Abstract
Cu (II) pollution has become a serious environmental issue in recent years due to the increasing discharge of wastewater containing copper. Consequently, it is necessary to treat copper-containing wastewater before it is discharged into the environment. Among the various methods of removing copper ions (Cu2+) from aqueous solutions, adsorption is considered to be one of the most efficient, economical and low-cost methods. In the present work, magnetic nanoparticles (Fe3O4 NPs) functionalized with silica (SiO2) and 2, 3-dimercaptosuccinic acid (DMSA) were synthesized using a facile coprecipitation method. The prepared DMSA@SiO2@Fe3O4 nanocomposite was evaluated as a magnetic adsorbent in the removal of Cu2+ from aqueous solutions. The nanocomposite exhibited fine colloidal stability, increased dispersibility and better magnetization as confirmed by zeta potential, dynamic light scattering (DLS) measurements and vibrating sample magnetometer (VSM). Using Design-Expert software, a statistical technique Box–Behnken design (BBD) and response surface methodology was employed to investigate Cu2+ removal using three adsorption parameters: pH, contact time and Cu2+ concentration. The maximum copper removal was found to be 97.1% under the optimal conditions at pH 7, contact time of 109 min and Cu2+ concentration of 163 ppm. Moreover, the adsorption process followed pseudo-first-order kinetics and was well fitted by Langmuir isotherm. Accordingly, this design procedure for making magnetic nanoadsorbents can be applied to a variety of environmental applications.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution has become one of the severe problems as a result of rapid industrial expansion and urbanization. This has largely deteriorated the water quality and can cause serious concerns for public health and the environment. Heavy metals such as lead (Pb), zinc (Zn), copper (Cu), arsenic (As), cadmium (Cd), chromium (Cr), nickel (Ni) and mercury (Hg) are naturally occurring elements having density at least five times that of water (Agarwal and Singh 2017; Tchounwou et al. 2012; Vareda et al. 2019). Industrial effluent from modern chemical industries focused on fossil fuels, batteries, metal plating, agrochemicals and pesticides is the primary source of heavy metal pollution in drinking water. Heavy metals are naturally poisonous, causing major health problems in humans and animals even when consumed at extremely low concentrations (Khan et al. 2020; Vardhan et al. 2019). Heavy metal ions are characterized by high toxicity, easy bioaccumulation and non-biodegradability in living creatures and food chains. They can be quickly absorbed by living creatures as they are soluble in aqueous environments (Kinuthia et al. 2020; Rehman et al. 2018). Several studies have shown that heavy metal ions interact with DNA and nuclear proteins, causing DNA damage and conformational changes that can lead to cancer, cell cycle regulation and apoptosis (Engwa et al. 2019). They can cause multiple organ failures and can cause carcinogenicity, teratogenicity and mutagenicity in humans (Bhat et al. 2019; Sharma and Agrawal 2005).
Copper (Cu) is a common heavy metal that is used in a variety of applications including electronic chips, batteries, cell phones, semiconductors, water pipes, fertilizer, pulp and paper sector, oil refining, electroplating, fungicides, pesticides, catalysts, alloy industry and metal processing items (Tamez et al. 2016). Copper is an essential element as a catalytic cofactor in redox chemistry for many proteins and acts as a micronutrient in human beings (Yruela, 2009). Globally, copper water pollution is a severe environmental problem due to an increase in copper-containing wastewater discharge in recent years. The permissible limit for the presence of copper in drinking water is 3 mg/L. High copper intake is associated with various health risks including negative effects on the nervous system, reproductive system, adrenal function, connective tissue and learning ability in newborn babies (Taylor et al. 2020). Excessive copper intake can also cause stomach aches, convulsions, cramps, severe vomiting, headache and paralysis (Gaetke and Chow 2003; Kumar et al. 2021). As a result, the removal of Cu2+ ions from wastewater is critical not only for the safety of water resources but also for the long-term survival of humans.
Copper removal from wastewater can be accomplished by a variety of processes including adsorption (Harja et al. 2020), membrane filtration (Mulungulungu et al. 2021), chemical precipitation (Ye et al. 2016), coagulation (Vasudevan et al. 2013), ion exchange (Siu et al. 2016), electrodialysis (de Morais Nepel et al. 2020) and bioremediation (Ahmed et al. 2022). A majority of these methods are not suitable for the removal of heavy metal ions from water resources due to their complicated mode of operation, secondary sludge generation, high operational costs and reusability difficulties. However, adsorption technology is a low-cost, efficient and cost-effective method for eliminating a variety of pollutants from aqueous solutions (Chai et al. 2021; Li et al. 2019). The synthesis of unique environmentally friendly adsorbent materials with many active sites for adsorption has emerged as one of the most relevant research area in recent times. A large number of existing studies in the broader literature have examined the application of different types of adsorbents such as zeolites, clays, fly ash, sawdust, industrial by-products, activated carbon, agricultural wastes, chitosan, limestone and alumina have been employed for Cu2+ removal (Krstić et al. 2018; Shahrashoub and Bakhtiari 2021). A considerable amount of attention has been given to magnetic nanoparticles (MNPs) based on iron oxide (Fe3O4) because of their high surface area, cost-effectiveness, high removal efficiency and ease with which they can be separated under an external magnetic field. Nanosized magnetic materials have recently gained a lot of attention due to their wide range of applications in adsorption, data storage, catalysis, drug administration and magnetic resonance imaging (Singh et al. 2020a, b; Zhu et al. 2018). Fe3O4 NPs are unstable and tend to aggregate which reduces their effectiveness in environmental applications. It has been demonstrated that surface functionalization with functional groups, surfactants, polymers and organic molecules can inhibit particle aggregation in the magnetite core and increase dispersion stability in suspension media (Maleki et al. 2018; Tian et al. 2018). There exists a considerable body of literature on applications of Fe3O4-based nanoadsorbents to adsorb Cu2+ ions as compared to conventional adsorbents. For instance, magnetic-activated carbon NPs were employed for Cu2+ removal from wastewater with a good adsorption capacity of 41.6 mg/g (Gu et al. 2019). Other magnetic nanoadsorbents based on surface functionalization of MNPs with carboxy-methyl chitosan, glycine, oligopeptides and biomass have tremendous potential to remove Cu2+ with good removal efficiency (Feitoza et al. 2014; Kandasamy 2017; Li et al. 2020; Plohl et al. 2019).
