Abstract
The present study deals with the characterization of essential oils from umbels and seeds of Algerian wild (bitter) fennel (Foeniculum vulgare Mill. Var vulgare) by determining the chromatographic profile, lethal dose (LD50), antioxidant and antimicrobial activities, as well as a kinetic modeling study of the extraction of the seed-based essential oils. The extraction of essential oils (EOs) was performed by hydrodistillation using Clevenger for 3.5 and 6 h for the umbels and seeds, respectively. The two mathematical models from the experimental data show a good fit with an R2 of 99.99 and 98.94%. GC/MS analyses of fennel EOs showed that fennel was rich in different oxygenated monoterpenes compounds. However, while fenchone was the main compound in fennel seeds (FSEO), fennel umbel EO (FUEO) mainly contained α-pinene, o-cymene, sylvestrene, fenchone, Endo-fenchyl acetate, and carvacrol. The acute toxicity study of FSEO showed a lethal dose (LD50) of 4.9085 ± 0.1213 g/kg body weight in mice. Based on the free radical scavenging method using BHT as a positive control, the IC50 values were 9.9658 ± 0.057 mg/mL and 0.4570 ± 0.0456 mg/mL for FSEO and BHT, respectively. The study of antimicrobial activity in two gram-negative bacteria: Echerichi coli (ATCC 8739), Pseudomonas aeruginosa (ATCC 9027), and one gram-positive bacterium: Bacilus subtilis (ATCC 6633), as well as two fungal strains: Candida albicans (ATCC 10,231), Saccaromyces cerevisiaes (ATCC 9763), revealed that the fungal strains were more susceptible to FSEO and showed a significant fungicidal effect. The results of this study highlight the high quality of Algerian wild fennel, and the possibility of recovering it for use in the pharmaceutical, cosmetic, and food industries.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditional medicine has used natural-based materials to treat various diseases. Recently, medicinal and aromatic plants (MAPs) have become a vital natural resource of medication not only in developing countries but all over the world (Yahaya Gavamukulya et al. 2014). Thus, the World Health Organization estimates that more than 80% of the world population uses traditional medicine to treat diseases, illnesses, and other health problems (Alabri et al. 2013; Alhakmani et al. 2013). MAPs have been the subject of essential studies on antimicrobials and natural antioxidants (Singh et al. 2006). It is worth noting that about 20% of plants known and studied for their pharmaceutical characteristics have improved the treatment of infections and cancers (Altemimi et al. 2017). Currently, scientists are placing great interest in natural plant-derived substances to replace synthetic antioxidants applied in industry. Indeed, numerous studies have proved that such synthetic compounds have side effects and become more dangerous for human health (Ahmed et al. 2019). Similarly, the resistance developed by pathogenic microorganisms to synthetic antibiotics leads to the exploitation and valorization of these natural gifted substances (Roby et al. 2012; Kwiatkowski et al. 2017), including the essential oils (EO) and non-volatile substances.
Wild fennel (Foeniculum vulgare Mill, Var Vulgare) is among the spontaneous species frequently applied in traditional medicine. Local population knows it as "BESBES LAKHLA or EL VESVES IYEGHZER" and widely uses it to treat digestive disorders such as dyspepsia, bloating, intestinal pain, and as a vasodilator. It is a biennial MAP belonging to the Apiaceae (Umbelliferae) family (Purkayastha et al. 2012), native to the shores of the Mediterranean (Burkhardt et al., 2015; Gheisari Zardak et al. 2016). Various countries have developed its culture in different areas of the world (Bedini et al. 2016), to widely use it in the food, pharmaceutical, perfumery, and cosmetics industries (Zuobing et al. 2017). Thus, fennel has a high commercial value because of its broad potential for therapeutic applications such as balsamic, cardiotonic, digestive, lactagogue, tonic, antispasmodic, anti-inflammatory, expectorant, diuretic and laxative (Damjanovic´, 2004; Mazandrani et al. 2015). Fennel can take part in the treatment of respiratory problems (Solana et al. 2014; Pacifico et al. 2015; Pavela et al. 2015), kidney stones (Gholami Zali et al. 2017; Rahimmalek et al. 2014), nervous disorders (Pavela et al. 2015; Hatami et al. 2017), menstrual disorders (Ostad et al. 2004, Pavela et al. 2015), menopausal disorders, hirsutism, osteoporosis (Hassanpour et al. 2017), and also has carminative, analgesic (Hatami et al 2017), antibacterial, antifungal, antithrombotic, antioxidant, estrogenic, antidiabetic, gastroprotective, hepatoprotective (Telci et al. 2009; Rather et al., 2012; Pavela et al., 2015; Gholami Zali et al. 2017), antitumor and cytoprotective properties (Majdoub et al. 2017).
The third European pharmacopeia has reported two fennel drugs based on bitter (Foeniculum Vulgare Miller spp. Vulgre var Vulgare) and sweet (Foeniculum vulgare Miller subsp. vulgare var. dulce (Miller) Thellung) fennel seeds (Damjanovic et al. 2004). Fennel is also considered a flavor used in the food, beverage, and cosmetics industry (Salama et al. 2013; Baby et al. 2016; Benmoussa et al. 2016; Zuobing et al. 2017).
Several studies reported that fennel EO shows chemical polymorphisms significantly different depending on variety (Diäaz-Maroto et al. 2005). Some authors have reported that trans anethole (Rahimmalek et al. 2014), fenchone (present in bitter varieties), (Damjanovic et al. et al. 2005; Hatami et al. 2017), estragol (methyl chavicol) (Diäaz-Maroto et al. 2005; M. Krizman et al., 2006; M., Hammouda et al., 2013), limonene (Akgul et al. 1988; Bahmani et al. 2015), and α-pinene are the main EO compounds (Senatore et al. 2013; Baby et al. 2016; Zuobing et al. 2017). Muckensturm et al. (1997), Gross et al. (2009), and Telci et al. (2009) recognized three chemotypes: the estragole type, the estragol/anethole type, and the anethole type, while Senatore et al. (2013) cited two chemotypes (trans-anethole and estragole) of bitter fennel.
In Algeria, phytochemical studies on local fennel EO are scarce. We cite those published by Zoubiri et al. (2010, 2011), Ouis et al. (2014) on the EO of cultivated fennel, and that of Lazouni et al. (2007) on wild fennel. This latter will be the object of the present study. In this context, we will present the chemical composition of their EO obtained from seeds and umbels (obtained after post-harvesting treatment). In the same way, we will illustrate a study on the toxicity of FSEO and their antioxidant and antimicrobial potential.
Materials and methods
Plant material
Identifying this plant at the National School of Agronomy (ENSA-Algiers) confirmed a wild fennel species. The wild fennel umbels (with mature green seeds) used in this study were harvested in November 2015 in the locality of Blida (Algeria), located 45 km southwest of Algiers. This plant material shade-dried is processed to separate the seeds from the umbels and then stored in paper bags until use.
