Abstract
A simple Schiff base fluorescent probe (SW) composed of oxime and salicylaldehyde units was designed and synthesized. The probe has high selectivity and sensitivity for Zn2+ ion in mixed solvent (DMSO/H2O, V/V = 9:1). The fluorescence has obvious redshift phenomenon and visible color change. The stoichiometric ratio of the probe to Zn2+ ion was confirmed to be 1:2 (mole) by 1H NMR, MS analysis and job curves. And the detection limit of fluorescence response of the SW to Zn2+ is down to 2.53 × 10–8 mol/L. At the same time, the probe SW can be applied to the test paper detection of Zn2+ ions under 365 nm UV light and the detection of Zn2+ ions in actual water samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc is the second most abundant transition metal ion in the human body after iron. It plays an important role in various physiological and pathological processes, including DNA synthesis, gene expression, enzyme regulation structure and neuron signal transmission (Wang et al. 2020a, b, c, d; Zhang et al. 2021). The lack of Zn2+ in adults can lead to neurological disorders, Alzheimer's disease and diabetes. Lack of Zn2+ in children can lead to decreased immune function, diarrhea and even death (Vetriarasu et al. 2019; Liu et al. 2020a, b). In recent years, zinc ion is widely used in electroplating industry, which causes more and more serious environmental pollution. Therefore, it is very important for human health and environment to design a high selective zinc ion detection probe (Zhao et al. 2019; Yu et al. 2017b, a). Although many chemical sensors for zinc detection have been studied before, new fluorescent probes for selective detection of zinc ions in physiological pH conditions and environmental systems are still in great demand. Due to its d10 configuration, Zn2+ sensor is usually interfered by other d10 metal ions (such as CdII and HgII) (Anand et al. 2018; Bian et al. 2021b, a). As we all know, Schiff base fluorescent probe is a kind of metal ion probe which is relatively simple to synthesize and widely used. It has the advantages of convenient operation, simple detection method, fast detection and high sensitivity, and has attracted great attention. Because of the C = N group in its structure, its rigid structure and fluorescence are enhanced after chelating with metal ions (Patil et al. 2018; Xu et al. 2021a, b).
In this paper, we designed and synthesized a fluorescent probe SW with high selectivity and sensitivity for the detection of Zn2+ ions, which can be used for visual detection. The probe has the advantages of simple preparation and low cost (Wei et al. 2020; Pannipara et al. 2018). It has a strong practical value for the test paper detection of Zn2+ ions under 365 nm UV light and the detection of Zn2+ ions in actual water samples.
Experimental
Materials and methods
The O-benzylhydroxylamine (99%), 4-aminoacetophenone (99%), and salicylic aldehyde (98%) used in the experiment were purchased from Alfa Aesar. The remaining reagents and solvents are all analytical reagents and can be used without further purification. The water used in the experiment is distilled water. The X-4 microscopic melting point instrument produced by Beijing Tyco Instrument Limited company was used for melting point measurement, and no calibration was performed before use. German Vario EL V3.00 automatic element analyzer was used for the analysis of C, H, and N elements. 1H NMR spectra were recorded in DMSO-d6 solution using Bruker AV series DRX-500 MHz nuclear magnetic resonance instrument. Fluorescence spectra were recorded using Hitachi (Japan) F-7000 fluorescence spectrophotometer. Ultraviolet–visible absorption spectrum is measured by Hitachi UV-3900 spectrometer. The B3LYP/6-31G function is used as the basis of geometric optimization, and the Gaussian 09 software program is used for DFT calculation.
By adding various metal cations in DMSO/H2O (V/V = 9:1) medium, and the probe SW concentration was kept constant (2.0 × 10–4 mol/L). All metal cation solutions (1.0 × 10–2 mol/L) Na+, Al3+, Ba2+, Ca2+, Cd2+, Co2+, Cr3+, Fe3+, Mg2+, Mn2+, Zn2+, Ni2+, Pb2+, Hg2+ and Cu2+ were prepared from the nitrate salts, while the Zn2+ solutions was prepared by Zn(NO3)2. The excitation wavelength used for fluorescence spectrometry is 368 nm, the entrance slit is 5 nm and the exit slit is 5 nm.