In the present work, the physical, chemical and surface properties of Fe3O4 NPs were tailored by silica (SiO2) and 2, 3-dimercaptosuccinic acid (DMSA) for selective removal of Cu2+ from wastewater. Different adsorption kinetics and isotherm models have been applied to interpret the results of a series of adsorption experiments. Further, the Box–Behnken design (BBD) and response surface methodology (RSM) were utilized to evaluate the interactive effects of independent variables like pH, initial copper concentration and contact time on copper removal process.
Experimental work
Chemical and reagents
Chemicals such as ferric chloride hexahydrate (FeCl3.6H2O; Himedia), ferrous chloride tetrahydrate (FeCl2.4H2O; Nice chemicals), sodium hydroxide solution (NaOH; 98% assay; Qualikems), hydrochloric acid (HCl, 37% assay; Rankem limited), ammonium hydroxide (NH4OH, Fisher Scientific), ethanol (Rankem limited), tetraethyl orthosilicate (TEOS), toluene (Fisher Scientific), dimethyl sulphoxide (DMSO; SRL scientific), DMSA (SRL scientific) and copper sulphate (CuSO4.5H2O; Himedia) were directly used as received without any further purification.
Synthesis of Fe3O4 NPs
The synthesis of Fe3O4 NPs was carried out using the co-precipitation method as previously reported in the scientific literature with slight modification (Yazid and Joon, 2019). Briefly, a mixture of ferric chloride hexahydrate (FeCl3.6H2O; 5.41 g) and ferrous chloride tetrahydrate (FeCl2. 4H2O, 1.99 g) were dissolved in 100 mL of distilled water and stirred uniformly over a stirrer at room temperature for half an hour. Further, HCl (0.75 mL) was added and kept over the stirrer for an additional stirring of 15 min. Later, 25 mL ammonia hydroxide (25%) solution was added in a drop-wise manner and continuously stirred for 2 h to precipitate the NPs. Further, the black precipitates were collected using a magnet, washed multiple times with distilled water and then dried overnight at 70 °C. The dried Fe3O4 NPs were stored at room temperature for further use.
Preparation of DMSA@SiO2@Fe3O4 nanocomposite
The synthesis of silica-coated Fe3O4 NPs was carried out using a method reported earlier with minor modification (Guo et al. 2015). In brief, 100 mg of Fe3O4 NPs was placed in 150 mL of ethanol and sonicated for half an hour. Further, aqueous ammonia and deionized water in ratio 3:47 were added to the above suspension and again kept for sonication for an additional half an hour. Later, 0.6 mL of tetraethyl orthosilicate (TEOS) was added with continuous stirring for 8 h. An external magnet was used to extract SiO2@Fe3O4 nanocomposites from the suspension. The collected nanocomposites were washed twice with deionized water and ethanol (100%) to remove any impurities.
The preparation of DMSA and silica-coated Fe3O4 nanocomposites was carried out using an already-reported method (Tian et al. 2018). Initially, 100 mg of SiO2@Fe3O4NPs was dissolved in toluene (10 mL) and sonicated for half an hour. Further, 10 mL of dimethyl sulphoxide was added and the contents were sonicated for additional 20 min. After sonication, 25 mg DMSA was added to the above solution and placed over a magnetic stirrer overnight. Further, a magnet was used to extract DMSA@SiO2@Fe3O4 nanocomposites from the solution. The collected nanocomposites were then washed multiple times with distilled water and ethanol. The nanocomposite was stored at room temperature for further use.
Characterization
The microscopic characterization of the prepared DMSA@SiO2@Fe3O4 nanocomposite adsorbent was carried out using Tecnai™ G2 high-resolution transmission electron microscopy (HRTEM). The elemental and chemical composition of the materials was studied with an energy-dispersive X-ray analysis (EDX) and Perkin-Elmer spectrum RX-1 Fourier transform-infrared (FTIR) spectrophotometer, respectively. The crystal structure of DMSA@SiO2@Fe3O4 nanocomposite was studied using X’Pert Pro PANalytical powder X-ray powder diffraction (XRD) in 2θ range of 5o to 80°. The magnetic measurements were carried out using a quantum design vibrating sample magnetometer (VSM). The colloidal stability, poly-dispersibility and hydrodynamic diameter of DMSA@SiO2@Fe3O4 nanocomposite were measured using the ZS-90 zeta sizer and Beckman Coulter Nano Dynamic Light Scattering (DLS) system. Further, the concentration of copper was determined by the Thermo scientific CAP RQ inductively coupled plasma-mass-spectrometer (ICP-MS).
