Abstract
Microemulsions of ionic liquids (ILs) are added to enhance the activity of peroxidase. From a catalytic standpoint, we provide the first research of the chemiluminescence (CL) features of luminol microencapsulated with horseradish peroxidase (HRP) in water–ionic liquid (W/IL) microemulsions. Water/AOT (sodium bis (2-ethyl-1-hexyl) sulfosuccinate)/hydrophobic IL [C8mim] [PF6] (1-octyl-3-methylimidazolium hexafluorophosphate)/1-hexanol constituted the system. Experiments demonstrated that the CL kinetic parameters, such as pseudo-first-order rise and fall rate constants for the chemiluminescence burst, CL intensity at time, maximum level intensity, total light yield, the intensity values at maximum CL, and time to reach maximum intensity, are dramatically affected as a function of W0 (water/surfactant), co-surfactant percentage, pH, and reactant concentrations. At W0 = 6, the maximum performance was achieved. The system exhibits more chemiluminescence properties than an enzymatic aqueous CL system and may be promoted without the need of an enhancer.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The enzyme horseradish peroxidase (HRP, EC 1.11.1.7) is well known for its high substrate specificity and efficiency in oxidizing a wide range of organic compounds, including phenols, biphenols, anilines, benzidines, and related heteroaromatic compounds. It is also used in immunohistochemistry, Western blots, dot blots, and ELISAs (Ryan et al. 1994). The appeal of HRP primary is due to its affordability, accessibility, low side product production, and action across a wide pH and temperature range (Ryan et al. 1994; Veitch 2004; Veitch and Smith 2000). However, this item significantly reduces the potential applications of enzyme in systems with little or no water content. Micro-heterogeneous systems such as micellar systems (Motlekar and Bhagwat 2001; Gȩbicka and Pawlak 1997; Parida et al. 1991; Passos et al. 2008; Ghasemi et al. 2023; Li et al. 2021) and microemulsions (Li and Huang 2021; Mahiuddin et al. 2005; Krickl et al. 2018; Tzika et al. 2011; Moniruzzaman et al. 2009; Bauduin et al. 2005; Ghasemi et al. 2022) have been developed to get around this obstacle.
The definition of a microemulsion is “a thermodynamically stable isotropic transparent solution of either a hydrophilic or hydrophobic nature, together with an amphiphilic component, their difference from conventional emulsions lying not only on their significantly smaller structural size (3–100 nm) but also on their thermodynamic stability, two properties which are translated in the long-lived stabilization of mixed polar/apolar systems which is not otherwise feasible” (Cates et al. 1988; Gradzielski et al. 2021; Kale and Deore 2017; Tartaro et al. 2020). The introduction of microemulsions as potential methods to enhance the enzymology in diverse media has occurred in recent years (Tzika et al. 2011; Moniruzzaman et al. 2009; Mitsou et al. 2017; Bose et al. 2022).
Despite these advantages, the organic solvents used in enzymatic microemulsions are very flammable and potentially harmful. Due to their unique properties such as low vapor pressure, wide liquid range, good dissolution properties, and high thermal stability, ionic liquids (ILs, salts melting below 100 °C) have attracted a lot of attention in recent years as a potential replacement for or way to mitigate the effects of organic solvents (Hejazifar et al. 2020). ILs retain the catalytic activity of a wide variety of enzymes. These include lipases, alcohol dehydrogenases, proteases, oxidoreductases, and many more. Compared to enzymes in molecular organic solvents, those in ILs are more active, stable, and selective, and they can be recovered and recycled more easily (Moniruzzaman et al. 2008a; Eastoe et al. 2005; Qiu and Texter 2008; Kuchlyan and Kundu 2016; Kaur et al. 2023).
