Abstract
Desalination of groundwater and brackish water reverse osmosis is becoming more common worldwide as a means of supplementing and diversifying fresh water supply. However, a key impediment to extensive reverse osmosis desalination adoption, particularly at inland sites, is the lack of economic and ecologically viable reject management alternatives. The reverse osmosis concentrate can harm the ecosystem by causing pH fluctuations, eutrophication, and the proliferation of hazardous metals that can cause various issues in the aquatic ecosystem and subsurface habitat degradation. Several alternative technologies have been explored to enhance reverse osmosis water recovery, limit the reject volume that must be disposed, and eliminate contaminants prior to beneficial uses or discharge. This review examines reject management options and technologies, including disposal, treatment, and beneficial usage. A comparative study reviewing all the plausible methodologies practically employed to economically and feasibly treat varied types of reverse osmosis concentrate is currently unavailable. This review also examines the suitability of the different treatment technologies for different types of reverse osmosis concentrate. The review also identifies important hurdles to a larger usage of desalination procedures, especially for inland applications, by critically reviewing reject management systems, treatment technologies, and beneficial uses. At last, conclusion and future perspectives are provided for researchers working in this field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Membrane-based desalination has gained popularity as an alternative to ensure a safe drinking water supply in the last century (Labhasetwar and Yadav 2023). Reverse Osmosis (RO) is one of the most preferred desalination techniques, accounting for 44% of global desalination capacity and 80% of the 15,000 desalination units installed worldwide (Greenlee et al. 2009). Inland areas benefit from brackish/groundwater desalination utilizing RO, ensuring safe drinking water supply. However, the RO Feed Water Recovery (FWR) is limited due to the presence of soluble compounds such as silica, carbonates, and sulphates of barium and calcium (Boerlage et al. 2000; Ning et al. 2006; Rahardianto et al. 2008). Another major problem encountered in water desalination is the Reverse Osmosis Concentrate (ROC) management and their disposal, particularly for inland desalination applications (Burbano et al. 2007). ROC can harm the ecosystem by causing pH fluctuations, eutrophication, proliferation of hazardous metals that can cause a variety of issues in the aquatic ecosystem and subsurface habitat degradation (Xevgenos et al. 2015; Petersen et al. 2018). ROC minimization, direct disposal, and reuse are only a few well-known practices used to manage ROC. The development of the desalination process is limited due to issues related to ROC disposal while considering the development of urban water infrastructure. As a result, while selecting a ROC management approach, factors like the cost, environmental concerns, complexity of regulations and laws, energy use, ease of installation, and operation of involved, must be analysed (Xu et al. 2013).
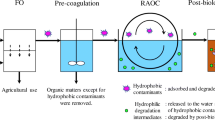
ROC treatment can be sustainably improved in three ways: add to the freshwater supply, reduce the amount of waste to be disposed, and extract valuable minerals. Conventional ROC disposal options are limited by environmental concerns such as dilution and required physical footprint of receiving water bodies. In the past, zero liquid discharge (ZLD) technologies were regarded as economically unrealistic and rarely put into practice (De Buren and Sharbat 2015). More cost-effective choices are now available due to the advancement in ZLD technologies. Membrane-based treatment techniques such as forward osmosis (FO) (Jamil et al. 2016), membrane distillation (MD) (Yadav et al. 2021a), electrodialysis (ED), and thermal-based technologies such as eutectic freeze crystallization (EFC) (van der Ham et al. 2004), wind-aided intensified evaporation (WAIV) (Gilron et al. 2003), multi-stage flash (Jeppesen et al. 2009) and multi-effect distillation are a few alternatives for ZLD or near-ZLD concentration management (Amutha 2017) (Fig. 1).
Several technologies have been investigated to enhance RO water recovery, limit the ROC volume that must be disposed, and recover minerals prior to discharge. These include chemical demineralization in the intermediate stage, followed by RO (Burbano et al. 2007; Gabelich et al. 2007; Qu et al. 2009b), biological removal, followed by RO (Hu et al. 2005; Crawley et al. 2012), and ED/ED Reversal (EDR) (Turek 2004; Shane Walker et al. 2014). The ROC conversion from waste to resource via these treatment methods may reduce cost and environmental hazards.
There are a few reviews on the treatment and management of ROC. However, they focus on solar evaporation (Subramani and Jacangelo 2014)(Subramani and Jacangelo 2014) and emerging technologies like hybrid desalination processes (Pérez-González et al. 2012; Lee et al. 2019) and crystallization treatment (Randall and Nathoo 2015). A comparative study reviewing all the plausible methodologies practically employed to economically and feasibly treat varied ROC is currently unavailable. This review examines the suitability of the different treatment technologies for ROC. The novelty of this review also lies in analysing the benefits and drawbacks of different treatment technologies. The purpose of this review is also to identify important hurdles to a larger usage of desalination procedures, especially for inland applications, by critically reviewing ROC management systems, treatment technologies, and beneficial use. This review focuses on the major treatment technologies for ROC treatment from the inland desalination plant. The review is divided into four sections, namely:
-
a.
Characteristics of ROC
-
b.
Challenges and opportunities of ROC on treatment technologies
-
c.
Benefits and drawbacks of various treatment technologies
-
d.
Conclusions and future perspectives
Characteristics of reverse osmosis concentrate
More than 15% of total treated water is discharged as ROC in RO technology. ROC contains a range of contaminants, including ammonia, sulphates, heavy metals, and metalloids, which have detrimental effects on humans, animals, and plants (Ersever et al. 2007a). Acids, biocides, biocide scavengers, antiscalants, antifoams, and corrosion inhibitors are typical chemicals employed in the feedwater pretreatment stage (membrane desalination), which also influence the physicochemical characteristics of ROC. Environmental factors such as temperature, pH, and ionic strength can significantly affect the amount of pollutants in desalination brine. Apart from this, the quality and amount of minerals/pollutants in the ROC also depend on the membrane pore size. Table 1 shows the characteristics of ROC from inland desalination plants. The pH of the ROC varies from 7 to 7.5, while the Total Dissolved Solids (TDS) varies from 2250 to 50,200. The temperature of the ROC varies from 24 to 28 °C, which depends upon the feed temperature (Xu et al. 2018; Kress et al. 2020). In general, the rate of ROC generation is between 20 and 30% of the sewage influent and between 5 and 20% for surface water. ROC contains high concentrations of TOC (> 40 mg L−1).
Challenges and opportunities for reverse osmosis concentrate treatment technologies
Biological treatment
Biological treatment methods are preferred over chemical treatments due to their cost-effectiveness and efficiency in meeting effluent regulations (Kim et al. 2016).
Biological denitrification
Biological denitrification, is the dissimulators’ microbiological conversion of nitrate (\({\text{NO}}_{3}^{ - }\)) and nitrite (\({\text{NO}}_{2}^{ - }\)) to gaseous nitrogen, where both \({\text{NO}}_{3}^{ - }\) and \({\text{NO}}_{2}^{ - }\) serve as electron acceptors in production of energy and the development of cells. Ersever et al. (2007a) employed biological nitrification–denitrification to remove nitrogen molecules from ROC using a high-rate Fluidized Bioactive Adsorber Reactor (FBAR). The experimental results showed a hydraulic retention period of 180 min. The nitrification process lowered the ammonia concentrations in the range of 90–110 mg N L−1 by over 95%. In another study by the same group, FBAR was used for sulphate and ammonia reduction and the removal of toxic metals and metalloids from ROC of a water reclamation plant (Ersever et al. 2007b). The elimination of ammonia was ~ 95%, with complete oxidation of \({\text{NO}}_{2}^{ - }\)–\({\text{NO}}_{3}^{ - }\) (Fig. 2).