Copper adsorption studies
Copper adsorption studies were performed using a series of batch adsorption experiments. The aqueous Cu2+ solution was prepared via diluting CuSO4.5H2O in distilled water at different concentrations. To conduct adsorption experiments, 0.03 g of DMSA@SiO2@Fe3O4 nanocomposite was kept in 25 mL copper solution in a 250-mL conical flask and placed over a shaker at room temperature. After the incubation time, the solution was centrifuged at 8500 rpm for 10 min and further exposed to an external magnetic field to separate the nanocomposite from the solution. Furthermore, 0.1 M NaOH and HCl solution were used to adjust the pH of the solutions whenever necessary. The ICP-MS was used to calculate the residual copper ion concentration in the separated solutions. The removal per cent (Y) of copper is determined by the given formula:
where C0 and Ce are the initial and equilibrium copper concentrations in the solution (mg/L), respectively.
Cu2+ adsorption capacity (qt) is the amount of Cu2+ adsorbed per unit mass of adsorbent (mg/g) at any time t. It was determined using the following equation:
where Co is the initial Cu2+ concentration (mg/L), Ct is the concentration of Cu2+ at time t (mg/L), V is the solution volume (L) and m is the amount of dry nanoparticles (adsorbent) added to the solution.
In experiments on batch kinetics adsorption, a known mass of 0.03 g of adsorbent and 25 mL of Cu2+ concentration (150 mg/L) were placed in a 250-ml conical flask and kept under a shaker at 100 rpm. The batch experiments were kept for different time intervals (15–120 min) at pH 7, and the adsorbent was magnetically separated from the solution. The experimental data obtained were fitted into pseudo-first-order, pseudo-second-order and intra-particle diffusion plot kinetic models to understand the adsorption kinetics of the process. All the rate constants and correlation coefficients involved in the kinetics were calculated and tabulated.
The adsorption studies of Cu2+ on nanocomposite were carried out by batch equilibrium experiments. The experiments were performed by addition of known amount (0.03 g) nanocomposite with 25 mL solution containing different Cu2+ concentrations (Co = 30–300 mg/L) at pH 7. After the system reached equilibrium, the equilibrium adsorption capacity (qe) and % Cu2+ removal were determined using the same equations mentioned earlier. The obtained experimental data were fitted into the adsorption isotherm models such as Langmuir, Freundlich and Temkin isotherms. Isotherm constants were calculated, and R2 values were determined.
Response surface methodology: systematic optimization
Response surface methodology (RSM) is the technique which explores the interrelationships between different input variables and one or more output/response variables. The basic idea of RSM is to use a series of designed experiments to obtain an optimal response. Optimization experimental designs are a collection of mathematical and statistical methodologies for developing, enhancing and optimizing processes. These designs can determine the best possible conditions for desired responses and also analyse the effects of individual independent parameters, their relative significance and the interaction of two or more factors (Ahmadi et al. 2014). Systematic optimization is one of the most often utilized methodologies due to its ability to build, improve and optimize processes, particularly in the face of complex interactions. It is also used to establish the optimal points of independent variables that are useful under ideal conditions as well as to assess their interactions. As a scientifically proven and risk-based approach to quality by design (QbD), it helps to understand and control the relationship between critical material parameters (CMPs) and critical quality attributes (CQAs). Factors, viz. contact time (X1), pH (X2) and copper concentration (X3), were chosen as the influential CMPs for factor screening studies and then systematically optimized by employing a second-order Box–Behnken design (Sultana et al. 2020). Furthermore, attempts were made to evaluate the % copper removal by DMSA@SiO2@Fe3O4 nanocomposite employing QbD paradigms. Response surface methodology was used to analyse the interrelationship (s) between CMPs and CQAs.