Water content
The assessment of the water content of the samples involved the use of 3 g of each grain sample to place in an oven at 105 °C under atmospheric pressure for 24 h (Bouallegue et al., 2015). The final samples used for the extraction processes had a water content of 11.90 ± 1.09 and 10.44 ± 0.14 g H2O/ 100 g−1 db (dry basis) for seeds and umbels, respectively.
Treatment and extraction
Extraction of essential oils
Extraction of essential oils (EO) from fennel seeds and umbels, used Clevenger type hydrodistillation with about 40 g and 60 g for 3.5 and 6 h as extraction time, respectively. The solid to liquid ratio was 1:10. with the extraction yield expressed as the ratio of the mass of EO obtained to the mass of dry plant material.
Kinetic modeling of fennel seed essential oils
Because of highly agitated dynamic extraction conditions, a negligible external resistance (NER) characterizes the hydrodistillation. The internal diffusion of water within the matrix was the limiting process. Thus, the literature proposes two mathematical kinetic models to simulate solid–liquid extraction (Benyoussef et al. 2013; Segovia et al. 2014). The first equation (Eq. 1) consists of one immersion term, while the second (Eq. 2) consists of two immersion terms for the studied matrix.
where Y is the yield at time t, Ymax is the maximum yield obtained at equilibrium (t = ∞); k, and k1 and k2 are the kinetic constants (min−1), f and (1 − f) are the fractions of solute diffusing at two different rates. The second model (Eq. 2) derives from the first model (Eq. 1) by assuming that the solid matrix has two extraction stages; surface interaction and internal diffusion or two types of cell structures; broken and intact. It is worth noticing that this part of the study did not imply umbels because of their poverty in EOs.
Chromatographic analyses of fennel essentials oils
Coupled Gas Chromatography / Mass Spectrometry (GC—MS) allows analyzing EO. In the present case, the mass detector was Hewlett Packard type (model 5973), the HP-5SM column (30 m * 0.25 mm * 0.25 μm) with a stationary phase at 5% phenyl and 95% dimethylpolysiloxane.
The injector temperatures, the GC–MS interface, and the ion source were maintained at 250 °C, 280 °C, and 230 °C, sequentially. The oven temperature programmed for the analysis was 60 °C (8 min), 60–250 °C (2 °C/min), and held at 250 °C for 15 min. The volume injected was 0.2 μL in split mode (1/20), and the carrier gas was helium (purity N60) at 0.5 μL/min. This system allowed identifying the compounds by comparing the retention indices calculated using the homologous series of C8-C28 n-alkanes, and their mass spectrum with those in the literature (Adams 2007) and NIST 02.L (National Institute of Standards and Technology) and Wiley 7n.1 libraries mass spectral libraries.
Biological investigation of fennel essentials oils
These different parts are related only to FSEO. Indeed, FUEO had such a low EO yield that the involving tests were only the yield and the GC–MS analyses without exploiting the kinetic and biological study part.
Acute toxicity study of fennel seeds essential oil
To estimate the lethal dose (LD50) of FSEO, a population of 30 NMR-I mice (25–30 g) was divided into 5 groups (6 mice each). All animals underwent a 12-h fast before the experiment. The first group served as vehicle control and received Twen80 (1%), and the other groups received a single oral dose of FSEO (3, 4, 5, and 6 g/kg) suspended in the vehicle (Turner 1965). Animal mortality and symptoms of toxicity were observed during the first 24 h and the next 22 days. After this period, the dead animals were counted to assess the LD50 using the method described by Miller and Tainter (Randhawa et al. 2009).
Free radical scavenging capacity of fennel seeds essential oil
The study of the antioxidant activity of FSEO was carried out using the free radical scavenging method DPPH (2, 2-diphenyl-1-picrylhydrazyl), and by adopting the protocol described in the literature (Kontogiorgis et al. 2016; Khaled Khodja et al. 2018). BHT (butylated hydroxytoluene) was used as a positive control. 3 ml of FSEO solution (1, 0.8, 0.6, 0.4 and 0.2 mg/mL) and that of BHT (1, 0.8, 0.6, 0.4 and 0.2 mg/mL) were added separately to 1 ml of DPPH solution (0.1 mM) prepared in ethanol. The mixture was shaken in the dark for 30 min, and then the absorbance (Abs) of each sample was measured at 517 nm against pure ethanol using a UV-1800 type SHIMADZO-UV spectrophotometer. The percentage of inhibition of the free radical DPPH was evaluated using the formula of percent inhibition:
where Abs blank is the absorbance of the control (containing all reagents except samples), and Abs sample is the absorbance of the sample incubated after 30 min. From the graph of the radical scavenging activity versus the concentration of the sample, it was possible to determine the sample concentration giving 50% of DPPH scavenging activity (IC50). These tests performed in triplicate allow giving IC50 value as the mean ± standard deviation (SD).
Antimicrobial study of fennel seeds essential oil
Microorganism tests
The antimicrobial activity of FSEO carried out at Sandal Research and Development Center in Algiers (Algeria) implied five microbial strains of the ATCC (American Type Culture Collection) type (Table 1).
Qualitative study of antimicrobial activity
The qualitative assessment of the antimicrobial activity of FSEO followed the disk diffusion method (Abdelli et al. 2016). It consists of detecting the sensitivity of microbial strains by direct contact with FSEO on an agar culture medium (Muller Hinton for bacteria and Sabouraud for fungi) inoculated by a microbial suspension. Sterile disks (6 mm) soaked with FSEO (15, and 20 µL) put on the surface, underwent an incubation at 37 °C for 24 h for bacteria and 48 h at 27 °C for fungi. The results exclusively come from measuring the diameter of the inhibition halos in mm. FSEO activity implied 4 levels of non-sensitive (0) for a diameter of less than 8 mm, moderately sensitive ( +) for a diameter of 8 to 14 mm, sensitive (+ +) for a diameter of 14 to 20 mm, and very sensitive (+ + +) for a diameter greater than 20 mm (Sfeir et al., 2013). Each strain includes two repetitions.
Quantitative study of antimicrobial activity
This part was performed only for yeasts, as they showed significant activity compared to bacteria strains, using the method cited by Hammer et al. (1999). A series of dilutions was prepared from the FSEO from 2 up to 0.03% in the appropriate media previously added with Tween 80 (to solubilize the FSEO in the culture medium) at a final concentration of 1% (Kwiatkowski et al. 2017). The sterile disks were placed aseptically in the dishes and soaked by the microbial suspension. After an adequate incubation, the concentration with no visible microbial growth reveals the minimum inhibitory concentration (MIC).
The disks corresponding to the concentrations ascending from the MIC were removed aseptically from the dishes and placed in the Petri dishes containing the appropriate agar to incubate for 48 h at 27 °C. The minimum fungicidal concentration (MFC) was the concentration with no visible microbial growth.
The MFC/MIC ratio allows the classification type of antimicrobial agent (FSEO): fungicidal (MFC/MIC less than 4) or bacteriostatic (greater than 4) (Sbayou et al. 2014; Bertella et al., 2018).