Synthesis of the probe SW
SW was synthesized routes shown in Scheme 1. O-benzylhydroxylamine (1.18 g, 9.0 mmol) and 4-aminoacetophenone (1.22 g, 9.0 mmol) were dissolved in anhydrous ethylalcohol (15 mL), and then 3 drops of glacial acetic acid were added to the mixed solution and refluxed at 65 °C for 6 h. The mixture solution is cooled, filtered to obtain a yellowish solid, washed with anhydrous ethanol/water (V/V = 1:4) and dried under vacuum (Sun et al. 2015; Wu et al. 2021), and obtained in 2.05 g of ({4-amino}phenyl)ethanone O-benzyl oxime as crystalline solid. Yield: 89.0%. M.p.351 ~ 352 K. Anal. Calcd. for C15H16N2O(%): C, 74.97; H, 6.71; N, 11.66. Found (%): C, 74.68; H, 6.80; N, 11.52.
({4-amino}phenyl)ethanone O-benzyloxime (1.51 g, 6 mmol) and salicylaldehyde (0.74 g, 6 mmol) were dissolved in ethanol (15 mL), and the mixed solution was stirred at 65 ℃ for 6 h. After cooling to room temperature, vacuum distillation and filtration were carried out. The precipitate was washed with ethanol/n-hexane (V/V = 1:4). After vacuum drying, 1.37 g SW dark yellow solid product was obtained (Fig S1). Yield: 61.3%. M.p. 229 ~ 230 ℃, Anal. Calcd. for C22H20N2O2 (%): C, 76.72; H, 5.85; N, 8.13. Found (%): C, 76.97; H, 5.23; N, 8.25. 1H NMR (500 MHz, DMSO-d6) δ 12.96 (s, 1H), 8.99 (s, 1H), 7.78—7.72 (m, 2H), 7.68 (dd, J = 7.7, 1.8 Hz, 1H), 7.47–7.36 (m, 7H), 7.35—7.29 (m, 1H), 7.03—6.95 (m, 2H), 5.22 (s, 2H), 2.25 (s, 3H).
Results and discussion
Fluorescence recognition of Zn2+ by probe SW
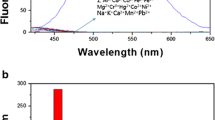
The response of probe SW to different metal cations was studied by the fluorescence method at room temperature (Li et al. 2021a, b, c). As shown in Fig. 1a, fifteen cations (Na+, Al3+, Ba2+, Ca2+, Cd2+, Co2+, Cr3+, Fe3+, Mg2+, Mn2+, Zn2+, Ni2+, Pb2+, Hg2+ and Cu2+) (1.0 × 10–2 mol/L) were added to the solution of probe SW (2.0 × 10–4 mol/L) (DMSO/H2O, V/V = 9:1), respectively. When the excitation wavelength is 368 nm, the probe SW has a weak fluorescence emission peak at 437 nm. After the addition of other metal ions, the intensity of emission peak has no obvious enhancement except Zn2+ ions. Furthermore, the emission peak was red-shifted from 437 to 502 nm and the intensity increased by 16 times upon addition of Zn2+ ions (Dong et al. 2017; Wang et al. 2020a). Under the UV lamp, the solution of 15 kinds of metal ions to be measured was added to DMSO/H2O solution (V/V = 9:1) of SW in turn, a strong bright green fluorescence is produced only added Zn2+ ions, and other metal cations have basically no obvious effect (Anand et al. 2017; Pan et al. 2020a, b), the fluorescence remains unchanged or quenched (Fig. 1b). It can prove that probe SW could selectively identify Zn2+ ion among other metal ions to be measured. It is helpful to study the high sensitivity of probe SW to Zn2+ ion by anti-interference experiment (Ozdemir 2016; Sun et al. 2019). In Fig. 2, after adding Zn2+ ion to probe SW solutions containing different metal ions, other metal cations had no markedly effect on the fluorescence recognition to Zn2+ ions except for the slight quenching of Cu2+ ion and Co2+ ion had a slight effect (Liu et al. 2020a). The results show that SW has good selectivity for Zn2+ ions.