Schematic of reverse osmosis concentrate nitrification, denitrification, and sulphate reduction processes. Reprinted with permission from (Ersever et al. 2007b)
Biological aerated filter (BAF)
The biological aerated filter (BAF) is a biological wastewater treatment device with a down/up flow, high rate, and fixed film design (Fig. 3). It is an effective method for removing organic waste and suspended particles (Choi and Lee 2015). Treating ROC with the BAF procedure effectively removes biological oxygen demand (BOD) (95.86%), while it proves less effective in removing chemical oxygen demand (COD) (88.95%) and suspended solids (81.12%) (Choi and Lee 2015). Kim et al. (2016) examined the effects of operational parameters such as load variation and water temperature for the nutrients and organic matters removal from ROC using the modified Ludzack-Ettinger and the sequencing batch reactor process. The sequencing batch reactor process showed better performance and is known for high load fluctuation conditions.
Schematic of the BAF process. Reprinted with permission from (Chang et al. 2009)
The biological treatment could be effective if one wishes to reuse the ROC. It can effectively reduce BOD and COD. These treatments depend upon operational parameters such as load variation and water temperature.
Electrodialysis/electrodialysis reversal
ED/EDR is based on ion-exchange membranes with electrochemical potential as a driving force (Acevedo et al. 2010). ED works by limiting ion movement using ion-selective membranes and leveraging increased ionic mobilities under an applied voltage (Tanaka 2007). ROC of lower COD is particularly well suited for this application (Virapan and Murugaiyan 2017). The feasibility of the ED process for ROC to produce salt and freshwater was studied by Jiang et al. (2014). Moreover, the effect of membrane characteristics, ROC content, direct current density, and mode of operation on desalination efficiency and the water recovery rate was explored using ion-exchange membranes. They achieved the FWR and desalination rates of 67.78% and 72.47%, respectively. The ion-exchange process was utilized by Acevedo (Acevedo et al. 2010) to remove silica from ROC. The silica removal was highly dependent on pH; the higher solubility was obtained below 2 and above 5.8 pH. They achieved a removal efficiency of 61% for silica removal. Zhang et al. (2009) used ED process to remove the nutrient ions and organic compounds from ROC. They achieved a high removal rate for nitrates and phosphate ions. Walker et al. (2014) evaluated the sensitivity of ED process while treating ROC (7.9–18.6 g L−1) with voltage variations and different membranes and recovered more than 78% of salts without any precipitation. The system showed a recovery of 95%. Korngold et al. (2009) utilized the ED process for concentrating ROC saturated with CaSO4 and/or silica from 1.5 to 10%. The ED process decreased the concentration of ROC to 18–20 mN with a recovery rate of 98%, which confirms its suitability for ROC treatment. Oren et al. (2010) investigated the performance of the EDR for brackish water RO brine and reported that the process efficiency improved with minimization of brine volume.
Due to its excellent energy efficiency, anti-scaling, anti-fouling properties, capacity to produce highly concentrated ROC, and ease of operation, ED/EDR are a feasible option to treat ROC. The EDR method can be used to improve RO recovery and minimize brine volume. When the ROC has high conductivity, ED using selective ion-exchange membranes or bipolar membrane ED can also be a promising treatment technique.
Forward osmosis
Due to the application of natural osmotic pressure to draw solutes, FO is a potential solution for improving FWR and dewatering ROC (Kazner et al. 2014) (Jamil et al. 2016). However, the regeneration of draw solutes from diluted draw solutions is a critical issue in the FO process as this process becomes energy intensive if the proper draw solutes and recycling process are not utilized. There are various studies available on draw solution recovery (McCutcheon et al. 2006; Bai et al. 2011; Su et al. 2012). FO or pressure-aided FO (PAFO) could be utilized for volume minimization, safe discharge of ROC and its reuse. Jamil et al. (2016) used PAFO (Fig. 4) to process the ROC from water reclamation plant. Prior to applying PAFO, organic fouling and scaling were reduced using pretreatment with granular activated carbon (GAC) and softening by HCl. Compared to raw ROC, the total organic carbon (TOC) and total inorganic carbon (TIC) were reduced by 90% and 85%, respectively. Moreover, 12 out of 14 organic micropollutants were below the detection limit from the treated ROC. They concluded that this process could be utilized for ROC volume reduction operating for long-term and with high-quality water and salts, in addition to the safe water discharge. Kazner et al. (2014) investigated the conditions under which FO can treat the ROC from water reclamation plants to recover useful salts. They reported that the scaling during the FO process could be controlled with the pH adjustment. Bell et al. (2017) evaluated the fouling behaviour and performance of the cellulose triacetate and polyamide thin-film composite membranes in the FO process. Both membranes rejected neutral hydrophobic substances by over 90%.
Schematic of a bench-scale PAFO system. Reprinted with permission from (Jamil et al. 2016)
Enhancing water recovery and dewatering ROC with FO is a feasible alternative and high-quality water and salts can be achieved. The pH adjustment controls the scaling during the FO process. Moreover, the fouling during ROC treatment can also be controlled by the surface charge of the membranes.
Nanofiltration
Nanofiltration (NF) is a pressure-driven membrane technique with a molecular weight cut-off between RO and ultrafiltration (Waite et al. 2005). Compared to RO technique, NF has various advantages, including lower operating pressures, high flux, cost-effectiveness, and lower operational and maintenance expenses (Llenas et al. 2011). Very recently, Ali (2021) employed NF process for the removal of divalent ions and reduced the load on subsequent RO membrane stages while treating ROC (Fig. 5). The results demonstrated that by utilizing the NF process, there was 79–89% of TDS and 96–98% of total hardness could be retained, while with CaSO4, Na2SO4, MgSO4, MgCl2, and NaCl salts concentration as 97.4, 97.3, 95.2, 93.4, and 79%, respectively. Istirokhatun et al. (2018) studied the performance of NF process for the removal of antiscalants from the ROC. Mousavi and Kargari ( 2022) investigated the feasibility of the NF process for treating the ROC and reported that the increased feed pressure and feed flow rate increased the rejection and permeate flux. Torma and Cséfalvay (2018) used NF process and achieved 41.5–66.6%, and > 96.6% recovery of the NaCl and Na2SO4, respectively.
Schematic of NF system. Reprinted with permission from (Ali 2021)
The ROC can be effectively treated by NF membranes. However, the performance of NF membranes while treating ROC depends upon the feed pressure and flow rate. Moreover, the NF of ROC can significantly reduce the RO load while mixing permeate of NF with RO feed.
Membrane distillation
MD utilizes a hydrophobic membrane and is a thermally-driven process in which water vapour is transported through membrane pores to permeate side (Yadav et al. 2021b). Furthermore, it is less reliant on the feed’s initial salinity and has a greater salt rejection ratio. Qu et al. (2009a) explored the feasibility of Direct Contact MD (DCMD) using hydrophobic polyvinylidene fluoride for ROC treatment. The overall water recovery increased to 98.8% from 50%. As a pretreatment, membrane fouling by calcium scaling was controlled using acidification and rapid precipitation softening. The primary ROC was concentrated by 40 times by preventing membrane fouling, and the overall recovery was increased to 98.8% (Qu et al. 2010). Yan et al. (2017) used a polyvinylidene fluoride membrane in the DCMD process (Fig. 6) to treat synthetic ROC at various feed temperatures and flow rates. A permeate conductivity of less than 11 µS cm−1 and a recovery rate of more than 70% were recorded. The flux reversibility varied from 88 to 96% (Fig. 7), indicating that the flushing could efficiently remove scaling.