Statistical analyses
All results included means ± standard deviation (SD), except the LD50 value, which gives the geometric means plus their respective confidence limits (95%). ANOVA analyses define the significant differences (P < 0.05%), then multiple comparison tests of Tukey’s post hoc test were applied between the averages. The statistical analyses have used Software XLStat (XLStat, Paris, France).
Results and discussion
Kinetic modeling of fennel seeds essential oil
The FSEOs’ kinetic presented in Fig. 1 notes three essential steps for the extraction process after heating the plant material for about 20 min. The first one with about 52% of FSEO was linear rapid (60 min). The second was hyperbolic slow (60 min to 360 min), including recovering about 94% of FSEO. The equilibrium stage corresponded to the last and final step (after 360 min). Subsequently, the chromatographic and biological measurements fixed this FSEO extraction time of 6 h.
The literature reported that the kinetics of FSEO extraction gives three specific steps: firstly, a linear increase in yield presenting extraction of surficial EO, then a slow evolution in yield, and finally, the horizontal line corresponding to the end of extraction (Benmoussa et al. 2016).
As shown in Fig. 1 and Table 2, the two mathematical models reasonably fit the extraction kinetics with significant values of adjusted R2. For the single-site model, it is worth noting a kinetic constant (k) value of 0.013 min−1, which reveals a rapid extraction till the equilibrium stage. This model explains the presence of one type on the matrix from which the EO diffuses with this constant rate until exhaustion.
For the two-site model, it is worth perceiving a first kinetic constant with k1 = 0.016 min−1, revealing that firstly we assisted quick extraction processing and the main fraction 80.7% (f = 0.807) of FSEO was achieved. However, a small value of the second kinetic constant k2 = 0.03 min−1 is noted; it reveals that we have a slow extraction of the remaining fraction of FSEO and reached the equilibrium extraction stage. However, it does not conclude that the unique-site model can better explain the kinetic profile of FSEO extraction.
Yield on essential oils from seeds and umbels of Algerian wild fennel
EOs obtained from umbels and seeds had a golden yellow (FUEO) and pale yellow (FSEO) color with a characteristic odor. The EO yield was about 0.293 ± 0.016% and 2.176 ± 0.002% for umbels and seeds, respectively. Stefanini et al. (2006a), Ilić et al. (2019), and Rezaei-Chiyaneh et al. (2019) reported that seeds were the richest organ in EOs. Our results are comparable to those reported in the literature for wild fennel of different origins (Table 3). The significant variability observed (Table 3) on EO yield is related to various factors such as environmental conditions, geographical origin, genetic factors, maturity and farming practice conditions, and extraction process and time (Telci et al. 2009; Bahmani et al. 2015; Benmousa et al. 2016; Abdellaoui et al. 2017; Ahmed et al. 2019).
Chromatographic profile of essential oils from seeds and umbels of Algerian wild fennel
Table 4 gathers the results of the chromatographic (Fig. 2) analyses of the EOs. We noted a significant difference for the EOs of seeds (FSEO) and umbels (FUEO) of Algerian wild fennel. Thus, while FSEO has oxygenated monoterpenes (85.476%) with fenchone (83.63%) as the main compounds and monoterpenes with limonene (8.695%) as another important compound, FUEO was rich in monoterpenes (57.755%), mainly α-pinene (23.513%), β-pinene (1.386%), Myrcene (1.994%), α-phellandrene (3.684%), o-cymene (18.309%) and sylvestrene (8.869%), and oxygenated monoterpenes (33.401%) whose; fenchone (13.637%), carvacrol (6.599%) and Endo-fenchyl acetate (13.165%).
Some compounds were present only in FSEO, such as sabinene, γ-terpinene, trans-pinenehydrate, and terpinene-4-ol, or only in FUEO like β-pinene, o-cymene, sylvetrene, Endo-fenchyl acetate, isobornyl acetate, carvacrol, and germacrene-D).
The compounds which were present in the two organs of the plant but in variable contents were principally: α-pinene (22.463, 1.307%), β-pinene (1.386, 0.062%), camphene (0.574, 0.357%), myrcene (1.994, 2.14%), α-phellandrene (3.684, 0.64%), and Fenchone (13.018, 83.63%), respectively.
These results agreed with other works which reported that the chemical composition was very variable between the different parts of the same plant (Senatore et al. 2013) and that seeds were richer in fenchone (4.75 to 13.85) compared to other parts of the plant (Akgul et al. 1988).
Despite the significant difference in the chemical composition of FSEO and FUEO, there was not a notable difference in refraction index values (Table 4) which were 1.4745 and 1.4618, respectively. This concords with the literature reported values (1.484 to 1.508) (Garnéro, 1996), but is different from that (1.55) cited for Indonesian FSEO (Damayanti et al. 2012).
Our results were different from those reported for wild fennel from different regions in the world (Table 5), for which the most abundant chemotypes were: trans-anethole, estragole, and fenchone. Besides, we noted that the Sylvestrene detected in FUEO was cited as a trace (1.7%) in the EO of Iranian fennel leaves (Shahmokhtar et al. 2017).
Also, it has been reported that the same variety can produce a variable EO chromatographic profile depending on genetic, climatic, geographic, and agronomic conditions (Badgujar et al. 2014; Zuobing et al. 2017; Bahmani et al. 2015; Rezaei-Chiyaneh et al. 2019), maturity stage of seed, soil quality, phytopathological insects, shine, rain, microorganisms (Stefanini et al. 2006b), the hydrodistillation method, the hydro module (Ilić et al. 2019), adaptive plant metabolism (Roby et al. 2012; Ahmed et al. 2019), which were reported for bitter fennel (Stefanini et al. 2006b) and vary according to the species, origin, and season (Bassyouni et al. 2018). On the other hand, we have exploited the same species (from Dellys; a small Mediterranean town in northern Algeria's coastal Boumerdes province at 50 km), which has shown a different chromatographic profile with the dominance of Fenchone (39.05–43.55%) and trans-anethole (34.79–42.86%) (Dahmani 2014). The dominance of fenchone, a typical Fennel EO compound (Napoli et al, 2010), gives excellent value to these EOs because of their importance in flavors, fragrances, and cosmetic and food preparations (Gonzalez-Rivera et al. 2015). Moreover, this composition is highly interesting for the pharmaceutical industry since the therapeutic effects of EOs correlate with their richness in oxygenated compounds (Yaldiz et al. 2019)..
