88.65° as represented in Fig.13b. ZnO-BTCA treated cotton fabric was hydrophilic in nature as per results revealed in Fig.13c; however, ZnO-SiO2 cotton performed a greater water contact angle than ZnO incorporated cotton fabric which was 124.5° (Fig.13d). Contact angle measurements also done after 10 washing cycles to study effect of 10 washing cycles on the surface texture for the ZnO incorporated, ZnO-BTCA treated, and ZnO-SiO2. As can be seen from the contact angle images shown in Fig.13c, e, g after 10 washing cycles, all except the ZnO-SiO2 cotton fabric are hydrophobic with contact angle of 120.5°, Fig.13g which is similar to the before washing cycles (124.5°). This suggest that the hydrophobic surface texture of the ZnO-SiO2 incorporated cotton fabrics is retained even after 10 washing cycles and it complements UV blocking property as shown in Fig. 5.
Wenzel and Cassie-Baxter model is significant for the study of wetting states of the structured surfaces. In described Wenzel model which is a completely wetted surface, the original control cotton fabric absorbed water whereas Fig.14b represented the Cassie-Baxter model i.e. non-wetted on the rough surface; ZnO-SiO2 incorporated cotton fabrics also performed the same Cassie-Baxter state on the surface. Figure 14c described the intermediate state of the surface between Wenzel and Cassie-Baxter states which is a partial or mixed wetting state of the surface, in our study, ZnO-incorporated cotton fabric performed the same state on the surface (Nagayama et al. 2020). Correlation study of the Cassie-Baxter and Wenzel model with the physical morphology of the cotton surfaces obtained from FESEM images in Fig. 8a–d would better understand deviations from hydrophilic to hydrophobic surfaces and the intermediate partial wetting surface of the nanomaterial incorporated cotton fabrics. Table 3 gives detailed comparision of multifunctional application of cotton fabrics.
To study the combustion properties of different nanomaterials incorporated in cotton fabrics compared with control cotton, a vertical flammability test (VFT) conducted. Each sample was evaluated 3 times, and the average data were represented. Images taken before and after VFT using a digital camera, corresponding data given in the following Table 4. VFT was studies observed that control cotton burned completely and no residue remained. Average time to capture flame (3.6 s) and flaming (9 s) performance increased in the case of nanomaterials incorporated cotton fabrics with increased char length. In the study observed, ZnO-SiO2 incorporated cotton fabric required more time to capture flame, it also greatly improved char length as compared to ZnO and ZnO-BTCA treated cotton fabrics. (Fig. 15)
Conclusions
ZnO, ZnO-BTCA treated, and ZnO-SiO2 nanomaterials have been successfully synthesized and incorporated in cotton fabrics and confirmed by UV-DRS, DLS, FTIR, XRD, FESEM, AFM, WCA, and VFT studies. ZnO incorporated cotton fabric performed good UV blocking, hydrophobic contact angle and flame time than control cotton fabric. ZnO-BTCA treated cotton where surface OH groups were cross-linked by using BTCA; however, there was no significant change observed in UV-DRS and wetting angle. According to the results, ZnO-SiO2 incorporated cotton fabric exhibited a significant enhancement in multifunctional properties of UV blocking, wettability, and flame time compared to control, ZnO incorporated and ZnO-BTCA cotton fabrics. The ZnO incorporated cotton fabrics demonstrated 1.7 times better UV-B blocking than the control cotton fabric; also, there was only a slight change observed in the transmittance of all nanomaterials incorporated cotton fabrics even after 10 washing cycles. ZnO incorporated cotton fabric surface behaved as hydrophobic in nature (WCA = 89°), after washing, it turned into hydrophilic surface and flame time of 7 ± 0.6 s. ZnO-SiO2 incorporated fabric performed better UV-B blocking ability which was 3.3 times greater than control cotton fabric. In the wettability study, ZnO-SiO2 was a significant hydrophobic nature (CA = 124.5°), after washing test, it retained hydrophobicity (WCA=120.5°) and flame time of 9 ± 1 s. Regarding effect of 10 washing cycles to the nanomaterial incorporated cotton fabrics, we can conclude that there is almost no effect of washing cycles on the texture of the ZnO-SiO2 incorporated cotton fabric; also, it retained good UV blocking property. Therefore, in this study, we conclude ZnO-SiO2 incorporated cotton possesses multifunctional properties, and the sonochemical method could be a better alternative for the direct incorporation of nanomaterials in cotton fabrics. The important areas of applications of these nanomaterials incorporated cotton fabrics can be beneficial in different aspects of society, likewise in protective clothing, UV blocking fabrics can be used against harmful UV radiations from the sun, in outdoor textiles hydrophobic and superhydrophobic fabrics are used to repel water and prevent moisture damage. In medical fields, hydrophobic and flame retardant cotton fabrics are used as a medical textile to prevent the spread of infection and protect against fire, as well as it also used in home textiles such as curtains for anti-dust, flame retardant properties. Nowadays, we are working on superhydrophobic cotton fabrics to enhance self-cleaning properties by increasing the water contact angle of the incorporated cotton fabrics also focusing on improving washing cycles and other properties of the developed multifunctional cotton fabrics by using a same sophisticated sonochemical method which helped to open a wide range of cotton fabrics and easy incorporation of the nanomaterials in cotton fabrics without significant damage to the original structure of the cellulose.