DCMD experimental set-up. Reprinted with permission from (Yan et al. 2017)
Feed temperature vs flux reversibility during ROC treatment (feed temperature; 50 °C and feed velocity; 0.25 m s−1). Reprinted with permission from (Yan et al. 2017)
Sanmartino et al. (2017) utilized the DCMD process to treat ROC and suggested that the chemical pretreatment of the ROC would be effective in increasing DCMD performance. As a result, the volume of discharged ROC can be significantly reduced, allowing for more effective management. Mericq et al. (2010) studied Vacuum MD (VMD) for the treatment of ROC (300 g L−1) with optimized operating conditions by simulations. High permeate flux was achieved even at very high salt concentrations. However, the calcium precipitation was observed at high concentrations, but the permeate flux was not decreased significantly. Temperature and concentration polarization showed less effect on permeate flux, and no organic or biofouling was observed even after 6–8 h of operation, attributed to membrane hydrophobicity.
Owing to the advantage of 100% theoretical rejection of the volatile compounds, the MD process can be effectively utilized as the treatment of the ROC for recovery of the fresh water and the valuable minerals. One more advantage of the MD process over other membrane processes is the less organic fouling as it is a thermal separation process.
Adsorption
Adsorption is a cost-effective and efficient process for removing various contaminants from wastewater and ROC (Al-Absi et al. 2021). Cost-effectiveness, accessibility, thermal and chemical stability, regeneration ability, high effective surface area, and ease of use are factors that researchers consider when choosing an adsorbent for a particular pollutant (Al-Saad et al. 2019). The unwanted competitive adsorption of organic matter in ROC can be avoided by utilizing high-silica zeolite (Jiang et al. 2018). Woo et al. (2019) designed and improved the performance of an adsorption desalination system (prototype) in which the adsorbent was fabricated by alumina silica gel. The system’s performance was independent of the ROC content, and the high-quality ultra-pure freshwater was recovered. To increase water recovery, Tao et al. (2011) designed a cost-effective ROC treatment process. Organic matter in the ROC was shown to be resistant to biodegradation, and biological activated carbon (BAC) was found to remove 15–27% of the TOC from the ROC. Jamil et al. (2019) compared the performance of the GAC and ion exchange resin adsorbents for ROC treatment for the removal of dissolved organic matter.
Adsorption is a cost-effective process for treating the ROC, which effectively adsorbs valuable minerals from the ROC. The most commonly used adsorbents are alumina, activated carbon, ion-exchange resins, silica gels, chitosan, and zeolites. The removal of organic micropollutants is possible with silica-based adsorbent.
Advanced oxidation processes
AOP can create extremely reactive hydroxyl radicals at normal room temperature and pressure, which are extremely suitable for destroying a broad spectrum of resistant organic molecules. The subsequent subsections cover a variety of AOP that can be used in ROC treatment.
Photocatalysis and photo-oxidation
Photo-oxidation involves a chain reaction involving numerous chemical processes in which the absorption of a photon results in the breakdown of free-radical products. In this process, the degradation of the organic compounds is controlled with the pH adjustment of the ROC, photocatalyst composition and energy bandgap (Tawfik et al. 2022). Several recent studies have focused on using UV/TiO2 and UV/ZnO to remove organic load from ROC (Zhou et al. 2011; Cemre Birben and Bekbolet 2019). Heterogeneous photocatalysis with TiO2 as a catalyst, among the several AOPs, has the benefit of allowing sunlight to be used. The use of TiO2 and ZnO, N-doped TiO2 ZnO, and TiO2/ZnO nanocomposite to degrade organic load from ROC was studied (Cemre Birben and Bekbolet 2019). The photocatalysts successfully demineralized municipal wastewater’s emergent pollutants in ROC in 60 min of operation (up to 85% removal of non-purgeable organic carbon) and in 6 h, about 95% of the organic matter was removed (Zhou et al. 2011).
Removal of organic compounds from the ROC is possible with the photocatalysis and photo-oxidation process. The use of TiO2 and ZnO, N-doped TiO2 ZnO, and TiO2/ZnO nanocomposite enhances the process efficiency of the degradation of the organic compounds from the ROC.
Electrochemical oxidation
Electricity is used in electrochemical water treatment systems to cause the removal of dissolved pollutants from water and can also treat ROC. Bagastyo et al. (2011b) examined the electrochemical oxidation of five Ti-coated IrO2–Ta2O5, RuO2–IrO2, Pt–IrO2, PbO2, and SnO2–Sb electrode materials as an anode to treat ROC formed during the advanced water treatment. The Ti/Pt–IrO2 anodes showed the best oxidation performance, followed by the Ti/SnO2–Sb and Ti/PbO2 anodes. Electrochemical oxidation using boron-doped diamond film as an electrode can oxidize substances in two ways: the direct transfer of electrons at the surface of the electrodes and hydroxyl radicals produced by water oxidation (Zhi et al. 2003). Van Hege et al. (2002) used electro-oxidative treatment for RO using boron-doped diamond as anode and Ti as cathode electrodes. Apart from this, the organic content and total ammonia nitrogen reduced greatly. Chaplin et al. (2010) used boron-doped diamond film electrodes to degrade N-nitrosodimethylamine in ROC. They studied the influence of dissolved organic carbon, Cl−, \({\text{HCO}}_{3}^{ - }\), and hardness on the N-nitrosodimethylamine degradation rate. The results indicated that the dissolved organic carbon, Cl− or \({\text{HCO}}_{3}^{ - }\) did not affect the degradation rate. Moreover, adding dissolved organic carbon and hydroxyl radicals to the ROC does not affect the degradation kinetics.
Capacitive deionization
The Capacitive Deionization (CDI) method removes inorganic chemicals from various water sources, including saltwater, groundwater, discharged water from industries, and wastewater (Pan et al. 2020). There are three phases involved in the CDI process cycle: purification, regeneration, and purge. In practice, the CDI method can recover more than 85% of the water (Lee et al. 2009a). Ng et al. (2008) used a biological activated carbon (BAC) column followed by the CDI for organic and inorganic removals. The bench-scale investigation revealed that employing BAC for 40 min of empty bed contact time removed 20% TOC, while the CDI method removed more than 90% of the conductivity (2.19 mS cm−1 to 164 µS cm−1). In the integrated BAC pretreatment and CDI process, removal efficiencies of 23.5% and 39.9% were achieved for TOC removal with BAC and BAC–ultrafiltration pretreatments, respectively. By lowering the pH (6.5) of the raw ROC, membrane and CDI fouling were minimized, resulting in increased operating duration (two times). TDS and ion removals were over 88 and 87%, respectively, for the CDI method, while PO4−3 and TOC removals were 52–81% and 50–63%, respectively (Lee et al. 2009a). In another study, Lee et al. (2009b) improved the biodegradability of ROC using CDI and ozone-BAC as a pretreatment method (Fig. 8). Using only ozonation, the average removal of COD and TOC was 64.9 ± 11.3% and 26.3 ± 7.5%, respectively, from the ROC. While using the combination ozone-BAC process, the removal of COD and TOC was 88.7 ± 9.1% and 69.8 ± 8.1%, respectively, from the ROC. Thus the combined pretreatment via ozone and BAC has a significant potential for decreasing fouling associated with the CDI and is a dedicated RO process for concentrate treatment.