Acute toxicity (LD50) of essential oils of Algerian wild fennel seeds
The therapeutic index of natural plant substances is set by studying the acute toxicity, which estimates the lethal dose (LD50) (Ahmed and Azmat 2014; Bertella et al. 2018). In the present study, the toxicity investigation of FSEO was performed in mice and revealed a value of LD50 of 4.9085 ± 0.1213 g/kg body weight. According to the Hodge and Sterner scale (Svarc-Gajiae et al. 2009; Ahmed and Azmat 2014), FSEO can be classified as mildly toxic since LD50 is between 500 and 5000 mg/kg. Although Mills et al. (2006) reported that LD50 of fennel EO varies from 1.3 to 4.5 g/kg, our results were better than those (1038 ml/kg, 3.12 g/kg, 3.8–1326 mg/kg rat body weight) reported in the literature (Albert-Puleo 1980; Ostad et al. 2001; Ozbek et al. 2005; European Medicines Agency 2008). Garnero (1996) also cited a LD50 of 4.52 mL/kg (4.06–5.05 mL/kg) in the acute oral toxicity of fennel EO in rats and greater than 5 g/kg in the acute dermal toxicity in rabbits. Several research works were devoted to studying the acute toxicity of EO of plant origin. The present study has shown that Algerian FSEO is safer than most of those reported for Lebanese Prangos Asperula Boiss (LD50 of 1.05 ml/kg) (Hilan et al. 2009), Brazilian EO (LD50 of 0.5 g/kg) of Mesosphaerum sidifolium (L'Hérit) (Rolim et al. 2017), Algerian Thymus Fontanesii Boiss (LD50 of 0.885 ± 0.08 g/kg) (Mouhi et al, 2017), and Algerian Artemisia herba-alba (LD50 of 0.615 g/kg) (Bertella et al. 2018).
Antioxidant activity of essential oils of Algerian wild fennel seeds
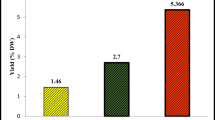
While DPPH free radical scavenging method could assess the in-vitro antioxidant activity of FSEO, BHT was used as a positive control. IC50 values were evaluated graphically (Fig. 3) and reordered in Table 6. BHT positive control showed better antioxidant capacity with IC50 value of 0.4570 ± 0.0456 mg/mL compared to that of FSEO with IC50 value of 9.9658 ± 0.057 mg/mL. This finding concord with previous studies which reported that synthetic antioxidants have a better antioxidant capacity (De Marino et al. 2007; Anwar et al. 2009; Abdellaoui et al. 2017). Our result was comparable to those reported by Abdellaoui et al. (2017) (10.62 ± 0.33 mg/mL) for Moroccan wild FSEO, but better than that obtained (13.08 ± 0.34 mg/mL) for the same cultivated species, and those cited for Egyptian FSEO (15.33 mg/mL) by Shahat et al. (2011). As well as for the aerial part (15.6 mg/mL) of Tajikistan fennel Khammassi et al. (2018) have reported an IC50 value ranging from 12 to 38.13 mg/mL for Tunisian wild SEO. Ahmed et al. (2019) noted IC50 of 15.66 mg/g and 141.82 mg/g, for the EO of Chinese and Egyptian sweet fennel seeds, respectively. However, Anwar et al. (2009) reported a strong antioxidant capacity (10.62 ± 0.33 μg/mL) from FSEO from Pakistan.
Many ongoing works on the antioxidant capacity of EO from fennel show a considerable variation that the authors justify by several factors such as the origin, the climatic conditions, the method, the duration of extraction, and the chemical composition of EO (Abdellaoui et al. 2017; Ahmed et al. 2019; Yaldiz et al. 2019). The study of the antioxidant activity of EO has taken a great interest of searchers to replace synthetic antioxidants used in the food and pharmaceutical industries. The present study encourages the interest in valuing the Algerian wild FSEO as a natural antioxidant that can replace synthetic antioxidants. It is worth highlighting that these herbal-based substances are safer, more accepted by consumers, and sometimes more practical.
Antimicrobial activity of essential oils of Algerian wild fennel seeds: Qualitative study of antimicrobial activity
The disk diffusion method is a method to highlight the antimicrobial capacity against the tested microbial strains. Figure 4 illustrates the results, and Table 7 highlights the sensitivity degrees of the tested strains against FSEO. We found that yeasts were more sensitive to FSEO than bacteria. Likewise, the dose used had a more significant impact on the diameter of the inhibition zone for yeasts than for bacteria. Elsewhere, some authors reported that the diameter of the inhibition zone depended on the EO dose. Both fungal strains were susceptible for 15 µl and 20 µl of FSEO. They registered inhibition zones of 20.1 ± 0.45 mm and 26 ± 0.375 mm with Candida albicans, 15.9 ± 1.2 mm and 27.083 ± 1.292 mm with Saccharomyces cerevisiaes, respectively. However, the gram-positive Bacillus subtilis strain was more sensitive than the moderately sensitive, gram-negative Escherichia coli strain, while Pseudomonas aeruginosa was resistant to the tested doses. In general, gram-negative strains are more resistant than gram-positive bacteria (Saviuc et al. 2012). Mashareq et al. (2016) reported the resistance of Pseudomonas aeruginosa to the EO (91.8% anethole) from fennel. This bacterium is known for its resistance because of its membrane's hydrophilic nature, which prevents the penetration of EO (Ilić et al. 2019). Our results agree with those found by A. Shahat et al. (2011) and Ilić et al. (2019), who noted significant antifungal activity of fennel EO against Candida Albicans. Ozken et al. (2006) showed variable fungistatic activity depending on the EO dose of bitter Turkish fennel. Boumahdi et al. (2020) also noted this finding by studying the same microbial strains with Algerian Pimpinella anisum essential oil.
Likewise, FSEO shows significant antifungal capacity against three fungal strains (Alternaria alter, Fusarium oxysporum, and Aspergillus flavus) (Javed et al. 2012). On the other hand, our results were lower than those found by Diao et al. (2014) for the antibacterial activity of fennel EO.
Quantitative study of antimicrobial activity
The disk diffusion method results showed that only fungal strains were sensitive to FSEO. This observation is crucial for determining the MIC and MFC for these strains. Table 8 presents the results with similar values of MIC (0.25%) and MFC (1%) for both tested fungal strains. The CMF/MIC ratio values, which do not exceed 4, can organize the fungicidal effects. Our results were higher than that found for Candida albicans (MIC of 0.5 and 0.4%) by Hammer et al. (1999) and by Gulfraz et al. (2008) for SEO, respectively. Moreover, Roby et al. (2012) and Ilić et al. (2019) noted for this strain a MIC of 10 µg/mL and 25 µg/mL with FSEO. The study of the antifungal activity of fennel EO against ten clinical strains of Candida albicans type showed a significant sensitivity of the strains which were resistant to fluconazole or sensitive to dependent doses (Bassyouni et al. 2018). Likewise, Kammasi et al. (2018) have reported a fungicidal activity of Tunisian wild fennel. The antimicrobial activity of EO depends on many factors, such as the method used, microbial strains, and chemical composition (structure and functional groups). Some authors (Javed et al. 2012, Bassyouni et al. 2018, and Ilić et al. 2019) reported that the antimicrobial activity of fennel EO was linked to anethol and/or its isomer and other compounds as fenchone, estragole, and limonene. Moreover, Senatore et al. (2013), Bertellla et al. (2018), and Ilić et al. (2019) also cited the presence of mono and sesqui-terpene hydrocarbons without neglecting synergistic interactions effects of minor compounds. Although the mechanism of their actions was not well studied, it may be due to the lipophilic character of EOs, which make them able to penetrate the cell membrane and mitochondria, causing structural damage enhancing permeability. That results in stopping the cell's biological metabolism and death (Khammassi et al. 2018; Ilić et al. 2019).