Results and discussion
Characterization
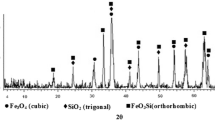
The surface morphology and shape of the prepared DMSA@SiO2@Fe3O4 nanocomposite were observed with HRTEM (Fig. 1a). It is possible to visualize a whitish layer of DMSA on the Fe3O4 NPs. Further, the EDX spectra (Fig. 1b) of DMSA@SiO2@Fe3O4 nanocomposite revealed a sulphur peak which conform that the presence of DMSA molecules on the nanocomposite. Figure 2 shows the FTIR spectra of Fe3O4 NPs, SiO2@Fe3O4 and DMSA@SiO2@Fe3O4 nanocomposite. All three spectra showed characteristic adsorption bands at 666.41, 620.21 and 620.26 cm−1, respectively corresponding to the Fe–O bond stretching vibrations indicating the presence of a magnetic core of iron oxide (Hwang et al. 2014). The strong absorption peaks at 3436.92 cm−1, 3416.15 cm−1 and 3414.79 cm−1 showed that the surface of nanocomposite had a sufficient amount of hydroxyl (–OH) groups indicating good water dispersibility (Mishra et al. 2014). The main amide group (C = O, C–N) is represented by the peak at 1617.93, 1618.29 and 1645.54 cm−1, respectively, in all spectra (Al‐Hayali and Cagrı‐Mehmetoglu 2022). Finally, the distinctive peaks at 1189.07 cm−1 in the DMSA@SiO2@Fe3O4 correspond to the characteristic peak of the carboxylic group that indicated the existence of DMSA coating, which is not present in Fe3O4 and SiO2@Fe3O4 (Kurian et al. 2015). Figure 3 shows the magnetization curve of DMSA@SiO2@Fe3O4 nanocomposite. The saturation magnetization (Ms) of the nanocomposite came around to be 80.00 emu/g which is sufficient for super para magnetism character. Due to fluctuations of magnetic moments caused by thermal energy, minimal retentivity (remanence) and zero coercively were seen in the curves of material. Figure 4a shows the XRD patterns of Fe3O4, SiO2@Fe3O4 NPs and DMSA@SiO2@Fe3O4 nanocomposite. The precise crystalline structure of Fe3O4 was represented by the sharpness of the peak. The magnetite phase of iron oxide is indicated by the peaks of Fe3O4 with distinctive maximum intensity at 35.50 (Predescu et al. 2018). The identification of peak at positions 35.50 [2θ] in both pristine and DMSA-coated Fe3O4 NPs indicated the presence of a magnetic iron oxide core, which has been reported earlier (Singh et al. 2020a, b; Yadav et al. 2020). The appearance of identical peaks in SiO2@Fe3O4 and DMSA@SiO2@Fe3O4 demonstrated that the silica and DMSA coating did not affect the crystalline state of the magnetite. In the liquid phase, dynamic light scattering (DLS) was used to determine the size and stability of the nanoparticles, as shown in Fig. 4(b, c). The average particle size and zeta potential of prepared DMSA@SiO2@Fe3O4 nanocomposite were found in the range of 109.7 nm and—25.6 mV. The zeta potential (surface charge) of the nanoparticles thus indicates their ability to form stable colloids.
Adsorption kinetics and isotherms
In order to investigate the adsorption behaviour of Cu2+ ions on DMSA@SiO2@Fe3O4, kinetic experiments were conducted. To investigate the adsorption kinetics of Cu2+, pseudo-first-order, pseudo-second-order and intra-particle diffusion kinetic models were used (Fig. 5 a-c). The linearized form of the pseudo-first-order kinetic model (Wang and Guo, 2020) is expressed as
where qe (mg/g) is the equilibrium adsorption capacity, qt is the adsorption capacity at any time t and k1 is pseudo-first-order rate constant (min−1). The kinetic parameters such as k1 and qe were determined from the slope and intercept, respectively, of the fitted linear plot of log (qe-qt) versus t.
Adsorption kinetic models fitted to experimental data a pseudo-first-order kinetics, b pseudo-second-order kinetics, c intra-particle diffusion plot; adsorption isotherms d Langmuir plot, e Freundlich isotherm plot, f Temkin isotherm. Note that the blue dots and the line represents experimental data and best fit, respectively
The linearized-integrated form of the pseudo-second-order equation is
where k2 is the rate constant for the pseudo-second-order adsorption process (g/mg min). The slope and intercept of the linear fitted plot of \(\frac{\mathrm{t}}{\mathrm{qt}}\) vs t was used to calculate the values of kinetic parameters such as qe and k2, respectively (Hubbe et al. 2019).
In the intra-particle diffusion model, Weber and Morris first found that the adsorption capacity varies with the square root of the adsorption time (t0.5) rather than with t (Ofomaja et al. 2020). It is given in the equation written below:
The values of kinetic parameters obtained from adsorption kinetics are shown in Table 1. From the interpretation of these models, it was confirmed that the adsorption of Cu2+ onto nanocomposite followed the first-order kinetics mechanism as observed from the R2 value (0.9955). The results suggest that the adsorption of Cu2+ is more likely to be inclined towards physisorption.
Various isotherm models like Langmuir isotherm, Freundlich isotherm and Temkin (Fig. 5 d, e, f) were employed to define the equilibrium nature of the adsorption of Cu2+ onto the nanocomposite. The linear form of the Langmuir equation for the adsorbent–adsorbate system is written as
A plot of Ce/qe versus Ce gives a straight line of slope 1/qm and intercepts 1/qmKL (Pavan Kumar et al. 2019). The adsorption parameters were calculated and tabulated.
The linearized form of the Freundlich isotherm is represented by the equation:
Temkin's isotherm model predicts that all molecules have a linear decrease in the heat with increasing adsorbent surface coverage for adsorption up to maximum binding energy (Batool et al. 2018). The model is given by the following equation:
where R is the universal gas constant (8.314 × 10–3 kJ/mol K), T is the temperature of the adsorption process (K), KT is the Temkin isotherm equilibrium binding constant (L/g) and b is the Temkin isotherm constant. Temkin plot was then used to calculate KT and b.
The Langmuir, Freundlich and Temkin isotherm constants are summarized in Table 1. The Langmuir isotherm model (R2 = 0.9966) was seen to fit the experimental data better than the Freundlich model (R2 = 0.985) and Temkin isotherm (R2 = 0.9714). Accordingly, the monolayer Langmuir adsorption isotherm seems to be the most appropriate explanation of the adsorption behaviour between DMSA@SiO2@Fe3O4 and Cu2+ with adsorption occurring over a homogeneous surface.