Schematic representation of the system for treatment of ROC using a ozonation, b combined ozonation, BAC column, and CDI unit. Reprinted with permission from (Lee et al. 2009b)
Coagulation
In coagulation, the negatively charged dissolved organic molecules bind to the positively charged coagulants (Jiang 2015; Yadav and Sinha 2022). The elimination of organics and colour from the ROC treated with ferric chloride coagulation was studied by Bagastyo et al. (2011a). The optimum dose of FeCl3 was 1.48 mM for 5 pH of ROC. The coagulation was efficient for colour removal (~ 79%) but not for nitrogen removal (~ 27%). Therefore, from the above studies, it is clear that coagulation can be a viable option for treating ROC.
Crystallization techniques
Crystallization is a technique for separating solids from liquids in which the solute crystallizes from the liquid feed and transforms into a pure crystalline solid (Randall et al. 2011). As a result, crystallization techniques are excellent for recovering both water and salt. Super saturation is the driving force for crystallization. The subsections cover major crystallization techniques used for ROC treatment.
Eutectic freeze crystallization
EFC works by lowering the stream temperature to the eutectic temperature when both salt and ice will crystallize. When ROC is cooled to the eutectic point, ice and salt crystals are separated, which can be easily separated due to density inequalities (Salvador Cob et al. 2014). EFC has several advantages over traditional separation procedures, including low energy usage, high-quality products, and the absence of extra chemicals. Salvador et al. (2014) examined NF, RO and EFC for ROC treatment and cation exchange to increase the recovery. When EFC was applied to the ROC, it formed ice and NaHCO3 (5.8%) at 3.9 °C. Randall et al. (2011) used EFC to treat the ROC obtained from a RO plant. Using EFC, the ROC was converted into ice, CaSO4 (98% purity), and NaSO4 (96.4% purity) with a total conversion rate of 99.9%. Multiple pure salts produced at their respective crystallization temperatures can avoid the major difficulties of a mixed salt product. Therefore, the EFC can be the final stage in the recovery processes from a saltwater mixture.
Membrane crystallization
In the membrane crystallization (MCr) process, the solution is supersaturated to achieve both solution separation and component solidification (Jiang et al. 2021) (Fig. 9). Owing to the advantage of the evaporation of only volatiles across the microporous membrane pores, the MCr process concentrates feed solutions (i.e. ROC) to supersaturation (Macedonio et al. 2013). Tun et al. (2005) successfully treated the highly salty water containing Na2SO4 and NaCl with MCr. Ji et al. (2010) studied the performance of an MCr on ROC emanating from seawater RO. The treatment of synthetic ROC resulted in a water recovery factor of 90% in addition to 21 kg m−3 of NaCl with cubic shape crystals and size in the range of 20–200 µm. During the treatment of real ROC, the dissolved organic matter reduced the quantity of salt crystallized and transmembrane flux by 20 and 8%, respectively. The growth rate of NaCl crystals developed from real seawater ROCs ranged from 0.8108 to 2.8108 m s−1, which was 15–23% slower than the rate of growth of NaCl crystals grown from synthetic ROC. Naidu et al. (2017) studied the feasibility of MCr for the treatment of ROC; they achieved 85% water recovery. The organics compound in the ROC decreased the membrane hydrophobicity resulting in a decrement of flux due to CaCO3 deposition on the membranes. Park et al. (2013) explored the MDCr for freshwater and salt recovery (CaSO4) from the ROC. However, the deposition of the CaSO4 crystals over the membrane declined the flux. Guan et al. (2012) proposed an MCr system for ROC treatment and achieving ZLD. They concluded that the feed flow rate must be high to avoid blockage membrane module. MCr can be the promising technology for water recovery and valuable slats from high TDS water, such as ROC, which contains valuable salts.
Membrane crystallizer setup. Reprinted with permission from (Yadav et al. 2022)
Vibratory shear-enhanced process
In vibratory shear-enhanced processing (VSEP), torsional vibration is applied to a membrane to improve separation and lessen membrane fouling (Leong et al. 2016). Solids and foulants rise off the membrane surface at high shear rates. Subramani et al. (2012) employed VSEP to treat the ROC to increase FWR. However, due to silica polymerization over the membrane surface, the FWR was limited (< 75%), with the flux in the range of 50–100 L m−2 h−1. Arola et al. (2019) achieved an overall recovery rate of up to 99.7% with VSEP and a cross-rotational filter while treating ROC from municipal wastewater. The overall FWR improved using the VSEP technique. Hence, VSEP enables the pathway to reduce more volume of ROC and increase recovery.
Evaporation techniques
Evaporation has been extensively applied for ROC treatment since they produce decontaminated water that may be discharged or even reused and solid waste that is easier to manage than the original waste (Arnal et al. 2005). Vyas et al. (2022) reviewed evaporation techniques for the valuable use of ROC by-products (salts) and the technical possibility of isolating them with the appropriate shape and purity. One strategy for ROC treatment that is commonly used is solar evaporation, particularly in the arid and semi-arid region’s inland desalination plants (Ahmed et al. 2000). A shallow line pond was used by Huang et al. (1999) for the ROC treatment using solar energy to evaporate water naturally. Arnal et al. (2005) investigated the viability of employing natural evaporation (without heat) to ROC from brackish desalination facilities as an alternative to classical evaporation. They reported that adsorbents could improve evaporation because adsorbents increase the surface area for evaporation. Wind-aided intensified evaporation (WAIV) is a unique alternative technique of natural evaporation (Hoque et al. 2010). WAIV is a less land-intensive way of reducing ROC by utilizing the drying force of the wind while avoiding the formation of microscopic droplets that might cause salt drift. In WAIV, ROC is recirculated in falling film over vertical hydrophilic surfaces generally positioned similarly to the wind direction (Macedonio et al. 2011). These hydrophilic surfaces cool near wet-bulb temperatures when exposed to dry winds. The schematic of WAIV shown in Fig. 10.
Hybrid processes
A hybrid process combines two or more techniques that improve the efficiency of the resulting treatment process. For the purification of ROC, several hybrid processes have been used. The most common hybrid process combines the membrane-based processes with other treatment processes, such as ozonation, coagulation, etc., which are applied before the membrane processes to get the desired efficiency in the resultant combination (Stylianou et al. 2015). AzadiAghdam et al. (2020) examined the effectiveness of the ED combined with FBC, ultrafiltration and coagulation/flocculation via ferric chloride. The treatments were found to be effective for the removal of calcium (84%), barium (93%), magnesium (> 99%) ions, as well as TOC (80%). During the treatment of primary ROC, accelerated precipitation softening was combined with a hydrophobic membrane in the DCMD process for drinking water supply during the 29th Olympic Games with high recovery (50%) (Qu et al. 2009b). Sodium hydroxide dosing induced and accelerated mineral precipitation, followed by solid–liquid separation, microfiltration, and DCMD. Elazhar et al. (2021) looked into the potential of using a hybrid NF-RO technique to remove hardness from brackish water with a greater recovery rate and less ROC. Liu et al. (2016) designed an NF–ED integrated system (Fig. 11) to separate monovalent and bivalent ions and concentrate the solution. The results revealed that the operating pressure and concentration of the feed solution have a significant impact on the ions rejection ratios and permeate flux. The maximum NaCl concentration in the concentrating cell was 160 g L−1, with a 70% NaCl recovery, whereas the combined concentration of K+, Ca2+, and Mg2+ was ~ 5 g L−1.
Schematic of NF–ED process. Reprinted with permission from (Liu et al. 2016)
For the treatment of ROC containing 19 organic micropollutants, Shanmuganathan et al. (2017) employed a hybrid system of submerged membrane filtration and GAC adsorption (Fig. 12). During a 10-day operation, 60–80% of the dissolved organic carbon was removed. Two micropollutants, namely, diethyltoluamide (less hydrophobic) and sulfamethoxazole (hydrophilic), were detected at 27 and 35 ng L−1 in the treated solution.