Because of its low background light, straightforward equipment, quicker reaction rate, and greater dynamic range, chemiluminescence (CL, i.e., the creation of light from a chemical reaction) methods have been extensively employed in clinical chemistry, biochemistry, and environmental chemistry (Garcia-Campana and Baeyens 2001; Lin and Yamada 2003; Yu and Zhao 2021; Biparva et al. 2014, 2016; Kazemi et al. 2012). The most effective chemiluminescent process that an enzyme can catalyze is the oxidation of luminol by hydrogen peroxide when HRP is present and mildly alkaline conditions are present (Misra and Squatrito 1982). When a luminol is oxidized, a 3-aminophthalate ion is created, which, when it returns to its ground state, emits light. The maximal emission wavelength, 425 nm, is employed in immunoassays, metabolic pathway monitoring, inorganic and organic component detection, enzymatic reaction evaluation, and blood detection at crime scenes (Misra and Squatrito 1982; Zhang et al. 2018; Nakamura and Nakamura 1998).
Surprisingly, only one publication has been reported on the peroxidase–luminol CL system in microemulsions (Puchkaev and Metelitsa 1993), and to the best of our knowledge, very few studies have been published on the use of microemulsions as media and for CL reactions (Thompson and McBee 1988; Cohen and Magdassi 1996; Kamyshny and Magdassi 1998; Ishimaru et al. 1998; Huang and Hohn 2010; Murillo Pulgarin et al. 2010). In this study, we used chemiluminescence for the first time to examine how HRP behaved when it was incubated with IL microemulsions. Then, emission was examined from a catalytic perspective. The implications of numerous physicochemical microemulsion factors that have an impact on the CL system are thoroughly examined.
Experimental
Materials
The surfactant AOT, sodium bis(2-ethyl-1-hexyl)sulfosuccinate (> 99%), horseradish peroxidase (HRP, EC 1.11.1.7), and luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) were bought from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) and applied without further purification. Hydrogen peroxide (H2O2) (35% W/W), [C8mim] [PF6] (1-octyl-3-methylimidazolium hexafluorophosphate), mannitol, desferrioxamine, α-tocopherol, NaN3, thiourea, and cysteamine were from Fluka (Basel, Switzerland). 1-Hexanol was provided from Romil (Romil, England). Tris-phosphate buffer was utilized via the work.
Apparatus
A Berthold tube luminometer was used to conduct the CL kinetic experiments (Berthold Detection System, Sirius L, Pforzheim, Germany). The kinetic chemiluminescence parameters were determined using the nonlinear least-squares curve fitting tool KINFIT. The appropriate intensity vs. time charts were used to analyze the experimental results, including the time to attain maximum intensity and maximum intensity. Studies on steady-state chemiluminescence were conducted using a Cary-Eclipse fluorescence spectrophotometer (Agilent, Australia). The spectroscopic research was done using T92 + (PG Instruments, UK). The Multimeter 8603 was used to test pH (AZ instrument, Taiwan). At a constant temperature of 27 °C, all tests were conducted. Dynamic light scattering (DLS) studies were done on a DLS Analyzer Nanotrac Wave II (Microtrac, USA).
Preparation of IL microemulsions
Dissolving HRP in hydrophobic ionic solutions is notoriously difficult. Possible solutions to this problem include using an appropriate surfactant to stabilize the water domains in an IL continuous phase (also known as W/IL microemulsions). The great stability and activity of the enzyme molecules in the micro-heterogeneous medium is a result of their isolation from the organic solvent by a layer of water and surfactant molecules (Moniruzzaman et al. 2009). The necessary quantity of AOT was dissolved in IL that included 10% (V/V) 1-hexanol. Next, a little bit of buffer solution was added to create a microemulsion. To assure the clarity and stability of the stock solution, it was mixed in a vortex mixer for one full minute. Microemulsions with the necessary water concentration were prepared by adding the appropriate volume of buffer solution (Moniruzzaman et al. 2008b), and the molar ratio of water-to-AOT (W0) was determined by subtracting the quantity of water soluble in pure IL without surfactant from the total water concentration.