Conclusion
EO extraction from Algerian wild fennel seeds and umbels by hydrodistillation has shown that the seeds were richer in EO, which agrees with that reported by previous studies. Mathematical modeling of FSEO kinetic extraction shows good results explaining the extraction phenomena. GC–MS analysis revealed new chromatographic profiles for the two studied organs. Fenchone (83.63%) and limonene (8.695%) are the major compounds of the FSEO, while α-pinene (23.513%), β-pinene (1.386%), Myrcene (1.994%), α-phellandrene (3.684%), o-cymene (18.309%), sylvestrene (8.869%), fenchone (13.637%), carvacrol (6.599%) and Endo-fenchyl acetate (13.165%) are the major compounds of FUEO. The FSEO was non-toxic with LD50 of 4.9085 ± 0.1213 g/kg and showed a good antioxidant capacity (IC50 of 9.9658 ± 0.057 mg/mL), matching previous studies. Likewise, a good antimicrobial activity has been noted, especially against fungal species. Finally, the present study results highlight the interest in valuing the Algerian wild fennel to replace the synthetic antioxidants used in industries (pharmaceuticals, cosmetics, and food) and other antimicrobial agents that present resistance problems developed by pathogenic microorganisms in hospitals and other environments. As a perspective of the whole study, which gave a new chromatographic profile of fennel EO, we plan to extend the future investigations and add to the present work other Algerian varieties from other regions, including very severe desert areas. We plan to perform a genetic identification of the fennel samples and a biological valorization of fennel EOs rich in interesting chemical compounds.
References
Abdellaoui M, Bouhlali E-DT, Kasrati A, El Rhaffari L (2017) The effect of domestication on seed yield, essential oil yield and antioxidant activities of fennel seed (Foeniculum vulgare Mill) grown in Moroccan oasis. J Assoc Arab Univ Basic Appl Sci 24:107–114. https://doi.org/10.1016/j.jaubas.2017.06.005
Abdelli M, Moghrani H, Aboun A, Maachi R (2016) Algerian Mentha pulegium L leaves essential oil: chemical composition, antimicrobial, insecticidal and antioxidant activities. Ind Crops Prod 94:197–205. https://doi.org/10.1016/j.indcrop.2016.08.042
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry, 4th ed
Ahmed M, Azmat A (2014) Acute toxicity (lethal dose 50 calculation) and histopathological effects of methanolic extract of berberis vulgaris in mice. World J Pharm Res 3(9):1439–1448
Ahmed AF, Shi M, Liu W, Kang W (2019) Comparative analysis of antioxidant activities of essential oils and extracts of fennel (Foeniculum vulgare Mill) seeds from Egypt and China. Food Sci Human Wellness 8(1):67–72. https://doi.org/10.1016/j.fshw.2019.03.004
Akgul A, Bayrak A (1988) Comparative volatile oil composition of various parts from Turkish bitter fennel (Foeniculum vulgare var.vulgare). Food Chem 30:319–323
Alabri THA, Al Musalami AHS, Hossain MA, Weli AM, Al-Riyami Q (2013) Comparative study of phytochemical screening antioxidant and antimicrobial capacities of fresh and dry leaves crude plant extracts of Datura metel L. J King Saud Univ Sci. https://doi.org/10.1016/j.jksus.2013.07.002
Albert-puleo M (1980) Fennel and anise as estrogenic agents. J Ethnopharmacol 2:337–344
Alhakmani F, Kumar S, Khan SA (2013) Estimation of total phenolic content, in-vitro antioxidant and anti-inflammatory activity of flowers of Moringa oleifera. Asian Pac J Trop Biomed 3(8):623–627
Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA (2017) Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. https://doi.org/10.3390/plants6040042
Anwar F, Ali M, Hussain AI, Shahid M (2009) Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare Mill.) seeds from Pakistan. Flavour Fragr J 24:170–176. https://doi.org/10.1002/ffj.1929
Baby KC, Ranganathana TV (2016) Effect of enzyme pre-treatment on extraction yield and quality of fennel (Foeniculum vulgare) volatile oil. Biocatal Agric Biotechnol 8:248–256. https://doi.org/10.1016/j.bcab.2016.10.001
Badgujar SB, Patel VV, Bandivdekar AH (2014) Foeniculum vulgare mill: a review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology Hindawi Publishing Corporation. BioMed Res Int 2014:32. https://doi.org/10.1155/2014/842674
Bahmani K, Darbandi AI, Ramshini HA, Moradi N, Akbari A (2015) Agro-morphological and phytochemical diversity of various Iranian fennel landraces. Ind Crops Prod 77:282–294. https://doi.org/10.1016/j.indcrop.2015.08.059
Bassyouni RH, Wali IE, Kamel Z, Kassim MF (2018) Fennel oil: A promising antifungal agent against biofilm forming fluconazole resistant Candida albicans causing vulvovaginal candidiasis. J Herb Med 15:100227. https://doi.org/10.1016/j.hermed.2018.08.002
Bedini S, Bougherra HH, Flamini G, Cosci F, Belhamel K, Ascrizzi R, Conti B (2016) Repellency of anethole- and estragole-type fennel essential oils against stored grain pests: the different twins. Bull Insectol 69(1):149–157
Benmoussa H, Farhat A, Romdhane M, Bouajila J (2016) Enhanced solvent-free microwave extraction of Foeniculum vulgare mill essential oil seeds using double walled reactor. Arab J Chem. https://doi.org/10.1016/j.arabjc.2016.02.010
Benyoussef E-H, Saibi S (2013) Influence of essential oil composition on water distillation kinetics. Flavour Fragr J 28:300–308. https://doi.org/10.1002/ffj.3169