Martinetti et al. (2009) studied vacuum-enhanced DCMD (VE-DCMD) combined with FO as potential processes for ROC treatment and FWR maximization. They examined two streams of the ROC with TDS (7.5 and 17.5 g L−1) with a draw solution (50 g L−1 NaCl). The FO and VE-DCMD processes independently recovered 90% and 81% water, respectively, from the ROCs, while the FWR with VE-DCMD + FO was 96 and 98% for the two streams of ROCs, respectively. For high saline concentrate treatment, osmotically assisted RO (OARO) has recently been proposed (Bartholomew et al. 2017). The OARO (RO + FO) showed 35–50% FWR and 6–19 kW h energy consumption per m−3 of purified water with a feed solution containing 100–140 g L−1 of NaCl.
Schematic of hybrid of submerged membrane system and GAC adsorption. Reprinted with permission from Shanmuganathan et al. (2017)
Benefits and drawbacks of ROC treatment technologies
There are several technologies available for treating ROC that have been discussed in previous sections. Each technique has its own set of benefits and drawbacks. However, some drawbacks associated with particular treatment technology can be eliminated by integrating other technologies or strategies. Table 2 compares various treatment technologies by listing their advantages and associated limitations encountered when employed.
Conclusions and future perspectives
The search for environmentally sustainable management methods has become a technological issue for the direct discharge of ROC from inland desalination plants. Several technologies and treatment processes are available to treat ROC from the inland desalination plant, which include biological treatment, adsorption, AOPs, membrane-based technology, and crystallization technologies. High-energy requirements and the adverse environmental impact are two alarming concerns of ROC discharge that must be tackled in future investigations and developments of ROC treatment, as these two challenges cannot be addressed separately. Increased public concerns about adverse environmental impacts, energy footprints and stricter discharge rules make disposal more complex and complicate the approval procedure. To overcome these problems, treatment of ROC from the inland desalination plant is necessary, and this article helps to select the best treatment method for different characteristics of ROC. Hence, ROC’s effective use should be prioritized. Reducing the volume of water or salt content in the ROC is a way to minimize the negative impacts.
Due to their simplicity and low operational costs, evaporation ponds are a solution. However, the evaporation rate is so low in moist regions that it is ineffectual to process huge volumes, which necessitates a large quantity of land. Compared to evaporation ponds, WAIV technology requires less land, although its availability has been shown only on a small scale. The ponds are also unsuitable for huge concentrations of ROC. Crystallization technology has progressed to an industrial level. However, the cost of energy is prohibitive at this time. Crystallization techniques can be combined with solar ponds or other residual heat sources. ED has been developed on an industrial scale to ROC using electricity solely as energy source, making them compatible with photovoltaic panels. However, when the ROC gets highly concentrated, ED works worse than other technologies due to scaling and less production of electric fields.
While evaluating potential ROC minimization technologies, the end-user must consider ROC characteristics, ROCs water recovery, disposal options, regulatory laws, infrastructure, and space. Several hybrid systems are being developed for ROC treatment and show promising results, but further testing is needed to ensure long-term operational reliability.
Data availability
The authors confirm that this article contains all the data supporting the findings of this study.
References
Acevedo CR, Gardea-Torresdey JL, Tarquin AJ (2010) Silica removal from brine by using ion exchange. World environmental and water resources congress 2010. American Society of Civil Engineers, Reston, pp 3529–3541
Ahmed M, Shayya WH, Hoey D et al (2000) Use of evaporation ponds for brine disposal in desalination plants. Desalination 130:155–168. https://doi.org/10.1016/S0011-9164(00)00083-7
Ahmed M, Arakel A, Hoey D et al (2003) Feasibility of salt production from inland RO desalination plant reject brine: a case study. Desalination 158:109–117. https://doi.org/10.1016/S0011-9164(03)00441-7
Al-Absi RS, Abu-Dieyeh M, Al-Ghouti MA (2021) Brine management strategies, technologies, and recovery using adsorption processes. Environ Technol Innov. https://doi.org/10.1016/j.eti.2021.101541
Ali MEA (2021) Nanofiltration process for enhanced treatment of RO brine discharge. Membranes (basel) 11:212. https://doi.org/10.3390/membranes11030212
Al-Saad K, El-Azazy M, Issa AA et al (2019) Recycling of date pits into a green adsorbent for removal of heavy metals: a fractional factorial design-based approach. Front Chem. https://doi.org/10.3389/fchem.2019.00552
Amutha K (2017) Sustainable chemical management and zero discharges. In: Muthu SS (ed) Sustainable fibres and textiles. Elsevier, Amsterdam, pp 347–366
Arnal JM, Sancho M, Iborra I et al (2005) Concentration of brines from RO desalination plants by natural evaporation. Desalination 182:435–439. https://doi.org/10.1016/j.desal.2005.02.036
Arola K, Van der Bruggen B, Mänttäri M, Kallioinen M (2019) Treatment options for nanofiltration and reverse osmosis concentrates from municipal wastewater treatment: a review. Crit Rev Environ Sci Technol 49:2049–2116. https://doi.org/10.1080/10643389.2019.1594519
AzadiAghdam M, Achilli A, Snyder SA, Farrell J (2020) Increasing water recovery during reclamation of treated municipal wastewater using bipolar membrane electrodialysis and fluidized bed crystallization. J Water Process Eng 38:101555. https://doi.org/10.1016/j.jwpe.2020.101555
Bagastyo AY, Keller J, Batstone DJ (2011a) Size fractionation characterisation of removed organics in reverse osmosis concentrates by ferric chloride. Water Sci Technol 63:1795–1800. https://doi.org/10.2166/wst.2011.379
Bagastyo AY, Radjenovic J, Mu Y et al (2011b) Electrochemical oxidation of reverse osmosis concentrate on mixed metal oxide (MMO) titanium coated electrodes. Water Res 45:4951–4959. https://doi.org/10.1016/j.watres.2011.06.039
Bai H, Liu Z, Sun DD (2011) Highly water soluble and recovered dextran coated Fe3O4 magnetic nanoparticles for brackish water desalination. Sep Purif Technol 81:392–399. https://doi.org/10.1016/j.seppur.2011.08.007
Bartholomew TV, Mey L, Arena JT et al (2017) Osmotically assisted reverse osmosis for high salinity brine treatment. Desalination 421:3–11. https://doi.org/10.1016/j.desal.2017.04.012
Bell EA, Poynor TE, Newhart KB et al (2017) Produced water treatment using forward osmosis membranes: evaluation of extended-time performance and fouling. J Memb Sci 525:77–88. https://doi.org/10.1016/j.memsci.2016.10.032