Preparation of reaction solutions
The HRP stock solutions were produced using the buffer solution. The peroxidase concentration was determined using spectrophotometric measurement, with a molar absorption coefficient of 9.1 × 104 M−1 cm−1 at 403 nm (Moniruzzaman et al. 2009). To prepare the H2O2 solutions for the experiment, the concentrated solution was diluted with buffer. For the luminol solutions, 1-mM Tris-buffer solution was used (pH 9.5). The following is an example of a typical experiment for enzymatic CL reactions using the AOT/water/IL/1-hexanol systems. It took 80 µL of luminol solution and measured amounts of buffer solution added to 1 mL of stock solution to get the desired W0. The solution was vortexed for 20 s to achieve macroscopic uniformity. After adding 40 µL of HRP, we gently mixed and kept the contents at 27 °C until they were ready to be blended with the reaction mixture at room temperature. To initiate the CL reaction, 50 µL of H2O2 were injected. The enzyme and substrate concentrations were stated as the total concentration, rather than the volume fraction of water droplets in the W/IL microemulsions or the partitioning coefficient of substrates in the bulk IL and microaggregate.
Results and discussion
Characteristics of CL system
Examining the kinetic parameters is essential to ascertain a more in-depth understanding of the chemiluminescence process. As a result, we employed a simple model pooled at the intermediate level (Dye and Nicely 1971; Hadd et al. 2000):
Both phases of the process are irreversible first-order reactions, as shown by the pools of reactants, intermediates, and products denoted by R, X, and P, respectively. The integrated rate equation of CL intensity vs. time is provided by: Where CL signal is proportional to the concentration of intermediate X.
As a result, I(t) represents the CL intensity at time t, M is the theoretical maximum intensity that would result from a complete conversion of the reactants into a CL-generating material, and Kr and Kf are the first-order rate constants for the rise (faster step) and fall (slower step) of the CL burst, respectively (Dye and Nicely 1971). This model’s additional benefits include the ability to estimate the intensity at maximum level (J) and the parameters M, Kr, and Kf. The time of maximum intensity (Tmax) and the total yield(Y) presented as follows:
Using the related CL intensity–time plots as a guide, the computerized nonlinear least-squares curve fitting tool KINFIT was utilized to assess the M, Kr, and Kf values (Dye and Nicely 1971). Using the discovered Kr, Kf, and M values, the remaining parameters J, Tmax, and Y were then deduced from Eqs. (3)–(5). The resulting intensity vs. time charts were then used to calculate the experimental time to obtain maximum intensity and maximum intensity.
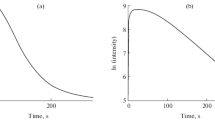
While maintaining constant the concentrations of the other factors, the effect of H2O2 concentration on the kinetic CL parameters was examined. Table 1 and Fig. 1 show the findings. It is clear that when the concentration of H2O2 is raised from 0.5 to 1.68 mM, both rate constants, CL intensity, and quantum yield clearly rise to their maximum value, while the duration to attain maximum CL decreases (Figs. 1a–d). The pseudo-first-order rise (Kr) and fall rate constant (Kf) linearly increases with the regression equations Kr = 0.0917 [H2O2] + 0.4598 with a relatively large intercept of about 0.4598 s−1 and equation Kf = 0.002 [H2O2] + 0.0058, respectively, which is representative of a first-order reaction that is zero-order at the hydrogen peroxide concentration used (Figs. 1e-f). All parameters decreased when concentration was raised to 1.68 mM, with the exception of duration to maximum CL. This finding may be explained by a number of factors: The polar substrate H2O2 is distributed efficiently in the IL phase, in contrast with how poorly it is soluble in a polar organic solvent. Here, the aqueous pseudo-phase’s concentration is decreased by H2O2 partitioning in the IL continuous phase, and the enzyme can only access the H2O2 that has been solubilized there (Azevedo et al. 2001). It is important to note that peroxide serves as an enzyme inhibitor at greater doses (Metelitza et al. 1992). Additionally, a greater peroxide concentration hinders the process by lowering superoxide anion concentrations, which accelerates the destruction of luminol (Hoshino and Hinze 1987).