Bertellaa A, Benlahcena K, Abouamama S, Pintob DCGA, Maamara K, Kihala M, Silvab AMS (2018) Artemisia herba-alba Asso. Essential oil antibacterial activity and acute toxicity. Ind Crops Prod 116:137–143. https://doi.org/10.1016/j.indcrop.2018.02.064
Boumahdi Y, Moghrani H, Nasrallah N, Ouarek S, Maachi R (2020) Microwave-assisted hydrodistillation of the essential oil from Algerian Pimpinella anisum seeds. Flavor Fragr. https://doi.org/10.1002/ffj.3614
Burkhardt A, Sintim HY, Gawdea A, Cantrell CL, Astatkie T, Zheljazkova VD, Schlegel V (2015) Method for attaining fennel (Foeniculum vulgare Mill.) seed oil fractions with different composition and antioxidant capacity. J Appl Res Med Aromat Plants 2(3):87
Dahmani K (2014) Contribution à l’étude de l’activité antimicrobienne des huiles essentielles des différentes espèces de fenouil algérienne. http://repository.usthb.dz:8080/handle/123456789/2564
Damayanti A, Setyawan E (2012) Essential oil extraction of fennel seed (Foeniculum vulgare) using steam distillation. Int J Sci Eng 3(2):12–14
Damjanovic B, Lepojevic Z, Zivkovic V, Tolic A (2004) Extraction of fennel (Foeniculum vulgare Mill.) seeds with supercritical CO2: comparison with hydrodistillation. Food Chem 92:143–149. https://doi.org/10.1016/j.Foodchem.2004.07.019
De Marino S, Gala F, Borbone N, Zollo F, Vitalini S, Visioli F, Iorizzi M (2007) Phenolic glycosides from Foeniculum vulgare fruit and evaluation of antioxidative activity. Phytochemistry 68:1805–1812. https://doi.org/10.1016/j.phytochem.2007.03.029
Diäaz-Maroto MC, Diäaz-Maroto IJ, Nchez-Palomo HES, Rez-Coello MSP (2005) Volatile components and key odorants of fennel (Foeniculum vulgare Mill.) and Thyme (Thymus vulgaris L.) oil extracts obtained by simultaneous distillation-extraction and supercritical fluid extraction. J Agric Food Chem 53:5385–5389. https://doi.org/10.1021/jf.050340
Diao WR, Hua QP, Zhang H, Xu JG (2014) Chemical composition antibacterial activity and mechanism of action of essential oil from seeds of fennel Foeniculum vulgare Mill. Food Control 35:109e116. https://doi.org/10.1016/j.foodcont.2013.06.056
Ehsanipour A, Razmjoo J, Zeinali H (2011) Effect of nitrogen rates on yield and quality of fennel (Foeniculum vulgare Mill.) accessions. Ind Crops Prod 35:121–125. https://doi.org/10.1016/j.indcrop.2011.06.018
Garnéro J (1996) Huiles essentielles, techniques de l’Ingénieur, traité Constantes physicochimiques, Technique d’ingénieur K 345–1 ; K 345–39
Gavamukulya Y, Abou-Elella F, Wamunyokoli F, AEl-Shemy H (2014) Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac J Trop Med 7(Suppl 1):S355–S363
Gheisari Zardak S, Dehnavi MM, Salehi A, Gholamhoseini M (2016) Responses of field grown fennel (Foeniculum vulgare Mill.) to different mycorrhiza species under varying intensities of drought stress. J Appl Res Med Arom Plants. https://doi.org/10.1016/j.jarmap.2016.09.004
Gholami Zali AG, Ehsanzadeh P (2017) Exogenous proline improves osmoregulation, physiological functions, essential oil, and seed yield of fennel. Ind Crops Prod 111:133–140. https://doi.org/10.1016/j.indcrop.2017.10.020
Ghouati Y, Belaiche T, Ouhssine M, Amechrouq A, Tahiri A, Chakir S (2014) Antimicrobial property of the essential oil from the Moroccan fennel fruits. Global J Pure Appl Chem Res 1(1):25–33
Gonzalez-Rivera J, Duce C, Falconieri D, Ferrari C, Ghezzi L, Piras A, Tine MR (2015) Coaxial microwave assisted hydrodistillation of essential oils from five different herbs (lavender, rosemary, sage, fennel seeds and clove buds): chemical composition and thermal analysis. Innov Food Sci Emerg Technol. https://doi.org/10.1016/j.ifset.2015.12.011
Gross M, Lewinsohn E, Tadmor Y, Bar E, Dudai N, Cohen Y, Friedman J (2009) The inheritance of volatile phenylpropenes in bitter fennel (Foeniculum vulgare Mill. var. vulgare, Apiaceae) chemotypes and their distribution within the plant. Biochem Syst Ecol 37:308–316. https://doi.org/10.1016/j.bse.2009.05.007
Gulfraz M, Mehmood S, Minhas N, Jabeen N, Kausar R, Jabeen K, Arshad G (2008) Composition and antimicrobial properties of essential oil of Foeniculum vulgare. Afr J Biotech 7(24):4364–4368
Hammer KA, Carson CF, Riley TV (1999) Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol 1999(86):985–990
Hammouda FM, Saleh MA, Abdel-Azim NS, Shams KA, Ismail SI, Shahat AA, Saleh IA (2013) Evaluation of the essential oil of foeniculum vulgare mill (fennel) fruits extracted by three different extraction methods by GC/MS. Afr J Tradit Complement Altern Med. 11(2):277–279. https://doi.org/10.4314/ajtcam.v11i2.8
Hassanpour A, Yousefian S, Askaripour M, Sharififar F, Ezzatabadipour M (2017) Ovarian protection in cyclophosphamide-treated mice by fennel. Toxicol Rep 4:160–164. https://doi.org/10.1016/j.toxrep.2017.03.002
Hatami T, Johnera JCF, Meireles MAA (2017) Investigating the effects of grinding time and grinding load on content of terpenes in extract from fennel obtained by supercritical fluid extraction. Ind Crops Prod 109:85–91. https://doi.org/10.1016/j.indcrop.2017.08.010
Hilan C, Bouaoun D, Aoun J, Sfeir R, Garabeth F (2009) Propriétés antimicrobiennes et toxicité par détermination de la DL50 de l’huile essentielle de Prangos asperula Boissier. Phytothérapie 7:8–14. https://doi.org/10.1007/s10298-008-0357-4
http://www.emea.europa.eu, Annual report of the European Medicines Agency, 2008. Doc. ref.: EMEA/330566/2009.