Availability of data and materials
All the data and materials are available in this article.
References
Arputharaj A, Nadanathangam V, Shukla SR (2017) A simple and efficient protocol to develop durable multifunctional property to cellulosic materials using in situ generated nano-ZnO. Cellulose 24:3399–3410
Ashar A, Bhutta ZA, Shoaib M, Alharbi NK, Fakhar-e-Alam M, Atif M, Ahmed AE (2023) Cotton fabric loaded with ZnO nanoflowers as a photocatalytic reactor with promising antibacterial activity against pathogenic E. coli. Arab J Chem 16(9):105084
Aslam S, Hussain T, Ashraf M, Tabassum M, Rehman A, Iqbal K, Javid A (2019) Multifunctional finishing of cotton fabric. Autex Res J 19(2):191–200
Belay A, Mekuria M, Adam G (2020) Incorporation of zinc oxide nanoparticles in cotton textiles for ultraviolet light protection and antibacterial activities. Nanomater Nanotechnol 10:1847980420970052
Chakrabarti S, Banerjee P (2015) Preparation and characterization of multifunctional cotton fabric by coating with sonochemically synthesized zinc oxide nanoparticle-flakes and a novel approach to monitor its self-cleaning property. J Text Inst 106(9):963–969
Chen D, Mai Z, Liu X, Ye D, Zhang H, Yin X, Zhou Y, Liu M, Xu W (2018) UV-blocking, superhydrophobic and robust cotton fabrics fabricated using polyvinylsilsesquioxane and nano-TiO2. Cellulose 25:3635–3647
Dalawai SP, Aly MAS, Latthe SS, Xing R, Sutar RS, Nagappan S, Ha CS, Sadasivuni KK, Liu S (2020) Recent advances in durability of superhydrophobic self-cleaning technology: a critical review. Prog Org Coat 138:105381
Gong X, Xiong Z, Chen X, Meng F, Wang H (2023) Multifunctional superamphiphobic cotton fabrics with highly efficient flame retardancy, self-cleaning, and electromagnetic interference shielding. ACS Appl Mater Interfaces 15(2):3395–3408
Heo JS, Eom J, Kim YH, Park SK (2018) Recent progress of textile-based wearable electronics: a comprehensive review of materials, devices, and applications. Small 14(3):1703034
Irfan M, Hussain H, Saleem B, Saleem M, Shukrullah S, Legutko S, Petrů J, Naz MY, Pagáč M, Rahman S, Khan R (2022) Evaluation of ultrasonically ZnO loading effect on photocatalytic self-cleaning, UV protection and antibacterial activity of plasma/citric acid-activated cotton fabric. Nanomaterials 12(12):2122
Jain D, Bhojiya AA, Singh H, Daima HK, Singh M, Mohanty SR, Stephen BJ, Singh A (2020) Microbial fabrication of zinc oxide nanoparticles and evaluation of their antimicrobial and photocatalytic properties. Front Chem 8:778
Javed A, Wiener J, Tamulevičienė A, Tamulevičius T, Lazauskas A, Saskova J, Račkauskas S (2021) One step in-situ synthesis of zinc oxide nanoparticles for multifunctional cotton fabrics. Materials 14(14):3956
Khan MZ, Baheti V, Ashraf M, Hussain T, Ali A, Javid A, Rehman A (2018) Development of UV protective, superhydrophobic and antibacterial textiles using ZnO and TiO2 nanoparticles. Fibers Polym 19:1647–1654
Kim T, Park C, Samuel EP, Kim YI, An S, Yoon SS (2022) Wearable sensors and supercapacitors using electroplated-Ni/ZnO antibacterial fabric. J Mater Sci Technol 100:254–264
Li Z, Dong J, Zhang H, Zhang Y, Wang H, Cui X, Wang Z (2021) Sonochemical catalysis as a unique strategy for the fabrication of nano-/micro-structured inorganics. Nanoscale Adv 3(1):41–72