The following phase was looking at how the W0 value (water content:concentration ratio of water to surfactant) affected the CL of the W/IL microemulsion. Water-in-IL microemulsions, like many W/O microemulsions, exhibit a spherical droplet form for which the droplet radius is directly proportional to W0 value. As a result, the W0 value may be changed to alter the microenvironment surrounding the enzyme. The formation of microemulsions in ionic liquids occurred between a W0 value of less than nine (Moniruzzaman et al. 2009; Metelitza et al. 1992), it should be highlighted. Above this water content, the system splits into two stages. Up until W0 = 6, the system was approaching a critical value for the generation of IL microemulsions and had reached an equilibrium value, as shown in Fig. 2 and Table 2, and this was assessed. The intensity vs. time curve in different W0s is shown in Fig. 2a. After mixing, the peak intensity rises quickly and reaches its peak in a few seconds. The light intensity gradually decreases from its peak over significantly longer times. The time to attain maximum CL was lowered by nearly six times, the CL intensity increased by about six times, and the rise in Kr was around 8.25 times (see Figs. 2b, c and Table 2). This finding may be explained by the enzyme’s proximity to the very minimum quantity of water required for maximum catalytic activity. For the enzyme to function well in microemulsions, it has to be suitably hydrated. At the low W0 value, a significant portion of water is firmly bonded to the AOT head groups (Azevedo et al. 2001). Because less water is available to hydrate the enzyme, the HRP reaction of luminol is better catalyzed and may experience structural changes with an increase in water concentration. Due to dilution of reactants at higher W0 (increased micelles and/or water pool), CL parameters reduced (Hoshino and Hinze 1987). In order to evaluation of size distribution of microemulsion droplet at optimum condition, DLS technique was performed. The size of (hydrodynamic diameter) distributions of the microemulsions obtained, which is one of the most commonly used methods to estimate the size distribution of the aggregates in microemulsions (Hyde 2001). As illustrated, the droplet is obviously spherical with average size of 29.21 nm.
The results of testing three different buffer concentrations (at 20, 30 and 50 mM) to ensure sufficient buffer capacity are shown in Fig. 3. The highest intensity and fastest increasing rate constant were reported at 50 mM, suggesting enhanced reactivity of superoxide anion. The influence of the pH of the aqueous pseudo-phase was studied in the CL microemulsions system. Since measuring the pH of a swimming pool’s water is problematic, the values provided below refer to the buffer's pH just before it is dissolved in IL microemulsions. We examined HRP-catalyzed oxidation of luminol in W/IL microemulsions as a function of pH at 50-mM buffer concentration, holding enzyme, and substrate concentrations constant. The usual pH profile of W/IL microemulsions is shown in Fig. 4 and tabulated below. At pHs 7.25 and 8.60, HRP CL oxidation of luminol was shown to be fastest both when no enhancer was present and when para-iodophenol was used. Previously, the CL process was reported by Metelitza et al. 1992, in a water–oil microemulsion at pH 9.5. Our results also showed that the optimal pH for HRP catalytic activity in the W/IL system was 9.5 (Table 3).
We analyzed how the amount of 1-hexanol present in W/IL microemulsions affected the CL system within (see Table 4 and Fig. 5). Generally speaking, 1-alcohols slow down enzyme reactions. For preparing W/IL microemulsions using AOT as the surfactant, 10% (V/V) 1-hexanol in IL is required (Moniruzzaman et al. 2009; Roy et al. 2006). Figure 5 and Table 4 show the result of progressively substituting 1-hexanol for IL in W/IL microemulsions at a constant surfactant concentration and W0 value. The intensity and rate constants of CL were found to be drastically diminished by increasing 1-hexanol concentration. Increasing 1-hexanol concentration is likely due to the following factors that contribute to low enzymatic activity. To begin, it stands to reason that the interfacial 1-hexanol concentration would increase proportionally to the total solution concentration. A higher concentration of 1-hexanol is clearly observed in the vicinity of the HRP active site. HRP is inactive because 1-alcohols can denature the enzyme structure. Second, it’s possible that the decreased catalytic activity of HRP was caused by the new microemulsion’s altered structure and rising 1-hexanol content. However, regardless of the 1-hexanol concentration used to produce the systems, all of the microemulsions were found to be stable and transparent.