Boerlage ŚF, Kennedy MD, Bremere I et al (2000) Stable barium sulphate supersaturation in reverse osmosis. J Memb Sci 179:53–68. https://doi.org/10.1016/S0376-7388(00)00504-4
Burbano AA, Adham SS, Pearce WR (2007) The state of full-scale RO/NF desalination-results from a worldwide survey. J Am Water Works Assoc 99:116–127. https://doi.org/10.1002/j.1551-8833.2007.tb07912.x
De Buren L, Sharbat A (2015) Inland desalination and brine management: Salt recovery and beneficial uses of brine. World environ water resour congr 2015 floods, droughts, ecosyst—proc 2015 world environmental and water resources congress 2015, p1219–1230. https://doi.org/10.1061/9780784479162.120
Cemre Birben N, Bekbolet M (2019) Role of emerging contaminants on solar photocatalytic treatment of organic matter in reverse osmosis concentrate. Catal Today 326:101–107. https://doi.org/10.1016/j.cattod.2018.10.048
Chang WS, Tran HT, Park DH, Zhang RH, Ahn DH (2009) Ammonium nitrogen removal characteristics of zeolite media in a Biological Aerated Filter (BAF) for the treatment of textile wastewater. J Ind Eng Chem 15(4):524–528. https://doi.org/10.1016/j.jiec.2009.01.009
Chaplin BP, Schrader G, Farrell J (2010) Electrochemical destruction of N -Nitrosodimethylamine in reverse osmosis concentrates using boron-doped diamond film electrodes. Environ Sci Technol 44:4264–4269. https://doi.org/10.1021/es903872p
Choi HJ, Lee SM (2015) Treatment of reverse osmosis concentrate by biological aerated filter. Desalin Water Treat 53:1188–1195. https://doi.org/10.1080/19443994.2013.850449
Choi JY, Kaufmann F, Rahardianto A, Cohen Y (2021) Desupersaturation of RO concentrate and gypsum removal via seeded precipitation in a fluidized bed crystallizer. Water Res 190:116766. https://doi.org/10.1016/j.watres.2020.116766
Crawley J, Jackson WA, Anderson T et al (2012) Evaluating RO performance with biological pretreatment of graywater. J Water Reuse Desalin 2:109–120. https://doi.org/10.2166/wrd.2012.075
de Luna MDG, Rance DPM, Bellotindos LM, Lu M-C (2017) Removal of sulfate by fluidized bed crystallization process. J Environ Chem Eng 5:2431–2439. https://doi.org/10.1016/j.jece.2017.04.052
Elazhar F, Elazhar M, El-Ghzizel S et al (2021) Nanofiltration-reverse osmosis hybrid process for hardness removal in brackish water with higher recovery rate and minimization of brine discharges. Process Saf Environ Prot 153:376–383. https://doi.org/10.1016/j.psep.2021.06.025
Ersever I, Ravindran V, Pirbazari M (2007a) Biological denitrification of reverse osmosis brine concentrates: I. Batch reactor and chemostat studies. J Environ Eng Sci 6:503–518. https://doi.org/10.1139/S07-021
Ersever I, Ravindran V, Pirbazari M (2007b) Biological denitrification of reverse osmosis brine concentrates: II. Fluidized bed adsorber reactor studies. J Environ Eng Sci 6:519–532. https://doi.org/10.1139/S07-009
Gabelich CJ, Williams MD, Rahardianto A et al (2007) High-recovery reverse osmosis desalination using intermediate chemical demineralization. J Memb Sci 301:131–141. https://doi.org/10.1016/j.memsci.2007.06.007
Gilron J, Folkman Y, Savliev R et al (2003) WAIV—wind aided intensified evaporation for reduction of desalination brine volume. Desalination 158:205–214. https://doi.org/10.1016/S0011-9164(03)00453-3
Greenlee LF, Lawler DF, Freeman BD et al (2009) Reverse osmosis desalination: water sources, technology, and today’s challenges. Water Res 43:2317–2348. https://doi.org/10.1016/j.watres.2009.03.010
Guan G, Wang R, Wicaksana F et al (2012) Analysis of membrane distillation crystallization system for high salinity brine treatment with zero discharge using aspen flowsheet simulation. Ind Eng Chem Res 51:13405–13413. https://doi.org/10.1021/ie3002183
Hoque S, Alexander T, Gurian PL (2010) Innovative technologies increase evaporation pond efficiency. IDA J Desalin Water Reuse 2:72–78. https://doi.org/10.1179/ida.2010.2.1.72
Hu JY, Song LF, Ong SL et al (2005) Biofiltration pretreatment for reverse osmosis (RO) membrane in a water reclamation system. Chemosphere 59:127–133. https://doi.org/10.1016/j.chemosphere.2004.09.096
Huang H, Shi M, Ge X (1999) The effect of a black insulation sheet on the evaporation rate from a shallow salt pond. Int J Energy Res 23:31–39. https://doi.org/10.1002/(SICI)1099-114X(199901)23:1%3c31::AID-ER449%3e3.0.CO;2-F
Istirokhatun T, Dewi MN, Ilma HI, Susanto H (2018) Separation of antiscalants from reverse osmosis concentrates using nanofiltration. Desalination 429:105–110. https://doi.org/10.1016/j.desal.2017.12.018
Jamil S, Jeong S, Vigneswaran S (2016) Application of pressure assisted forward osmosis for water purification and reuse of reverse osmosis concentrate from a water reclamation plant. Sep Purif Technol 171:182–190. https://doi.org/10.1016/j.seppur.2016.07.036
Jamil S, Loganathan P, Kandasamy J et al (2019) Removal of dissolved organic matter fractions from reverse osmosis concentrate: comparing granular activated carbon and ion exchange resin adsorbents. J Environ Chem Eng 7:103126. https://doi.org/10.1016/j.jece.2019.103126
Jeppesen T, Shu L, Keir G, Jegatheesan V (2009) Metal recovery from reverse osmosis concentrate. J Clean Prod 17:703–707. https://doi.org/10.1016/j.jclepro.2008.11.013
Ji X, Curcio E, Al Obaidani S et al (2010) Membrane distillation-crystallization of seawater reverse osmosis brines. Sep Purif Technol 71:76–82. https://doi.org/10.1016/j.seppur.2009.11.004
Jiang J-Q (2015) The role of coagulation in water treatment. Curr Opin Chem Eng 8:36–44. https://doi.org/10.1016/j.coche.2015.01.008
Jiang C, Wang Y, Zhang Z, Xu T (2014) Electrodialysis of concentrated brine from RO plant to produce coarse salt and freshwater. J Memb Sci 450:323–330. https://doi.org/10.1016/j.memsci.2013.09.020
Jiang N, Shang R, Heijman SGJ, Rietveld LC (2018) High-silica zeolites for adsorption of organic micro-pollutants in water treatment: a review. Water Res 144:145–161. https://doi.org/10.1016/j.watres.2018.07.017
Jiang X, Shao Y, Sheng L et al (2021) Membrane crystallization for process intensification and control: a review. Engineering 7:50–62. https://doi.org/10.1016/j.eng.2020.06.024
Kazner C, Jamil S, Phuntsho S et al (2014) Forward osmosis for the treatment of reverse osmosis concentrate from water reclamation: process performance and fouling control. Water Sci Technol 69:2431–2437. https://doi.org/10.2166/wst.2014.138
Kim IH, Il LS, Kim DK (2016) Biological treatment of reverse osmosis concentrate from low salinity water. Desalin Water Treat 57:7667–7678. https://doi.org/10.1080/19443994.2015.1043593
Korngold E, Aronov L, Daltrophe N (2009) Electrodialysis of brine solutions discharged from an RO plant. Desalination 242:215–227. https://doi.org/10.1016/j.desal.2008.04.008
Kress N, Gertner Y, Shoham-Frider E (2020) Seawater quality at the brine discharge site from two mega size seawater reverse osmosis desalination plants in Israel (Eastern Mediterranean). Water Res 171:115402. https://doi.org/10.1016/j.watres.2019.115402
Labhasetwar PK, Yadav A (2023) Membrane based point-of-use drinking water treatment systems. IWA Publishing, London