In order to quantitatively evaluate the fluorescence sensing behavior of SW, we carried out fluorescence titration experiment. As shown in Fig. 3, the Zn2+ solution (1.0 × 10−3 mol/L) was gradually added to the probe SW solution (2.0 × 10−4 mol/L) (Upadhyay et al. 2018; Wang et al. 2021a, b), the free SW exhibited a weak emission peak at 437 nm, a new peak emission present at 507 nm and its intensity gradually increased when the concentration of Zn2+ ion increases continuously (Wang et al. 2017; Chang et al. 2020). When the Zn2+ content reaches 0.5 equivalent, the fluorescence emission intensity reaches the maximum, indicating that the optimal binding ratio of Zn2+ to probe SW is 1:2. Bringing the results of titration experiments into the Benesi–Hildebrand equation \(\{1/(F-{F}_{0})=1/({F}_{\mathrm{max}}-{F}_{0}\)) + \({1/K}_{d}\)[C]\(\times {1/(F}_{\mathrm{max}}-{F}_{0}\))} (Sudipa et al. 2019; Xu et al. 2021a), and the binding constant is calculated as Ka = 7.7 × 104 M−1 (Fig. S2a). Where, F0, F and Fmax are the fluorescence intensity without Zn2+, the fluorescence intensity at any given Zn2+ concentration and the fluorescence intensity after titration saturation, respectively. The limit of detection LOD for Zn2+ toward SW was calculated using LOD = 3σ/slope and was found to be 2.53 × 10−8 mol/L (Fig. S2b) (Wang et al. 2020b; Purkait et al. 2019). Among them, the standard deviation σ was calculated by measuring five consecutive fluorescence intensities of probe SW, and the slope was obtained by plotting the relationship between the emission intensity of SW and the concentration of Zn2+ (Zhang et al. 2020; Bian et al. 2021b; Fan et al. 2020). The calculated results are lower than the acceptable limit (7.0 × 10−6 mol/L) of WHO drinking water (Lu et al. 2020; Kang et al. 2019b). Compared with other Zn2+ sensors reported previously, the detection limit is lower and the sensitivity is higher (Table 1).
In order to test and verify the rapid detection performance of probe SW for Zn2+, the Zn2+ response time experiment was carried out (Zhang et al. 2019). As shown in Fig. S3, after adding Zn2+, the response time of probe SW was 3 min (Pan et al. 2020a; Li et al. 2021a). The fluorescence reversibility studied by adding Zn2+ and ethylenediaminetetraacetic acid (EDTA, c = 1.0 × 10–3 mol/L) to SW solution. In Fig S4, when EDTA was introduced to SW and Zn2+ mixed solution, the fluorescence intensity at 502 nm was diminished. Then, Zn2+ solution was added again, and the fluorescence intensity was close to the initial fluorescence value, and the above experimental steps were repeated, and the fluorescence intensity continued to decrease and increase (Wang et al. 2021a; Mu et al. 2020). Thus, the probe SW can monitor Zn2+ reversibly.
UV–Vis spectroscopic studies of Zn2+ by probe SW
In the UV–Vis spectrum, as shown in Fig S5a, the free ligand SW exhibited a weak absorb peak at 425 nm. When Zn2+ ions were added to probe SW solution, a new absorption peak appeared at 458 nm, and the solution changed from colorless to yellow (Fig. S5b). At the same time, the addition of Co2+, Fe3+ and Cu2+ metal ions also slightly changed the color. Therefore, UV–Vis spectrum can be used as an assistant method to detect the Zn2+ by this probe SW. As shown in Fig. S6, the Zn2+ solution (1.0 × 10−3 mol/L) was gradually added to the probe SW solution (2.0 × 10−4 mol/L) for UV–visible titration experiments (Wang et al. 2020c; Liu et al. 2018). With the continuous increase of Zn2+ concentration, a new absorption peak appears at 458 nm, while the absorption peak at 352 nm gradually decreases. At the same time, an isoabsorptive point appeared at 285 nm and until the content of Zn2+ solution reached 0.5 equivalents, the peaks at 458 nm and 352 nm remained unchanged (Fig. S7b). The UV–Vis spectral characteristics support the molar ratio of metal to ligand of 1:2, and the association constant Ka = 5.6 × 104 M−1 (Fig. S7a).