Louris E, Sfiroera E, Priniotakis G, Makris R, Siemos H, Efthymiou C, Assimakopoulos MN (2018) Evaluating the ultraviolet protection factor (UPF) of various knit fabric structures. In: IOP conference series: materials science and engineering (vol 459, no 1, p 012051). IOP Publishing
Ma J, Niu T, Wang Y, Sun D, Zhang X, Fang L (2023) Fabrication of multifunctional cotton fabrics with antibacterial, hydrophobic, and dyeing performance. ACS Appl Mater Interfaces 15(44):51727–51736
Mirjalali S, Peng S, Fang Z, Wang CH, Wu S (2022) Wearable sensors for remote health monitoring: potential applications for early diagnosis of Covid-19. Adv Mater Technol 7(1):2100545
Mohammadipour-Nodoushan R, Shekarriz S, Shariatinia Z, Heydari A, Montazer M (2023) Improved cotton fabrics properties using zinc oxide-based nanomaterials: a review. Int J Biol Macromol 242:124916
Nagayama G, Zhang D (2020) Intermediate wetting state at nano/microstructured surfaces. Soft Matter 16(14):3514–3521
Nesa SHS, Tarangini K (2023) A review on augmentation of natural fabric materials with novel bio/nanomaterials and their multifunctional perspectives. Hybrid Adv 2:100020
Noman MT, Wiener J, Saskova J, Ashraf MA, Vikova M, Jamshaid H, Kejzlar P (2018) In-situ development of highly photocatalytic multifunctional nanocomposites by ultrasonic acoustic method. Ultrason Sonochem 40:41–56
Noor N, Mutalik S, Younas MW, Pragya A, Hu X, Ho CY, Fei B (2023) Sonochemical coating of textiles with zinc oxide: robust, silver-seeded growth on cotton for effective UV shielding. ACS Appl Eng Mater 1(12):3254–3267
Noralian Z, Gashti MP, Moghaddam MR, Tayyeb H, Erfanian I (2021) Ultrasonically developed silver/iota-carrageenan/cotton bionanocomposite as an efficient material for biomedical applications. Int J Biol Macromol 180:439–457
Park J (2020) Functional fibers, composites and textiles utilizing photothermal and joule heating. Polymers 12(1):189
Perelshtein I, Perkas N, Gedanken A (2017) Bar-Ilan University, Ramat Gan, Israel. Handbook of antimicrobial coatings, p 235
Qian J, Dong Q, Chun K, Zhu D, Zhang X, Mao Y, Culver JN, Tai S, German JR, Dean DP, Miller JT (2023) Highly stable, antiviral, antibacterial cotton textiles via molecular engineering. Nat Nanotechnol 18(2):168–176
Rilda Y, Safitri R, Putri YE, Refinel R, Agustien A, Leaw WL, Nur H (2019) Hexamethyldisiloxane-modified ZnO–SiO2-coated superhydrophobic textiles for antibacterial application. J Chin Chem Soc 66(6):594–599
Rilda Y, Damara D, Putri YE, Refinel R, Agustien A, Pardi H (2020) Pseudomonas aeruginosa antibacterial textile cotton fiber construction based on ZnO–TiO2 nanorods template. Heliyon 6(4):e03710
Sadr FA, Montazer M (2014) In situ sonosynthesis of nano TiO2 on cotton fabric. Ultrason Sonochem 21(2):681–691
Saravanan S, Dubey RS (2020) Synthesis of SiO2 nanoparticles by sol–gel method and their optical and structural properties. Rom J Inf Sci Technol 23(1):105–112
Seeharaj P, Pasupong P, Detsri E, Damrongsak P (2018) Superhydrophobilization of SiO2 surface with two alkylsilanes for an application in oil/water separation. J Mater Sci 53:4828–4839
Sfameni S, Hadhri M, Rando G, Drommi D, Rosace G, Trovato V, Plutino MR (2023) Inorganic finishing for textile fabrics: recent advances in wear-resistant UV protection and antimicrobial treatments. Inorganics 11(1):19