Lee LY, Ng HY, Ong SL et al (2009a) Integrated pretreatment with capacitive deionization for reverse osmosis reject recovery from water reclamation plant. Water Res 43:4769–4777. https://doi.org/10.1016/j.watres.2009.08.006
Lee LY, Ng HY, Ong SL et al (2009b) Ozone-biological activated carbon as a pretreatment process for reverse osmosis brine treatment and recovery. Water Res 43:3948–3955. https://doi.org/10.1016/j.watres.2009.06.016
Lee S, Choi J, Park YG et al (2019) Hybrid desalination processes for beneficial use of reverse osmosis brine: current status and future prospects. Desalination 454:104–111. https://doi.org/10.1016/j.desal.2018.02.002
Leong J, Tan J, Heitz A, Ladewig BP (2016) Use of vibratory shear enhanced processing to treat magnetic ion exchange concentrate: a techno-economic analysis. Desalination 383:46–52. https://doi.org/10.1016/j.desal.2016.01.002
Liu J, Yuan J, Ji Z et al (2016) Concentrating brine from seawater desalination process by nanofiltration-electrodialysis integrated membrane technology. Desalination 390:53–61. https://doi.org/10.1016/j.desal.2016.03.012
Llenas L, Martínez-Lladó X, Yaroshchuk A et al (2011) Nanofiltration as pretreatment for scale prevention in seawater reverse osmosis desalination. Desalin Water Treat 36:310–318. https://doi.org/10.5004/dwt.2011.2767
Macedonio F, Katzir L, Geisma N et al (2011) Wind-Aided Intensified Evaporation (WAIV) and Membrane Crystallizer (MCr) integrated brackish water desalination process: advantages and drawbacks. Desalination 273:127–135. https://doi.org/10.1016/j.desal.2010.12.002
Macedonio F, Quist-Jensen CA, Al-Harbi O et al (2013) Thermodynamic modeling of brine and its use in membrane crystallizer. Desalination 323:83–92. https://doi.org/10.1016/j.desal.2013.02.009
Martinetti CR, Childress AE, Cath TY (2009) High recovery of concentrated RO brines using forward osmosis and membrane distillation. J Memb Sci 331:31–39. https://doi.org/10.1016/j.memsci.2009.01.003
McCutcheon JR, McGinnis RL, Elimelech M (2006) Desalination by ammonia–carbon dioxide forward osmosis: influence of draw and feed solution concentrations on process performance. J Memb Sci 278:114–123. https://doi.org/10.1016/j.memsci.2005.10.048
Mericq JP, Laborie S, Cabassud C (2010) Vacuum membrane distillation of seawater reverse osmosis brines. Water Res 44:5260–5273. https://doi.org/10.1016/j.watres.2010.06.052
Mickley MC (2006) Membrane concentrate disposal: practices and regulation. Desalin Water Purif Res Dev Progr 123:298
Mousavi SS, Kargari A (2022) Water recovery from reverse osmosis concentrate by commercial nanofiltration membranes: a comparative study. Desalination 528:115619. https://doi.org/10.1016/j.desal.2022.115619
Naidu G, Jeong S, Choi Y, Vigneswaran S (2017) Membrane distillation for wastewater reverse osmosis concentrate treatment with water reuse potential. J Memb Sci 524:565–575. https://doi.org/10.1016/j.memsci.2016.11.068
Ng HY, Lee LY, Ong SL et al (2008) Treatment of RO brine–towards sustainable water reclamation practice. Water Sci Technol 58:931–936. https://doi.org/10.2166/wst.2008.713
Ning RY, Tarquin A, Trzcinski M, Patwardhan G (2006) Recovery optimization of RO concentrate from desert wells. Desalination 201:315–322. https://doi.org/10.1016/j.desal.2006.06.006
Oren Y (2008) Capacitive deionization (CDI) for desalination and water treatment—past, present and future (a review). Desalination 228:10–29. https://doi.org/10.1016/j.desal.2007.08.005
Oren Y, Korngold E, Daltrophe N et al (2010) Pilot studies on high recovery BWRO-EDR for near zero liquid discharge approach. Desalination 261:321–330. https://doi.org/10.1016/j.desal.2010.06.010
Pan S-Y, Haddad AZ, Kumar A, Wang S-W (2020) Brackish water desalination using reverse osmosis and capacitive deionization at the water-energy nexus. Water Res 183:116064. https://doi.org/10.1016/j.watres.2020.116064
Park Y-S, Lee C-K, Kim S-K et al (2013) Effect of temperature difference on performance of membrane crystallization-based membrane distillation system. Desalin Water Treat 51:1362–1365. https://doi.org/10.1080/19443994.2012.705052
Pérez-González A, Urtiaga AM, Ibáñez R, Ortiz I (2012) State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res 46:267–283. https://doi.org/10.1016/j.watres.2011.10.046
Petersen KL, Frank H, Paytan A, Bar-Zeev E (2018) Impacts of seawater desalination on coastal environments. Sustain Desalin Handb Plant Sel Des Implement. https://doi.org/10.1016/B978-0-12-809240-8.00011-3
Qu D, Wang J, Fan B et al (2009a) Study on concentrating primary reverse osmosis retentate by direct contact membrane distillation. Desalination 247:540–550. https://doi.org/10.1016/j.desal.2008.08.004
Qu D, Wang J, Wang L et al (2009b) Integration of accelerated precipitation softening with membrane distillation for high-recovery desalination of primary reverse osmosis concentrate. Sep Purif Technol 67:21–25. https://doi.org/10.1016/j.seppur.2009.02.021
Qu D, Wang J, Hou D et al (2010) Concentrating primary reverse osmosis concentrate by direct contact membrane distillation. Water Sci Technol Water Supply 10:403–410. https://doi.org/10.2166/ws.2010.121
Rahardianto A, McCool BC, Cohen Y (2008) Reverse osmosis desalting of inland brackish water of high gypsum scaling propensity: kinetics and mitigation of membrane mineral scaling. Environ Sci Technol 42:4292–4297. https://doi.org/10.1021/es702463a
Ramdin M, Morrison ART, De Groen M et al (2019) High pressure electrochemical reduction of co2 to formic acid/formate: a comparison between bipolar membranes and cation exchange membranes. Ind Eng Chem Res 58:1834–1847. https://doi.org/10.1021/acs.iecr.8b04944
Randall DG, Nathoo J (2015) A succinct review of the treatment of reverse osmosis brines using freeze crystallization. J Water Process Eng 8:186–194. https://doi.org/10.1016/j.jwpe.2015.10.005
Randall DG, Nathoo J, Lewis AE (2011) A case study for treating a reverse osmosis brine using eutectic freeze crystallization-approaching a zero waste process. Desalination 266:256–262. https://doi.org/10.1016/j.desal.2010.08.034
Salvador Cob S, Genceli Güner FE, Hofs B et al (2014) Three strategies to treat reverse osmosis brine and cation exchange spent regenerant to increase system recovery. Desalination 344:36–47. https://doi.org/10.1016/j.desal.2014.03.009
Sanmartino JA, Khayet M, García-Payo MC et al (2017) Treatment of reverse osmosis brine by direct contact membrane distillation: chemical pretreatment approach. Desalination 420:79–90. https://doi.org/10.1016/j.desal.2017.06.030
Shane Walker W, Kim Y, Lawler DF (2014) Treatment of model inland brackish groundwater reverse osmosis concentrate with electrodialysis—Part III: sensitivity to composition and hydraulic recovery. Desalination 347:158–164. https://doi.org/10.1016/j.desal.2014.05.034
Shanmuganathan S, Johir MAH, Listowski A et al (2016) Sustainable processes for treatment of waste water reverse osmosis concentrate to achieve zero waste discharge: a detailed study in water reclamation plant. Procedia Environ Sci 35:930–937. https://doi.org/10.1016/j.proenv.2016.07.076