The detection mechanism of the probe SW for Zn2+
1H NMR titration experiment was carried out in DMSO-d6 (Long et al. 2020). As shown in Fig. 4, with the addition of Zn2+ from 0 to 0.5 equivalent, the phenolic hydroxyl protons H1 (δ = 12.87 ppm) gradually disappeared until completely disappeared, indicating the deprotonation process of hydroxyl induced by Zn2+. With the addition of Zn2+, azomethine H2 (HC = N) shifts from 9.02 ppm to 8.65 ppm, which may be due to the coordination between imine-N and Zn2+. In the complex SW-Zn2+, the 1H NMR titration data supports 1:2 metal–ligand ratio (Xu et al. 2020; Yu et al. 2017a). Job's plot further analyzes the coordination ratio between SW and Zn2+ (Fig. S8), when the mole fraction of Zn2+ ion is 0.345, the fluorescence intensity reaches the maximum value, showing that the binding ratio of Zn2+ ion to SW is 1:2 (Liu et al. 2020b; Xue et al. 2019).
As shown in Scheme 2, the fluorescence enhancement recognition mechanism of Zn2+ by probe SW may be mainly due to the presence of Zn2+ hindering the PET (light-induced electron transfer) effect of SW. Owing to the rotation of imine group (-CH = N) in free SW, there is a very weak fluorescence in SW solution. The addition of Zn2+ ions can coordinate with the probe SW, which inhibits the free rotation of imine group of SW molecules and produces CHEF effect (Liu et al. 2018; Zhang et al. 2018), resulting enhanced fluorescence. The mass spectrum peak of the complex appeared at 751.22 (Fig. S9), which further confirmed that the complex was consistent with the conjecture.
DFT computation
In order to further study the geometry and interaction of SW and Zn2+, density functional theory (DFT) calculation was carried out. The geometry structures of SW and SW-Zn2+ were optimized by 6-31G/LanL2DZ basic setting program using Gausans-09 software (Feng et al. 2021; Rout et al. 2019). As shown in Fig. 5, one the Zn2+ ion coordinated with two ligand SW molecules. Among, the coordination atoms are hydroxyl O atoms and the imine N atoms on C = N groups of two SW molecules, respectively. The LUMO and HOMO energies of SW were −1.551 eV and −5.659 eV, respectively. The energy gap (∆E = ELUMO-EHOMO) was 4.108 eV, and the SW molecule was delocalized on the entire conjugated skeleton except for the oxime phenyl group. After SW coordinating with Zn2+, LUMO and HOMO energies were −1.931 eV and −5.386 eV, respectively. Correspondingly, the energy gap was 3.455 eV, and the electron density was mainly delocalized on the two Schiff groups and mainly on entire salicylaldehyde and aminoacetophenone conjugated skeleton except for the oxime phenyl group. The electron density of HOMO–LUMO transition showed the fluorophore-metal charge transfer, indicating that with the PET effect, the excited electrons were readily averted to metal ions (Cui et al. 2019; Li et al. 2021b). The decrease of ∆E indicates that there is good coordination ability between Zn2+ and SW and formed a stable environment.
Practical application of probe SW
In practical application, the probe SW is designed as a Zn2+ responsive strip sensor with good selectivity, and it can quickly and simply detect Zn2+ ions by changing the wavelength, which has great practical value (Kang et al. 2019a). The filter strips were immersed in DMSO/H2O solution of probe SW for 1 h, and dried at low temperature, then immersed in different concentrations of Zn2+ ions and other metal ions for 30 min, and the changes were observed under 365 nm UV lamps (Diao et al. 2018). As shown in Fig. 6a, with the increase of Zn2+ concentration, the color of the test paper changed from colorless to green. Whereas the other metal ions did not cause obvious changes in the color of test paper except Zn2+ ions (Fig. 6b), indicating that SW probe had high selectivity for Zn2+.