Investigating the effects of altering HRP concentration on the CL parameters produced the results shown in Table 5 and Fig. 6. Under the experimental circumstances, there is obviously a good association between solution HRP catalytic activity and a linear relationship for enzyme concentrations between 0.008 m and 0.16 m. The nonlinear correlation and waning intensity at increasing enzyme concentrations indicate additional reaction limiting condition (Bhandari et al. 2010).
Possible mechanism
Luminol (5-amino-2, 3 dihydrophthalazine-1, 4-dione) emits light by a chemiluminescent process that involves the splitting of an oxygen–oxygen bond (Bhandari et al. 2010). The light produced by this reaction occurs at 425 nm. As part of the HRP–luminol–H2O2 test, HRP reacts with H2O2 by oxygen transfer. Equations (6) and (7) show how HRP is transformed into HRP-I and HRP-II in the presence of luminol LH− and peroxide. The luminol radical, L•−, is formed when one of these intermediates removes an electron from luminol (see Eq. 9). After entering a complicated chemical route, luminol radicals ultimately provide diazaquinone L, luminol endoperoxide LO2−2, excited 3-aminophthalate ion AP−2, and nitrogen gas Eqs. (10–12). 3-aminophthalate dianion (AP−2) is formed when AP−2 is decomposed into it and light (Eq. (14)) (Roswell and White 1978; Rauhut et al. 1966; Seitz 1978).
Some antioxidants were utilized as scavengers to research the potential mechanism of CL in W/IL microemulsion: Superoxide radicals are dealt with by superoxide dismutase (SOD), hydroxyl radicals are dealt with by mannitol and desferrioxamine, singlet oxygen is dealt with by α-tocopherol and NaN3, and hypochlorite is dealt with by thiourea and cysteamine (Nakamura and Nakamura 1998; Oosthuizen and Greyling 1999). The results of the experiments were comparable to those of aqueous mediums.
Various amounts of water have a drastic effect on CL parameters. By increasing water content up to W0 = 6, the CL parameters were increased. At upper value, these parameters were noticeably decrease. This phenomena can be attributed that the stability of the dianion be affected by water content that led to change the monoanion–dianion equilibrium of Eqs. (11) and (12) (decrease dianion concentration) (Hoshino and Hinze 1987; Gorsuch and Hercules 1972; Fujiwara and Kumamaru 1994). It is important to note that the rate constant, maximum intensity, and quantum yield are all greatly improved by IL microemulsion.
Conclusion
Hence, for the first time, we surveyed the biocatalyzed chemiluminescence reaction of luminol in water–ionic liquid microemulsion. Some general conclusions are provided from these results:
-
In contrast with aqueous medium, the chemiluminescence reaction of luminol catalyzed by HRP may be carried out in water-in-ionic liquid microemulsion with enhanced rate constant, intensity, and quantum yield.
-
Based on kinetic studies and SOD testing, the chemiluminescence process is comparable to that seen in aqueous media.
-
HRP-catalyzed chemiluminescence reaction in W/IL can be enhanced without any enhancer.
-
High background emission, auto-oxidation of luminol, spontaneous decomposition of peroxide, or interferences from potentially reductive species present in solution are some issues that are frequently encountered under the strongly basic conditions necessary for the CL production process in aqueous solution and can be effectively reduced by using W/IL microemulsion.