Shanmuganathan S, Loganathan P, Kazner C et al (2017) Submerged membrane filtration adsorption hybrid system for the removal of organic micropollutants from a water reclamation plant reverse osmosis concentrate. Desalination 401:134–141. https://doi.org/10.1016/j.desal.2016.07.048
Sluys JTM, Verdoes D, Hanemaaijer JH (1996) Water treatment in a membrane-assisted crystallizer (MAC). Desalination 104:135–139. https://doi.org/10.1016/0011-9164(96)00036-7
Stylianou SK, Szymanska K, Katsoyiannis IA, Zouboulis AI (2015) Novel water treatment processes based on hybrid membrane-ozonation systems: a novel ceramic membrane contactor for bubbleless ozonation of emerging micropollutants. J Chem 2015:1–12. https://doi.org/10.1155/2015/214927
Su J, Chung T-S, Helmer BJ, de Wit JS (2012) Enhanced double-skinned FO membranes with inner dense layer for wastewater treatment and macromolecule recycle using Sucrose as draw solute. J Memb Sci 396:92–100. https://doi.org/10.1016/j.memsci.2012.01.001
Subramani A, Jacangelo JG (2014) Treatment technologies for reverse osmosis concentrate volume minimization: a review. Sep Purif Technol 122:472–489. https://doi.org/10.1016/j.seppur.2013.12.004
Subramani A, DeCarolis J, Pearce W, Jacangelo JG (2012) Vibratory shear enhanced process (VSEP) for treating brackish water reverse osmosis concentrate with high silica content. Desalination 291:15–22. https://doi.org/10.1016/j.desal.2012.01.020
Tanaka Y (2007) Chapter 3 Bipolar membrane electrodialysis. pp 405–436
Tao G, Viswanath B, Kekre K et al (2011) RO brine treatment and recovery by biological activated carbon and capacitive deionization process. Water Sci Technol 64:77–82. https://doi.org/10.2166/wst.2011.604
Tawfik A, Alalm MG, Awad HM et al (2022) Solar photo-oxidation of recalcitrant industrial wastewater: a review. Environ Chem Lett 20:1839–1862. https://doi.org/10.1007/s10311-022-01390-4
Torma CZ, Cséfalvay E (2018) Nanofiltration and electrodialysis: alternatives in heavy metal containing high salinity process water treatment. Chem Pap 72:1115–1124. https://doi.org/10.1007/s11696-018-0433-7
Tun CM, Fane AG, Matheickal JT, Sheikholeslami R (2005) Membrane distillation crystallization of concentrated salts—flux and crystal formation. J Memb Sci 257:144–155. https://doi.org/10.1016/j.memsci.2004.09.051
Turek M (2004) Electrodialytic desalination and concentration of coal-mine brine. Desalination 162:355–359. https://doi.org/10.1016/S0011-9164(04)00069-4
van der Ham F, Seckler MM, Witkamp GJ (2004) Eutectic freeze crystallization in a new apparatus: the cooled disk column crystallizer. Chem Eng Process Process Intensif 43:161–167. https://doi.org/10.1016/S0255-2701(03)00018-7
Van Hege K, Verhaege M, Verstraete W (2002) Indirect electrochemical oxidation of reverse osmosis membrane concentrates at boron-doped diamond electrodes. Electrochem Commun 4:296–300. https://doi.org/10.1016/S1388-2481(02)00276-X
Virapan SR, Murugaiyan V (2017) Treatment of reverse osmosis reject water from industries. Int J Appl Environ Sci 12:489–503
Vyas BG, Labhasetwar PK, Yadav A, Paital AR (2022) A compendium of evaporation techniques for salt production from seawater and sub-soil brine. Chem Pap 2022:1–16. https://doi.org/10.1007/S11696-022-02363-1
Waite T, Fane A, Schäfer A (2005) Nanofiltration: principles and applications. Elsevier, Amsterdam
Woisetschläger D, Humpl B, Koncar M, Siebenhofer M (2013) Electrochemical oxidation of wastewater - Opportunities and drawbacks. Water Sci Technol 68:1173–1179. https://doi.org/10.2166/wst.2013.366
Woo SY, Lee HS, Ji H et al (2019) Silica gel-based adsorption cooling cum desalination system: focus on brine salinity, operating pressure, and its effect on performance. Desalination 467:136–146. https://doi.org/10.1016/j.desal.2019.06.016
Xevgenos D, Vidalis A, Moustakas K et al (2015) Sustainable management of brine effluent from desalination plants: the SOL-BRINE system. Desalin Water Treat 53:3151–3160. https://doi.org/10.1080/19443994.2014.933621
Xu P, Cath TY, Robertson AP et al (2013) Critical review of desalination concentrate management, treatment and beneficial use. Environ Eng Sci 30:502–514. https://doi.org/10.1089/ees.2012.0348
Xu X, Lin L, Ma G et al (2018) Study of polyethyleneimine coating on membrane permselectivity and desalination performance during pilot-scale electrodialysis of reverse osmosis concentrate. Sep Purif Technol 207:396–405. https://doi.org/10.1016/j.seppur.2018.06.070
Yadav A, Sinha N (2022) Organic polymers for drinking water purification. In: Hashmi MSJ (ed) Encyclopedia of materials: plastics and polymers. Elsevier, Amsterdam, pp 997–1003
Yadav A, Labhasetwar PK, Shahi VK (2021) Membrane distillation using low-grade energy for desalination: a review. J Environ Chem Eng 9:105818. https://doi.org/10.1016/j.jece.2021.105818
Yadav A, Labhasetwar PK, Shahi VK (2021) Fabrication and optimization of tunable pore size poly(ethylene glycol) modified poly(vinylidene-co-hexafluoropropylene) membranes in vacuum membrane distillation for desalination. Sep Purif Technol 271:118840. https://doi.org/10.1016/j.seppur.2021.118840
Yadav A, Labhasetwar PK, Shahi VK (2022) Membrane distillation crystallization technology for zero liquid discharge and resource recovery: opportunities, challenges and futuristic perspectives. Sci Total Environ 806:150692. https://doi.org/10.1016/j.scitotenv.2021.150692
Yan Z, Yang H, Qu F et al (2017) Reverse osmosis brine treatment using direct contact membrane distillation: effects of feed temperature and velocity. Desalination 423:149–156. https://doi.org/10.1016/j.desal.2017.09.010
Zhang Y, Van der Bruggen B, Pinoy L, Meesschaert B (2009) Separation of nutrient ions and organic compounds from salts in RO concentrates by standard and monovalent selective ion-exchange membranes used in electrodialysis. J Memb Sci 332:104–112. https://doi.org/10.1016/j.memsci.2009.01.030
Zhi J-F, Wang H-B, Nakashima T et al (2003) Electrochemical incineration of organic pollutants on boron-doped diamond electrode. Evidence for direct electrochemical oxidation pathway. J Phys Chem B 107:13389–13395. https://doi.org/10.1021/jp030279g
Zhou T, Lim T-T, Chin S-S, Fane AG (2011) Treatment of organics in reverse osmosis concentrate from a municipal wastewater reclamation plant: feasibility test of advanced oxidation processes with/without pretreatment. Chem Eng J 166:932–939. https://doi.org/10.1016/j.cej.2010.11.078
Acknowledgements
CSIR-CSMCRI PRIS no 170/2022.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
RVP, RG, BGV, PL, AY—conceptualization, methodology, data curation, writing—original draft, visualization, investigation, writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
We consent to publish the paper in this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, R.V., Gajera, R., Vyas, B.G. et al. Compendium of technologies for the treatment of reverse osmosis concentrate from inland desalination plants. Chem. Pap. 77, 5623–5639 (2023). https://doi.org/10.1007/s11696-023-02867-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02867-4