In order to test the practicability of Zn2+ ions in real water samples, drinking water, tap water and Yellow River Water were collected for sensing experiments (Sarkar et al. 2020). All samples were filtered through 0.2 mm filter membrane and tested for three times. The calibration curve was obtained by measuring the Zn2+ ions concentration (Ke et al. 2020). As shown in Table 2, the detection results show that the probe SW can effectively detection Zn2+ ions and has high recovery (98%-103%), good analytical precision (RSD < 3%), which meets the detection requirements. Therefore, the probe SW can be effectively used for the detection of Zn2+ ion concentration in actual water samples and has practical value in environmental analysis.
Conclusions
In summary, a simple Schiff base ligand SW has been designed and synthesized, and it has higher sensitivity and selectivity to Zn2+ in DMSO/H2O (v/v = 9:1) solution. And the detection limit of the SW to Zn2+ is down to 2.53 × 10−8 mol/L, which was lower than the limited value defined by WHO. By means of 1H NMR, MS analysis and theoretical calculation, we obtained the binding mode of probe SW to Zn2+ is 2:1. In addition, the probe SW can be used for the detection of Zn2+ ions in the test paper under the UV light at 365 nm and the detection of Zn2+ ions in actual water samples, which has a potential application prospect.
Data Availability
The data sets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
Anand T, Ashok Kumar SK, Sahoo SK (2017) Vitamin B6 cofactor derivative: a dual fluorescent turn-on sensor to detect Zn2+ and CN− ions and its application in live cell imaging. Chem Select 2:7570–7579. https://doi.org/10.1002/slct.201701024
Anand T, Ashok Kumar SK, Sahoo SK (2018) A novel Schiff base derivative of pyridoxal for the optical sensing of Zn2+ and cysteine. Photochem Photobiol 17:414–422. https://doi.org/10.1039/C7PP00391A
Bian RN, Wang JF, Xu X, Dong WK, Ding YJ (2021) Investigation of mononuclear, dinuclear, and trinuclear transition metal (II) complexes derived from an asymmetric Salamo-based ligand possessing three different coordination modes. Appl Organomet Chem 35:e6040. https://doi.org/10.1002/aoc.6040
Bian RN, Xu X, Feng T, Dong WK (2021) A novel O-phenanthroline-based bis(half-salamo)-like chemical sensor: For rapid and efficient continuous recognition of Cu2+, HPO42- and H2PO4-. Inorg Chim Acta 516:120098. https://doi.org/10.1016/j.ica.2020.120098
Chang J, Zhang SZ, Wu Y, Zhang HJ, Sun YX (2020) Three supramolecular trinuclear Nickel(II) complexes based on Salamo-type chelating ligand: Syntheses, crystal structures, solvent effect, Hirshfeld surface analysis and DFT calculation. Transit Met Chem 45:279–293. https://doi.org/10.1007/s11243-020-00379-8.26
Cui YF, Zhang Y, Xie KF, Dong WK (2019) A newly synthesized heterobimetallic NiII-GdIII salamo-BDC-based coordination polymer: Structural characterization, DFT calculation, fluorescent and antibacterial properties. Crystals 9:596. http://creativecommons.org/licenses/by/4.0/.