Ilić DP, Stanojević LP, Troter DZ, Stanojević JS, Danilović BR, Nikolić VD, Nikolić LB (2019) Improvement of the yield and antimicrobial activity of fennel (Foeniculum vulgare Mill.) essential oil by fruit milling. Ind Crops Prod 142:111854. https://doi.org/10.1016/j.indcrop.2019.111854
Javed S, Mushtaq S, Khokhar I, Ahmad R, Haider MS (2012) Comparative antimicrobial activity of clove and fennel essential oils against food borne pathogenic fungi and food spoilage bacteria. Afr J Biotech 11(94):16065–16070. https://doi.org/10.5897/AJB11.3058
Khaled Khodja N, Boulekbache L, Chegdani F, Dahmani K, Bennis F, Madani K (2018) Chemical composition and antioxidant activity of phenolic compounds and essential oils from Calamintha nepeta L. J Complement Integr Med. https://doi.org/10.1515/jcim-2017-0080
Khammassi M, Loupassaki S, Tazarki H, Mezni F, Slama A, Tlili N, Zaouali Y, Mighri H, Jamoussi H, Khaldi A (2018) Variation in essential oil composition and biological activities of Foeniculum vulgare Mill populations growing widely in Tunisia. J Food Biochem 42:12532. https://doi.org/10.1111/jfbc.12532
Kontogiorgis C, Deligiannidou GI, Hadjipavlou-Litina D, Lazari D, Papadopoulos A (2016) Antioxidant protection: the contribution of proper preparation of fennel (Foeniculum vulgare Mill.) beverage. Ind Crops Prod 79:57–62. https://doi.org/10.1016/j.indcrop.2015.10.020
Krizman M, Baricevi D, Prosek M (2006) Fast quantitative determination of volatile constituents in fennel by headspace-gas chromatography. Analytica Chimica Acta 557:267–271. https://doi.org/10.1016/j.aca.2005.09.067
Kwiatkowski P, Mnichowska-Polanowska M, Pruss A, Masiuk H, Dziecioł M, Giedrys-Kalemba M, Sienkiewicz M (2017) The effect of fennel essential oil in combination with antibiotics on staphylococcus aureus strains isolated from carriers. Burns 43(7):1544–1551. https://doi.org/10.1016/j.burns.2017.04.014
Lazouni HA, Benmansour A, Chabane Sari D, Smahi MDE (2006) Valeurs nutritives et toxicité du foenicululm vulgare miller. Afrique Sci 02(1):94–101
Lazouni HA, Benmansour A, Taleb-Bendiab SA, Chabane Sari D (2007) Composition des constituants des huiles essentielles et valeurs nutritives du Foeniculum vulgare Mill. Sci Technol C–N 25:7–12
Majdoub N, El-Guendouz S, Rezgui M, Carlier J, Costa C, Bettaieb Ben Kaab L, Miguel MG (2017) Growth, photosynthetic pigments, phenolic content and biological activities of Foeniculum vulgare Mill., Anethum graveolens L. and Pimpinella anisum L. (Apiaceae) in response to zinc. Ind Crops Prod 109:627–636. https://doi.org/10.1016/j.indcrop.2017.09.012
Mashareq MK, Amira ME, Zenab AA, Ali IA, Fathy IR (2016) Evaluating antimicrobial and antioxidant activities of volatile oils extracted from anise, fennel and spearmint plants. J Agric Res Kafr El-Sheikh Univ 42(2):196–209
Mazandrani HA, Javadian SR, Bahram S (2015) The effect of encapsulated fennel extracts on the quality of silver carp fillets during refrigerated storage. Food Sci Nutr 4(2):298–304. https://doi.org/10.1002/fsn3.290
Mills E, Duguoa JJ, Perri D, Koren G, Saunders PR (2006) Herbal medicines in pregnancy and lactation an evidence-based approach. Taylor & Francis Medical
Mouhi L, Moghrani H, Nasrallah N, Amrane A, Maachi R (2017) Anti-inflammatory activity of essential oil of an endemic Thymus fontanesii Boiss & Reut with chemotype carvacrol and its healing capacity on gastric lesions. J Food Biochem 41:e12359. https://doi.org/10.1111/jfbc.12359
Moura LS Jr, Carvalho RN, Stefanini MLC, Meireles MAA (2005) Supercritical fluid extraction from fennel (Foeniculum vulgare): global yield, composition and kinetic data Meireles. J Supercrit Fluids 35:212–219. https://doi.org/10.1016/j.supflu.2005.01.006
Muckensturm B, Foechterlen D, Reduron JP, Hildenbrand DM (1997) Phytochemical and chemotaxonomic studies of Foeniculum vulgare. Biochem Syst Ecol 25(4):353–358
Napoli EM, Curcuruto G, Ruberto G (2010) Screening the essential oil composition of wild Sicilian fennel. Biochem Syst Ecol 38:213–223. https://doi.org/10.1016/j.bse.2010.01.009
Ostad SN, Soodi M, Shariffzadeh M, Khorshidi N, Marzban H (2001) The effect of fennel essential oil on uterine contraction as a model for dysmenorrhea, pharmacology and toxicology study. J Ethnopharmacol 76:299–304
Ostad SN, Khakinegad B, Sabzevari O (2004) Evaluation of the teratogenicity of fennel essential oil (FEO) on the rat embryo limb buds culture. Toxicology in Vitro. vol 18. pp 623–627. https://doi.org/10.1016/j.tiv.2004.02.008
Ouis N, Hariri A, El abed DZ (2012) Effect of the essential oils from parsley and fennel seeds on the growth of Lactobacillus Casei Subsp rhamnosus. J Biotechnol Biomaterial. https://doi.org/10.4172/2155-952X.1000130
Ouis N, Hariri A, El abed D (2014) Composition chimique de l’huile essentielle du Fenouil (Foeniculum vulgare) de la région de Mascara. Phytochemistry & Bioactives Substances. ISSN 2170-1768
Ozbek H (2005) The anti-inflammatory activity of Foeniculum vulgare L. essential oil and investigation of its medium lethal dose in rats and mice. Int J Pharmacologie 1(4):329–331
Ozcan MM, Chalchat JC, Arslan D, Ates A, Unver A (2006) Comparative essential oil composition and antifungal effect of bitter fennel (Foeniculum vulgare ssp. piperitum) fruit oils obtained during different vegetation. J Med Food J Med Food 9(4):552–561
Pacifico S, Galasso S, Piccolella S, Kretschmer N, Pan SP, Nocera P, Lettieri A, Bauer R, Monaco P (2015) Winter wild fennel leaves as a source of anti-inflammatory and antioxidant polyphenols. Arab J Chem 11:513–524. https://doi.org/10.1016/j.arabjc.2015.06.026
Pavela R, Zabkaa M, Bednárb J, Tríska J, Vrchotov N (2015) New knowledge for yield, composition and insecticidal activity ofessential oils obtained from the aerial parts or seeds of fennel (Foeniculum vulgare Mill.). Ind Crops Prod 83:275–282. https://doi.org/10.1016/j.indcrop.2015.11.090
Piccaglia R, Marotti M (2001) Characterization of Some Italian Types of wild (Fennel Foeniculum vulgare Mill). J Agric Food Chem 49(1):239–244
Purkayastha S, Narain R, Dahiya P (2012) Evaluation of antimicrobial and phytochemical screening of Fennel, Juniper and Kalonji essential oils against multi drug resistant clinical isolates. Asian Pac J Trop Biomed 2(3):S1625–S1629. https://doi.org/10.1016/S2221-1691(12)60465-1
Rahimmalek M, Maghsoudi H, Sabzalian MR, Ghasemi Pirbalouti A (2014) Variability of essential oil content and composition of different iranian fennel (Foeniculum vulgare Mill.) accessions in relation to some morphological and climatic factors. J Agr Sci Tech 16:1365–1374
Randhawa MA (2009) Calculation of LD50 values from the method of Miller and Tainter, 1944. J Ayub Med Coll Abbottabad 21(3):184–185
Rather MA, Dar BA, Sofi SN, Bhat BA, Qurishi MA (2012) Foeniculum vulgare: a comprehensive review of its traditional use phytochemistry pharmacology and safety. J Arabjc. https://doi.org/10.1016/j.arabjc.2012.04.011