Sharifalhoseini Z, Entezari MH, Shahidi M (2018) Sonication affects the quantity and the morphology of ZnO nanostructures synthesized on the mild steel and changes the corrosion protection of the surface. Ultrason Sonochem 41:492–502
Sun S, Xu P, Chen ZH, Xiao QR, Qiang XL, Shi XL (2023) “One stone three birds”: a multifunctional cotton fabric with favorable self-cleaning, photothermal effect and Joule heating properties. Appl Surf Sci 623:156961
Svirinovsky A, Perelshtein I, Natan M, Banin E, Gedanken A (2018) Imparting superhydrophobic and biocidal functionalities to a polymeric substrate by the sonochemical method. Ultrason Sonochem 44:398–403
Wang YW, Shen R, Wang Q, Vasquez Y (2018) ZnO microstructures as flame-retardant coatings on cotton fabrics. ACS Omega 3(6):6330–6338
Wang Y, Baheti V, Khan MZ, Viková M, Yang K, Yang T, Militký J (2022) A facile approach to develop multifunctional cotton fabrics with hydrophobic, self-cleaning and UV protection properties using ZnO particles and fluorocarbon. J Text Inst 113(10):2238–2248
Yang H, Zhang Q, Chen Y, He Y, Yang F, Lu Z (2018) Microwave-ultrasonic synergistically assisted synthesis of ZnO coated cotton fabrics with an enhanced antibacterial activity and stability. ACS Appl Bio Mater 1(2):340–346
Yazhini KB, Prabu HG (2015) Antibacterial activity of cotton coated with ZnO and ZnO-CNT composites. Appl Biochem Biotechnol 175:85–92
Zhang D, Williams BL, Becher EM, Shrestha SB, Nasir Z, Lofink BJ, Santos VH, Patel H, Peng X, Sun L (2018) Flame retardant and hydrophobic cotton fabrics from intumescent coatings. Adv Composit Hybrid Mater 1:177–184
Zhou X, Sun S, Zhang C, Wang XY, Li YL, Jiang Y (2020) Facile fabrication of durable superamphiphobic PET fabrics. J Coat Technol Res 17:711–718
Acknowledgements
Thankful to Chhatrapati Shahu Maharaj, Research, Training and Human Development Institute (SARTHI), Pune, for providing SRF. The authors would like to acknowledge RUSA (Centre for Nano fabrics) for financial support and also thankful to Sophisticated Analytical Instrument Facilities (SAIF) in Common Facility Centre (CFC) of Shivaji University, Kolhapur, Maharashtra, for providing XRD, FTIR, TGA, and AFM facility accessed through I-STEM (Indian Science, Technology, and Engineering facilities Map) portal, supported by the office of the Principal Scientific Adviser to the Govt. of India.
Funding
The authors declare that no funds, grants, or other support were received for this manuscript.
Author information
Authors and Affiliations
Contributions
Pranita Magadum: Experimental, data analysis, data interpretation, writing original data, and visualization. Abhishek Chavan: Data analysis and data writing. Kiran Kumar Sharma: Supervision, Conceptualization, methodology, project administrations, and funding acquisition. Shivaji Tayade: Editing and investigations. Ajit Kamble: Data investigation. All authors discussed and wrote the manuscript for final communication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have approved this submission for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Magadum, P., Chavan, A., Tayade, S. et al. Multifunctional properties of sonochemically in-situ synthesized and incorporated ZnO/ZnO–SiO2 nanomaterials in cotton fabric. Chem. Pap. 78, 4673–4688 (2024). https://doi.org/10.1007/s11696-024-03390-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-024-03390-w