Adsorption mechanism
The functionalized Fe3O4 NPs have the greatest potential for heavy metal removal (Osman et al. 2019). From the above discussions, it can be concluded that Langmuir's monolayer adsorption isotherm best described the adsorption behaviour between DMSA@SiO2@Fe3O4 and Cu2+. The chemical compound dimercaptosuccinic acid (DMSA) has two sulphydryl groups and is analogous to dimercaprol, which is used in metal chelation (Shukla and Mittal, 2015). In DMSA@SiO2@Fe3O4 nanocomposite, DMSA molecules are oxidized around Fe3O4 NPs and a cage of disulphide-cross-linked DMSA molecules is formed around the Fe3O4 core, giving the particles a negative surface charge due to an overabundance of carboxylate groups (Cao et al. 2015). Negatively charge DMSA interacted with positively charge Cu2+ ions through electrostatic interaction to achieve chelation. As DMSA has a dithiol structure, it can embrace and shield the deleterious and 'soft' Cu2+ species. Therefore, DMSA has a strong copper binding ability to form a DMSA-Cu2+ complex.
Experimental design using RSM
The BBD was used to fit the obtained data into the second-order quadratic polynomial model with significant correlation coefficients and insignificant "lack of model fitness" values. Table 2 enlists the experimental runs and factors selected at different levels, namely low (− 1), medium (0) and high (+ 1) with a total of 17 experimental runs as per BBD for subsequent experimental studies including quintuplicate trials at the centre point (0, 0) runs. Significant interactions between the examined input factors and output variables (CQAs) were inferred from the polynomial models' coefficient analyses. Further, to investigate the interactions among the three key parameters (time, pH and copper concentration) that had a greater impact on % copper removal in the sample. A total of 17 experiments were conducted on each important component at three distinct levels (− 1, 0 and + 1). Equation 9 shows the primary impacts as well as the interactions between the chosen parameters.
where, β0, β1, β2 and β3 represent the intercept and coefficients of factors, viz.,\({X}_{1}\),\({X}_{2}\) and \({X}_{3}\), respectively, and β4, β5 and β6 represent the coefficients of interaction terms between X1 and X2, X1 and X3, X2 and X3, respectively, and β7, β8 and β9 are the corresponding coefficients of quadratic terms, \({X}_{1}^{2},{X}_{2}^{2}\mathrm{and }{X}_{3}^{2}\). The coefficients of several quadratic model terms used in the analysis of individual CQAs are listed in Table 3, along with R2 values and statistical significance (p) levels. Figure 6 depicts the 3D-response surface and matching 2D-contour plots for each of the different levels of the chosen CMAs such as pH, contact time and adsorbate concentration, on the CQAs (% copper removal) of synthesized DMSA@SiO2@Fe3O4 nanocomposite. These essential response surface methodology (RSM) techniques made it possible to create a thorough scientific understanding of the potential factor-response relationships between the selected CMPs and the relevant CQAs.
Figure 6a represents the curvilinear 3D-response surface plots between pH and contact time on the % removal capacity of the prepared nanocomposite. On moving towards acidic pH with increasing the contact time, % Cu removal was decreased sharply. Besides, keeping minimum contact time constant, the % removal capacity of adsorbent decreased with an increasingly acidic environment. While keeping low pH constant, with a gradual increase in contact time, negligible change in % removal capacity was observed. Figure 6b indicates the analogous result for the capacity of % removal of Cu2+ ions from aqueous solutions.
Figure 6c shows the curvilinear 3D-response plot between Cu2+ concentration and contact time on the effect of % Cu2+ removal. Maximum removal of copper was observed at an intermediate level of both factors. The corresponding analogous 2-D plot result was observed as depicted in Fig. 6d.
Figure 6e depicts the curvilinear 3D-response plot between Cu2+ concentration and pH of the medium on the effect of % Cu2+ removal by the adsorbent. With decreasing Cu2+ concentration, a gradual increase in % Cu2+ removal was observed. While increasing the pH of the medium, maximum % Cu2+ removal was observed at the intermediate level followed by a declining pattern observed at high pH. While keeping low pH constant, with decreasing Cu2+ concentration, a plateau pattern was observed up to mid-level followed by a declining trend in % Cu2+ removal capacity by the prepared nanocomposite. Figure 6f displays the corresponding 2D-contour plot of the 3D-response plot.
An overlay plot was created by superimposing the contour plots of individual responses to obtain the optimal operating conditions for Cu2+ removal by the nanocomposite. Figure 7a, b, c depicts the overlay space of the optimized formulation obtained after numerical and graphical optimization for maximum Cu2+ removal from aqueous solutions. Overall, results showed the mutual effects of different input variables such as contact time, pH and concentration on % Cu2+ removal from aqueous solutions. To get the intended outcomes, several CQAs were "traded off" to determine the most acceptable circumstances. The overlay space indicated the maximum % Cu2+ removal was obtained as 97.1% at pH of 7, Cu2+ ions concentration of 163 ppm and contact time of 109 min, as demarcated in the plot. In the past, several studies have been conducted on the adsorption of copper (II) by composite materials. Table 4 gives a performance comparison of the current work with the reported adsorbents for Cu2+ removal from aqueous solutions. Performing direct comparisons of copper adsorption capacity with different studies would be unfair because subsequent adsorption capacities are dependent on adsorption environmental conditions including pH of the solution, contact time and adsorbate concentration.