Rezaei-Chiyaneh E, Amirnia R, Machiani MA, Javanmard A, Maggi F, Morshedloo MR (2019) Intercropping fennel (Foeniculum vulgare L.) with common bean (Phaseolus vulgaris L.) as affected by PGPR inoculation: a strategy for improving yield, essential oil and fatty acid composition. Scientia Horticulturae 261:10895. https://doi.org/10.1016/j.scienta.2019.108951
Roby MHH, Sarhan MA, Selim KAH, Khalel KI (2012) Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.). Ind Crops Prod 44:437–445. https://doi.org/10.1016/j.indcrop.2012.10.012
Rodríguez-Solana R, Salgadoc JM, Domíngueza JM, Cortés-Diéguez S (2014) Characterization of fennel extracts and quantification of estragole: Optimization and comparison of accelerated solvent extractionand Soxhlet techniques. Ind Crops Prod 52:528–536. https://doi.org/10.1016/j.indcrop.2013.11.028
Rolim TL, Meireles DRP, Batista TM, Gomes de Sousa TK, Mangueira VM, Albuquerque de Abrantes R, Rodrigues Pita JCL, Xavier AL, Costa VCO, Batista LM, Sobral da Silva M, Sobral MV (2017) Toxicity and antitumor potential of Mesosphaerum sidifolium (Lamiaceae) oil and fenchone, its major component. BMC Complement Altern Med. https://doi.org/10.1186/s12906-017-1779-z
Salama ZA, El Baz FK, Gaafar AK, Zaki MF (2013) Antioxidant activities of phenolics, flavonoids and vitamin C in two cultivars of fennel (Foeniculum vulgare Mill.) in responses to organic and bio-organic fertilizers. J Saudi Soc Agric Sci 14:91–99. https://doi.org/10.1016/j.jssas.2013.10.004
Saviuc C, Marinas I, Grumezescu AM, Bleotu C, Chifiriuc C, Mihaaiescu D, Lazar V (2012) Phytochemical composition of fennel fruits essential oil and its influence in prokaryotic cells growth and pathogenic features. Biointerface Res Appl Chem 2(2):300–305
Sbayou H, Ababou B, Boukachabine K, Manresa A, Zerouali K, Amghar S (2014) Chemical composition and antibacterial activity of Artemisia herba-alba and Mentha pulegium essential oils. J Life Sci 8(1):35–41
Segovia FJ, Luengo E, Corral-Pérez JJ, Raso J, Almajano MP (2014) Improvements in the aqueous extraction of polyphenols from borage (Borago officinalis L.) leaves by pulsed electric fields: pulsed electric fields (PEF) applications. Ind Crops Prod 65:390–396. https://doi.org/10.1016/j.indcrop.2014.11.010
Senatore F, Oliviero F, Scandolera E, Taglialatela-Scafati O, Roscigno G, Zaccardelli M, De Falco E (2013) Chemical composition, antimicrobial and antioxidant activities of anethole-rich oil from leaves of selected varieties of fennel [Foeniculum vulgare Mill. ssp. vulgare var. azoricum (Mill.) Thell]. Fitoterapia 90:214–219. https://doi.org/10.1016/j.fitote.2013.07.021
Sharopov F, Valiev A, Satyal P, Gulmurodov I, Yusufi S, Setzer WN, Wink M (2017). Cytotoxicity of the Essential Oil of Fennel (Foeniculum vulgare) from Tajikistan. Foods, 6 : 73. https://doi.org/10.3390/foods6090073.
Sfeir J, Lefrançois C, Baudoux D, Derbré S, Licznar P (2013) In Vitro antibacterial activity of essential oils against streptococcus pyogenes. Evidence-Based Complementary Altern Med 2013:269161. https://doi.org/10.1155/2013/269161
Shahat AA, Ibrahim AY, Hendawy SF, Omer EA, Hammouda FM, Abdel-Rahman FH, Saleh MA (2011) Chemical composition, antimicrobial and antioxidant activities of essential oils from organically cultivated fennel cultivars. Molecules 16:1366–1377. https://doi.org/10.3390/molecules16021366
Shahmokhtar MK, Armand S (2017) Phytochemical and biological studies of fennel (Foeniculum vulgare Mill) from the South West Region of Iran (Yasouj). Nat Prod Chem Res. https://doi.org/10.4172/2329-6836.1000267
Singh G, Maury S, de Lampason MP, Catalan C (2006) Chemical constituents, antifungal and antioxidative potential of Foeniculum vulgare volatile oil and its acetone extract. Food Control 17:745–752
Stefanini MB, Ming LC, Marques MOM, Facanali R, Meireles MAA, Moura LS, Marchese JA, Sousa LA (2006a) Essential oil constituents of different organs of fennel (Foeniculum vulgare var. vulgare). Rev Bras Pl Med Botucatu 8:193–198
Stefanini MB, Ming LC, Marques MOM, Meireles MAA, Moura LS, Marchese JA (2006b) Seed productivity, yield and composition of the essential oil of fennel Foeniculum vulgare var. dulcis in the season of the year. Rev Bras Pl Med Botucatu 8:86–90
Švarc-Gajić J (2009) General toxicology. Nova Science Publishers Inc, New York
Telci I, Demirtas I, Sahin A (2009) Variation in plant properties and essential oil composition of sweet fennel (Foeniculum vulgare Mill.) fruits during stages of maturity. Ind Crops Prod 30:126–130. https://doi.org/10.1016/j.indcrop.2009.02.010
Telci I, Dirican A, Elmastas M, Akşit H, Demirtas I (2019) Chemical diversity of wild fennel populations from Turkey. J Appl Res Med Arom Plants 13:100201. https://doi.org/10.1016/j.jarmap.2019.02.002
Turner RA (1965) Screening methods in pharmacology. Academic Press, New York
Yaldiz G, Camlica M (2019) Variation in the fruit phytochemical and mineral composition and phenolic content and antioxidant activity of the fruit extracts of different fennel (Foeniculum vulgare L) genotypes. Ind Crops Prod 142:111852. https://doi.org/10.1016/j.indcrop.2019.111852
Zoubiri S, Baaliouamer A (2011) Chemical composition and insecticidal properties of some aromatic herbs essential oils from Algeria. Food Chem 129:179–182. https://doi.org/10.1016/j.foodchem.2011.04.033
Zoubiri S, Baaliouamer A, Seba N, Chamouni N (2010) Chemical composition and larvicidal activity of Algerian Foeniculum vulgare seed essential oil. Arab J Chem 7:480–485. https://doi.org/10.1016/j.arabjc.2010.11.006
Zuobing X, Jiaying C, Yunwei N, Feng C (2017) Characterization of the key odorants of fennel essential oils of different regions using GC–MS and GC–O combined with partial least squares regression. J Chromatogr 1063:226. https://doi.org/10.1016/j.jchromb.2017.07.053
Acknowledgements
This study was supported by the Laboratory of Reaction Engineering of USTHB and the Laboratory of Microbiology of Algerian Research and Development Centre of the SAIDAL group.
Author information
Authors and Affiliations
Contributions
KD Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing-original draft, Writing-review & editing. HM Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, writing-review & editing. ND Methodology, review. SO Supervision of the antimicrobial study. KA Validation, review & editing KA Methodology, supervision, validation, review & editing.
Corresponding author
Ethics declarations
Conflict of interest
We do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dahmani, K., Moghrani, H., Deghbar, N. et al. Algerian wild fennel essential oils: chromatographic profile, acute toxicity, antioxidant, and antimicrobial activities. Chem. Pap. 76, 1639–1652 (2022). https://doi.org/10.1007/s11696-021-02008-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-02008-9