-
The employment of ILs in microemulsion may boost the sensitivity of the CL system in analytical and biological applications due to the ascend in chemiluminescence yield.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Azevedo AM, Fonseca LP, Graham DL, Cabral JM, Prazeres DM (2001) Behaviour of horseradish peroxidase in AOT reversed micelles. Biocatal Biotransform 19(3):213–233
Bauduin P, Touraud D, Kunz W, Savelli M-P, Pulvin S, Ninham BW (2005) The influence of structure and composition of a reverse SDS microemulsion on enzymatic activities and electrical conductivities. J Colloid Interface Sci 292(1):244–254
Bhandari A, Kim W, Hohn K (2010) Luminol-based enhanced chemiluminescence assay for quantification of peroxidase and hydrogen peroxide in aqueous solutions: effect of reagent pH and ionic strength. J Environ Eng 136(10):1147–1152
Biparva P, Abedirad SM, Kazemi SY (2014) ZnO nanoparticles as an oxidase mimic-mediated flow-injection chemiluminescence system for sensitive determination of carvedilol. Talanta 130:116–121
Biparva P, Abedirad SM, Kazemi SY, Shanehsaz M (2016) Chemiluminescence recognition of berberine triggered by biomimetically synthesized silver nanoparticles. Sens Actuators B Chem 234:278–285
Bose AL, Bhattacharjee D, Goswami D (2022) Mixed micelles and bicontinuous microemulsions: promising media for enzymatic reactions. Colloids Surf B 209:112193
Cates M, Andelman D, Safran S, Roux D (1988) Theory of microemulsions: comparison with experimental behavior. Langmuir 4(4):802–806
Cohen S, Magdassi S (1996) Chemiluminescence in microemulsions: effect of phase composition. Langmuir 12(16):3759–3762
Dye JL, Nicely VA (1971) A general purpose curve fitting program for class and research use. J Chem Educ 48(7):443
Eastoe J, Gold S, Rogers SE, Paul A, Welton T, Heenan RK, Grillo I (2005) Ionic liquid-in-oil microemulsions. J Am Chem Soc 127(20):7302–7303
Fujiwara T, Kumamaru T (1994) Catalytic effect of rhodium (III) on the chemiluminescence of luminol in reverse micelles and its analytical application. Anal Chim Acta 292(1–2):151–157
Garcia-Campana A, Baeyens W (2001) Chemiluminescence in analytical chemistry. Marcel Dekker, Inc., New York, pp 621. ISBN: 0–8247–0464–9. In.
Gȩbicka L, Pawlak J (1997) Kinetic investigations of horseradish peroxidase in AOT/n-heptane reverse micelles. J Mol Catal B Enzym 2(4–5):185–192
Ghasemi H, Mozaffari S, Mohammadghasemi H, Jemere AB, Nazemifard N (2022) Microfluidic platforms for characterization of crude oil emulsion stability. Can J Chem 100(7):484–494
Ghasemi H, Afshang M, Gilvari T, Aghabarari B, Mozaffari S (2023) Rapid and effective removal of heavy metal ions from aqueous solution using nanostructured clay particles. Results Surf Interfaces. https://doi.org/10.1016/j.rsurfi.2023.100097
Gorsuch JD, Hercules D (1972) Studies on the chemiluminescence of luminol in dimethylsulfoxide and dimethylsulfoxide-water mixtures. Photochem Photobiol 15(6):567–583
Gradzielski M, Duvail M, de Molina PM, Simon M, Talmon Y, Zemb T (2021) Using microemulsions: formulation based on knowledge of their mesostructure. Chem Rev 121(10):5671–5740
Hadd AG, Seeber A, Birks JW (2000) Kinetics of two pathways in peroxyoxalate chemiluminescence. J Org Chem 65(9):2675–2683
Hejazifar M, Lanaridi O, Bica-Schröder K (2020) Ionic liquid based microemulsions: a review. J Mol Liq 303:112264
Hoshino H, Hinze WL (1987) Exploitation of reversed micelles as a medium in analytical chemiluminescence measurements with application to the determination of hydrogen peroxide using luminol. Anal Chem 59(3):496–504