Conclusion and future perspective
In this work, DMSA-functionalized MNPs were used as effective magnetic nanoadsorbents in the removal of toxic heavy metals such as Cu2+ ions from aqueous solutions. The properties of synthesized nanocomposite were studied using a zeta analyser, DLS, EDX, HRTEM, FTIR, VSM and XRD. According to the kinetic studies, the adsorption reaction follows a pseudo-first-order kinetic model. According to the study of the adsorption isotherm, the Langmuir model was more consistent with the experimental data than the Freundlich model, implying the monolayer adsorption between nanoadsorbent and Cu2+ ions. For optimization of the copper removal process, BBD and RSM were employed. The adsorptive removal of copper was investigated as a function of four variables: pH, contact time and adsorbate concentration. The levels of the following three variables were found to be optimal for the maximum coper removal (97.1%) at pH 7, the initial adsorbate concentration of 163 ppm and the contact time of 109 min. In future, nanomaterial-based filter based on DMSA@SiO2@Fe3O4 nanocomposite can be used as potential material for copper removal from wastewater.
References
Adeli M, Yamini Y, Faraji M (2017) Removal of copper, nickel and zinc by sodium dodecyl sulphate coated magnetite nanoparticles from water and wastewater samples. Arab J Chem 10:S514–S521
Agarwal M, Singh K (2017) Heavy metal removal from wastewater using various adsorbents: a review. J Water Reuse Desalin 7(4):387–419
Ahmadi A, Heidarzadeh S, Mokhtari AR, Darezereshki E, Harouni HA (2014) Optimization of heavy metal removal from aqueous solutions by maghemite (γ-Fe2O3) nanoparticles using response surface methodology. J Geochem Explor 147:151–158
Ahmed SF, Mofijur M, Parisa TA, Islam N, Kusumo F, Inayat A, Badruddin IA, Khan TY, Ong HC (2022) Progress and challenges of contaminate removal from wastewater using microalgae biomass. Chemosphere 286:131656
Al-Hayali JHD, Cagrı-Mehmetoglu A (2022) Development of nanocomposite biodegradable film containing iron nanoparticles biosynthesised by Saccharomyces cerevisiae. Int J Food Sci Technol 57:3616
Al-Jabri MTK, Devi MG, Al Abri M (2018) Synthesis, characterization and application of magnetic nanoparticles in the removal of copper from aqueous solution. Appl Water Sci 8(8):1–7
Asthana A, Verma R, Singh AK, Susan MABH (2016) Glycine functionalized magnetic nanoparticle entrapped calcium alginate beads: A promising adsorbent for removal of Cu (II) ions. J Environ Chem Eng 4(2):1985–1995
Batool F, Akbar J, Iqbal S, Noreen S, Bukhari SNA (2018) Study of isothermal, kinetic, and thermodynamic parameters for adsorption of cadmium: an overview of linear and nonlinear approach and error analysis. Bioinorg Chem Appl. https://doi.org/10.1155/2018/3463724
Bhat SA, Hassan T, Majid S (2019) Heavy metal toxicity and their harmful effects on living organisms–a review. Int J Med Sci Diagn Res 3:106
Cao Y, Skaug MA, Andersen O, Aaseth J (2015) Chelation therapy in intoxications with mercury, lead and copper. J Trace Elem Med Biol 31:188–192
Chai WS, Cheun JY, Kumar PS, Mubashir M, Majeed Z, Banat F, Ho S-H, Show PL (2021) A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J Clean Prod 296:126589
Covaliu CI, Stoian O, Matei E, Paraschiv G, Tanasa E, CATRINA, G. A. (2021) Research on Copper Ions Removal from Wastewater Using Fe3O4 and Fe3O4-PVP Hybrid Nanomaterials. Mater Plastice 58(1):154–166
de Morais Nepel TC, Landers R, Vieira MGA, de Almeida Neto AF (2020) Metallic copper removal optimization from real wastewater using pulsed electrodeposition. J Hazard Mater 384:121416
Engwa GA, Ferdinand PU, Nwalo FN, Unachukwu MN (2019) Mechanism and health effects of heavy metal toxicity in humans. Poisoning Mod W-New Tricks Old Dog 10:70
Feitoza NC, Goncalves TD, Mesquita JJ, Menegucci JS, Santos M-KM, Chaker JA, Cunha RB, Medeiros AM, Rubim JC, Sousa MH (2014) Fabrication of glycine-functionalized maghemite nanoparticles for magnetic removal of copper from wastewater. J Hazard Mater 264:153–160
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189(1–2):147–163
Ghaemi N, Madaeni SS, Daraei P, Rajabi H, Zinadini S, Alizadeh A, Heydari R, Beygzadeh M, Ghouzivand S (2015) Polyethersulfone membrane enhanced with iron oxide nanoparticles for copper removal from water: application of new functionalized Fe3O4 nanoparticles. Chem Eng J 263:101–112
Gong J-L, Wang X-Y, Zeng G-M, Chen L, Deng J-H, Zhang X-R, Niu Q-Y (2012) Copper (II) removal by pectin–iron oxide magnetic nanocomposite adsorbent. Chem Eng J 185:100–107