Huang C-C, Hohn KL (2010) Tetrakis (dimethylamino) ethylene chemiluminescence (TDE CL) characterization of the CMC and the viscosity of reversed microemulsions. J Phys Chem B 114(8):2685–2694
Hyde ST (2001) Identification of lyotropic liquid crystalline mesophases. Handb Appl Surf Colloid Chem 2:299–332
Ishimaru N, Lin J-M, Yamada M (1998) Luminol-free chlorine chemiluminescence in an oil-in-water microemulsion medium. Anal Commun 35(2):67–69
Kale SN, Deore SL (2017) Emulsion micro emulsion and nano emulsion: a review. Syst Rev Pharm 8(1):39
Kamyshny A, Magdassi S (1998) Chemiluminescence immunoassay in microemulsions. Colloids Surf B 11(5):249–254
Kaur M, Singh M, Singh G, Singh A, Kaur G, Mehta SK, Kang TS (2023) Water-pluronic-ionic liquid based microemulsions: preparation, characterization and application as micro-reactor for enhanced catalytic activity of Cytochrome-c. Colloids Surf B 222:113034
Kazemi SY, Abedirad SM, Vaezi Z, Ganjali MR (2012) A study of chemiluminescence characteristics of a novel peroxyoxalate system using berberine as the fluorophore. Dyes Pigm 95(3):751–756
Krickl S, Touraud D, Bauduin P, Zinn T, Kunz W (2018) Enzyme activity of horseradish peroxidase in surfactant-free microemulsions. J Colloid Interface Sci 516:466–475
Kuchlyan J, Kundu N (2016) Ionic liquids in microemulsions: formulation and characterization. Curr Opin Colloid Interface Sci 25:27–38
Li X, Huang X (2021) Good’s buffer ionic liquid tunes the phase behavior of an anionic surfactant SDBS-stabilized n-octane–water microemulsion and the stability of the solubilized horseradish peroxidase. Soft Matter 17(35):8086–8094
Li W, Taylor MG, Bayerl D, Mozaffari S, Dixit M, Ivanov S, Seifert S, Lee B, Shanaiah N, Lu Y (2021) Solvent manipulation of the pre-reduction metal–ligand complex and particle-ligand binding for controlled synthesis of Pd nanoparticles. Nanoscale 13(1):206–217
Lin J-M, Yamada M (2003) Microheterogeneous systems of micelles and microemulsions as reaction media in chemiluminescent analysis. TrAC, Trends Anal Chem 22(2):99–107
Mahiuddin S, Renoncourt A, Bauduin P, Touraud D, Kunz W (2005) Horseradish peroxidase activity in a reverse catanionic microemulsion. Langmuir 21(12):5259–5262
Metelitza D, Eryomin A, Shibaev V (1992) Enhanced chemiluminescence in the oxidation of luminol and an isoluminol cortisol conjugate by hydrogen peroxide in reversed micelles. J Biolumin Chemilumin 7(1):21–26
Misra HP, Squatrito PM (1982) The role of superoxide anion in peroxidase-catalyzed chemiluminescence of luminol. Arch Biochem Biophys 215(1):59–65
Mitsou E, Xenakis A, Zoumpanioti M (2017) Oxidation catalysis by enzymes in microemulsions. Catalysts 7(2):52
Moniruzzaman M, Kamiya N, Nakashima K, Goto M (2008a) Water-in-ionic liquid microemulsions as a new medium for enzymatic reactions. Green Chem 10(5):497–500
Moniruzzaman M, Kamiya N, Nakashima K, Goto M (2008b) Formation of reverse micelles in a room-temperature ionic liquid. ChemPhysChem 9(5):689–692
Moniruzzaman M, Kamiya N, Goto M (2009) Biocatalysis in water-in-ionic liquid microemulsions: a case study with horseradish peroxidase. Langmuir 25(2):977–982
Motlekar NA, Bhagwat SS (2001) Activity of horseradish peroxidase in aqueous and reverse micelles and back-extraction from reverse-micellar phases. J Chem Technol Biotechnol Int Res Process, Environ Clean Technol 76(6):643–649
Murillo Pulgarin JA, Bermejo LFG, Durán AC (2010) Evaluation of the antioxidant activity of vegetable oils based on luminol chemiluminescence in a microemulsion. Eur J Lipid Sci Technol 112(12):1294–1301