Gu S-Y, Hsieh C-T, Gandomi YA, Yang Z-F, Li L, Fu C-C, Juang R-S (2019) Functionalization of activated carbons with magnetic Iron oxide nanoparticles for removal of copper ions from aqueous solution. J Mol Liq 277:499–505
Guo X, Mao F, Wang W, Yang Y, Bai Z (2015) Sulfhydryl-modified Fe3O4@ SiO2 core/shell nanocomposite: synthesis and toxicity assessment in vitro. ACS Appl Mater Interfaces 7(27):14983–14991
Harja M, Buema G, Lupu N, Chiriac H, Herea DD, Ciobanu G (2020) Fly ash coated with magnetic materials: Improved adsorbent for Cu (II) removal from wastewater. Materials 14(1):63
Hosseini S, Amini S, Khodabakhshi A, Bagheripour E, Van der Bruggen B (2018) Activated carbon nanoparticles entrapped mixed matrix polyethersulfone based nanofiltration membrane for sulfate and copper removal from water. J Taiwan Inst Chem Eng 82:169–178
Hubbe MA, Azizian S, Douven S (2019) Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: a review. BioResources 14(3):7582–7626
Hwang S, Umar A, Dar G, Kim S, Badran R (2014) Synthesis and characterization of iron oxide nanoparticles for phenyl hydrazine sensor applications. Sens Lett 12(1):97–101
Jadidian R, Parham H, Haghtalab S, Asrarian R (2014) Removal of copper from industrial water and wastewater using magnetic iron oxide nanoparticles modified with benzotriazole. Adv Mater Res 829:742
Kandasamy R (2017) A novel single step synthesis and surface functionalization of iron oxide magnetic nanoparticles and thereof for the copper removal from pigment industry effluent. Sep Purif Technol 188:458–467
Khan M, Javed M, Rehman M, Urooj M, Ahmad M (2020) Heavy metal pollution and risk assessment by the battery of toxicity tests. Sci Rep 10(1):1–10
Khan J, Lin S, Nizeyimana JC, Wu Y, Wang Q, Liu X (2021) Removal of copper ions from wastewater via adsorption on modified hematite (α-Fe2O3) iron oxide coated sand. J Clean Prod 319:128687
Kinuthia GK, Ngure V, Beti D, Lugalia R, Wangila A, Kamau L (2020) Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: community health implication. Sci Rep 10(1):1–13
Krstić V, Urošević T, Pešovski B (2018) A review on adsorbents for treatment of water and wastewaters containing copper ions. Chem Eng Sci 192:273–287
Kumar V, Pandita S, Sidhu GPS, Sharma A, Khanna K, Kaur P, Bali AS, Setia R (2021) Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 262:127810
Kurian G, Meyyappan A, Banu S (2015) One step synthesis of iron oxide nanoparticles via chemical and green route–an effective comparison. Int J Pharm Pharm Sci 7(1):70–74
Li W, Mu B, Yang Y (2019) Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology. Biores Technol 277:157–170
Li N, Li Z, Zhang L, Shi H, Li J, Zhang J, Zhang Z, Dang F (2020) One-step fabrication of bifunctional self-assembled oligopeptides anchored magnetic carbon nanoparticles and their application in copper (II) ions removal from aqueous solutions. J Hazard Mater 382:121113
Mahmouda AS, Youssefa NA, Abo El Nagab AO, Selimc M (2019) Removal of copper ions from wastewater using magnetite loaded on active carbon (AC) and oxidized active carbon (OAC) Support. J Sci Res Sci 36(1):226–247
Maleki A, Taheri-Ledari R, Soroushnejad M (2018) Surface functionalization of magnetic nanoparticles via palladium-catalyzed Diels–Alder approach. ChemistrySelect 3(46):13057–13062
Mishra D, Arora R, Lahiri S, Amritphale SS, Chandra N (2014) Synthesis and characterization of iron oxide nanoparticles by solvothermal method. Prot Met Phys Chem Surf 50(5):628–631
Mulungulungu GA, Mao T, Han K (2021) Efficient removal of high-concentration copper ions from wastewater via 2D g-C3N4 photocatalytic membrane filtration. Colloids Surf, A 623:126714
Ofomaja AE, Naidoo EB, Pholosi A (2020) Intraparticle diffusion of Cr (VI) through biomass and magnetite coated biomass: a comparative kinetic and diffusion study. S Afr J Chem Eng 32(1):39–55
Osman MS, Noorossaidy NA, Azis N, Salim NS, Zaini NA, Bashah NAA (2019) Synthesis of dimercaptosuccinic acid (DMSA) coated magnetic nanoparticles: feasibility on dye decolorization and heavy metal adsorption. IOP Conf Ser: Mater Sci Eng 551:012125
Pavan Kumar G, Malla KA, Yerra B, Srinivasa Rao K (2019) Removal of Cu (II) using three low-cost adsorbents and prediction of adsorption using artificial neural networks. Appl Water Sci 9(3):1–9
Plohl O, Ajdnik U, Gyergyek S, Ban I, Vesel A, Glaser TK, Zemljič LF (2019) Superior stability and high biosorbent efficiency of carboxymethylchitosan covalently linked to silica-coated core-shell magnetic nanoparticles for application in copper removal. J Environ Chem Eng 7(1):102913
Predescu AM, Matei E, Berbecaru AC, Pantilimon C, Drăgan C, Vidu R, Predescu C, Kuncser V (2018) Synthesis and characterization of dextran-coated iron oxide nanoparticles. R Soc Open Sci 5(3):171525