Nakamura M, Nakamura S (1998) One-and two-electron oxidations of luminol by peroxidase systems. Free Radic Biol Med 24(4):537–544
Oosthuizen MM, Greyling D (1999) Antioxidants suitable for use with chemiluminescence to identify oxyradical species. Redox Rep 4(6):277–290
Parida S, Parida GR, Maitra AN (1991) Studies on the catalytic activity of horseradish peroxidase hosted in Aerosol OT reverse micelles containing cholesterol. Colloids Surf 55:223–229
Passos ML, Saraiva MLM, Lima JL (2008) Enzymatic oxidation in aqueous and micellar media based on horseradish peroxidase–hydrogen peroxide system using a SIA manifold. Talanta 77(2):484–489
Puchkaev A, Metelitsa D (1993) Luminol Oxidation in detergent-free microemulsions (hexane–isopropanol–water) catalyzed by horseradish peroxidase or hemin. Biochem-New York-Engl Transl Biohim 58(10):1125–1133
Qiu Z, Texter J (2008) Ionic liquids in microemulsions. Curr Opin Colloid Interface Sci 13(4):252–262
Rauhut M, Semsel A, Roberts B (1966) Reaction rates, quantum yields, and partial mechanism for the chemiluminescent reaction of 3-Aminophthalhydrazide with aqueous alkaline hydrogen peroxide and persulfate1. J Org Chem 31(8):2431–2436
Roswell DF, White EH (1978) [36] The chemiluminescence of luminol and related hydrazides. Methods in enzymology, vol 57. Elsevier, Amsterdam, pp 409–423
Roy S, Dasgupta A, Das PK (2006) Tailoring of horseradish peroxidase activity in cationic water-in-oil microemulsions. Langmuir 22(10):4567–4573
Ryan O, Smyth MR, Fagain CO (1994) Horseradish peroxidase: the analyst’s friend. Essays Biochem 28:129–146
Seitz WR (1978) Chemiluminescence detection of enzymically generated peroxide. Methods in enzymology, vol 57. Elsevier, Amsterdam, pp 445–462
Tartaro G, Mateos H, Schirone D, Angelico R, Palazzo G (2020) Microemulsion microstructure (s): a tutorial review. Nanomaterials 10(9):1657
Thompson RB, McBee SES (1988) Peroxyoxalate chemiluminescence in microemulsions. Langmuir 4(1):106–110
Tzika ED, Christoforou M, Pispas S, Zervou M, Papadimitriou V, Sotiroudis TG, Leontidis E, Xenakis A (2011) Influence of nanoreactor environment and substrate location on the activity of horseradish peroxidase in olive oil based water-in-oil microemulsions. Langmuir 27(6):2692–2700
Veitch NC (2004) Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry 65(3):249–259
Veitch NC, Smith AT (2000) Horseradish peroxidase. Adv Inorg Chem 51:107–162
Yu W, Zhao L (2021) Chemiluminescence detection of reactive oxygen species generation and potential environmental applications. TrAC, Trends Anal Chem 136:116197
Zhang Z, Lai J, Wu K, Huang X, Guo S, Zhang L, Liu J (2018) Peroxidase-catalyzed chemiluminescence system and its application in immunoassay. Talanta 180:260–270
Author information
Authors and Affiliations
Contributions
SMA worked in: methodology, investigation, formal analysis, writing—original draft preparation, and confirming of the final version. MS helped in: conceptualization, supervision, resources, reviewing, writing—original draft preparation, and confirming of final version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Ethics approval
This research is not involving human or animal subject. The Razi University committee has confirmed that no ethical approved is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abedirad, S.M., Shamsipur, M. Chemiluminescence of horseradish peroxidase in water–ionic liquid microemulsion: an approach from catalytic point of view. Chem. Pap. 77, 4659–4669 (2023). https://doi.org/10.1007/s11696-023-02815-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02815-2