Rehman K, Fatima F, Waheed I, Akash MSH (2018) Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem 119(1):157–184
Shahrashoub M, Bakhtiari S (2021) The efficiency of activated carbon/magnetite nanoparticles composites in copper removal: Industrial waste recovery, green synthesis, characterization, and adsorption-desorption studies. Microporous Mesoporous Mater 311:110692
Sharma RK, Agrawal M (2005) Biological effects of heavy metals: an overview. J Environ Biol 26(2):301–313
Shukla J, Mittal BR (2015) Dimercaptosuccinic acid: A multifunctional cost effective agent for imaging and therapy. Indian J Nucl Med 30(4):295
Singh H, Bhardwaj N, Arya SK, Khatri M (2020a) Environmental impacts of oil spills and their remediation by magnetic nanomaterials. Environ Nanotechnol, Monit Manag 14:100305
Singh H, Jain A, Kaur J, Arya SK, Khatri M (2020b) Adsorptive removal of oil from water using SPIONs–chitosan nanocomposite: kinetics and process optimization. Appl Nanosci 10(4):1281–1295
Siu P, Koong LF, Saleem J, Barford J, McKay G (2016) Equilibrium and kinetics of copper ions removal from wastewater by ion exchange. Chin J Chem Eng 24(1):94–100
Sulaiman S, Azis R, a. S., Ismail, I., Man, H. C., Yusof, K. F. M., Abba, M. U., & Katibi, K. K. (2021) Adsorptive removal of copper (II) ions from aqueous solution using a magnetite nano-adsorbent from mill scale waste: synthesis, characterization, adsorption and kinetic modelling studies. Nanoscale Res Lett 16(1):1–17
Sultana N, Hossain S, Mohammed ME, Irfan M, Haq B, Faruque M, Razzak S, Hossain M (2020) Experimental study and parameters optimization of microalgae based heavy metals removal process using a hybrid response surface methodology-crow search algorithm. Sci Rep 10(1):1–15
Tamez C, Hernandez R, Parsons J (2016) Removal of Cu (II) and Pb (II) from aqueous solution using engineered iron oxide nanoparticles. Microchem J 125:97–104
Taylor AA, Tsuji JS, Garry MR, McArdle ME, Goodfellow WL, Adams WJ, Menzie CA (2020) Critical review of exposure and effects: Implications for setting regulatory health criteria for ingested copper. Environ Manage 65(1):131–159
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Mol, Clin Environ Toxicol 101:133–164
Tian Q-H, Wang X-Y, Mao F-F, Guo X-Y (2018) Absorption performance of DMSA modified Fe3O4@ SiO2 core/shell magnetic nanocomposite for Pb2+ removal. J Cent South Univ 25(4):709–718
Vardhan KH, Kumar PS, Panda RC (2019) A review on heavy metal pollution, toxicity and remedial measures: current trends and future perspectives. J Mol Liq 290:111197
Vareda JP, Valente AJ, Durães L (2019) Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: a review. J Environ Manage 246:101–118
Vasudevan S, Lakshmi J, Kamaraj R, Sozhan G (2013) A critical study on the removal of copper by an electrochemically assisted coagulation: equilibrium, kinetics, and thermodynamics. Asia-Pac J Chem Eng 8(1):162–171
Wang J, Guo X (2020) Adsorption kinetic models: Physical meanings, applications, and solving methods. J Hazard Mater 390:122156
Yadav VK, Ali D, Khan SH, Gnanamoorthy G, Choudhary N, Yadav KK, Thai VN, Hussain SA, Manhrdas S (2020) Synthesis and characterization of amorphous iron oxide nanoparticles by the sonochemical method and their application for the remediation of heavy metals from wastewater. Nanomaterials 10(8):1551
Yazid NA, Joon YC (2019) Co-precipitation synthesis of magnetic nanoparticles for efficient removal of heavy metal from synthetic wastewater. AIP Conf Proc 2124(1):020019
Ye L, Chai L, Li Q, Yan X, Wang Q, Liu H (2016) Chemical precipitation granular sludge (CPGS) formation for copper removal from wastewater. RSC Adv 6(115):114405–114411
Yruela I (2009) Copper in plants: acquisition, transport and interactions. Funct Plant Biol 36(5):409–430
Zhu K, Ju Y, Xu J, Yang Z, Gao S, Hou Y (2018) Magnetic nanomaterials: chemical design, synthesis, and potential applications. Acc Chem Res 51(2):404–413
Acknowledgements
The authors acknowledge DBT Builder Programme (BT/INF/22/SP41295/2020) at Panjab University for financial assistance. Dr Neha Bhardwaj acknowledges the DST INSPIRE faculty grant for support. The authors also acknowledge the Sophisticated Analytical Instrumentation Facility (SAIF) and Central Instrumentation Facility (CIL) at Panjab University, Chandigarh, for providing an instrumentation facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, D., Singh, H., Jain, A. et al. Response surface methodology for removal of copper (II) ions from aqueous solutions by DMSA@SiO2@Fe3O4 nanocomposite. Chem. Pap. 77, 1907–1920 (2023). https://doi.org/10.1007/s11696-022-02588-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02588-0