Diao HP, Guo LX, Liu W, Feng LH (2018) A novel polymer probe for Zn(II) detection with ratiometric fluorescence signal. Spectrochim Acta Part A 196:274–280. https://doi.org/10.1016/j.saa.2018.02.036
Dong WK, Akogun FS, Zhang Y, Sun YX, Dong XY (2017) A reversible “turn-on” fluorescent sensor for selective detection of Zn2+. Sensor Actuat B 238:723–734. https://doi.org/10.1016/j.snb.2016.07.047
Fan L, Qin JC, Li CR, Yang ZY (2020) Two similar Schiff-base receptor based quinoline derivate: Highly selective fluorescent probe for Zn(II). Spectrochimica Acta Part A 236:118347. https://doi.org/10.1016/j.saa.2020.118347
Feng T, Li LL, Li YJ, Dong WK (2021) A half-salamo-based pyridine-containing ligand and its novel NiII complexes including different auxiliary ligands: Syntheses, structures, fluorescence properties, DFT calculations and Hirshfeld surface analysis. Acta Cryst B 77:168–181. https://doi.org/10.1107/S2052520620016157
Kang TT, Wang HP, Wang XJ, Feng LH (2019a) A facile Zn(II) probe based on intramolecular charge transfer with fluorescence red-shift. Microchem J 148:442–448. https://doi.org/10.1016/j.microc.2019.05.035
Kang T, Wang H, Wang X, Feng L (2019b) A facile Zn(II) probe based on intramolecular charge transfer with fluorescence red-shift. Microchem J 148:442–448. https://doi.org/10.1016/j.microc.2019.05.035
Ke HS, Wei W, Yang YS, Wu HP, Zhang YQ, Xie G, Chen SP (2020) A trinuclear zinc coordination cluster exhibiting fluorescence, colorimetric sensitivity, and recycling of silver ion and detection of cupric ion. Inorg Chem 21:24–39. https://doi.org/10.1021/acs.inorgchem.9b03169
Li YJ, Guo SZ, Feng T, Xie KF, Dong WK (2021) An investigation into three-dimensional octahedral multi-nuclear Ni(II)-based complexes supported by a more flexible salamo-type ligand. J Mol Struct 1228:129796. https://doi.org/10.1016/j.molstruc.2020.129796
Li P, Yao GX, Li M, Dong WK (2021) Influence of different counteranions on supramolecular self-assemblies, Hirshfeld surfaces analyses and fluorescence properties of three multinuclear Cu(II) salamo-based complexes. Polyhedron 195:114981. https://doi.org/10.1016/j.poly.2020.114981
Li RY, Wei ZL, Wang L, Zhang Y, Ru JX (2021) A new salamo-based fluorescence probe to visually detect aluminum(III) ion and bio-imaging in zebrafish. Microchem J 162:105720. https://doi.org/10.1016/j.microc.2020.105720
Liu LM, Yang ZY (2018) A rhodamine and chromone based “turn-on” fluorescent probe (RC1) for Zn(II) in aqueous solutions and its application. J Photoch Photobio A 364:558–563. https://doi.org/10.1016/j.jphotochem
Liu YJ, Qiu DL, Pan H, Li MG, Chen HB, Li HM (2018) A high selective fluorescent probe for colourimetric recognition of cyanide anion based on heptamethine cyaninetriphenylamine conjugate. J Photoch Photobio A 364:151–158. https://doi.org/10.1016/j.jphotochem.2018.06.018
Liu C, Wei ZL, Mu HR, Dong WK, Ding YJ (2020) A novel unsymmetric bis(salamo)-based chemosensor for detecting Cu2+ and continuous recognition of amino acids. J Photoch Photobio A 397:112569. https://doi.org/10.1016/j.jphotochem.2020.112569
Liu C, An XX, Cui YF, Xie KF, Dong WK (2020) Novel structurally characterized hetero‐bimetallic [Zn(II)2M(II)] (M=Ca and Sr) bis(salamo)-type complexes: DFT calculation, Hirshfeld analyses, antimicrobial and fluorescent properties. Appl Organomet Chem 34:e5272. https://orcid.org/0000-0003-1249-5808.
Long RQ, Tang C, Yang Z, Fu QC, Xu JJ, Tong CY, Shi SY, Guo Y, Wang DJ (2020) Natural hyperoside based novel light-up fluorescent probe with AIE and ESIPT characteristics for on-site and long-term imaging of β-galactosidase in living cells. J Mater Chem C 00:1–5. https://doi.org/10.1039/D0TC01981J
Lu MM, Qiu SY, Cui SQ, Pu SZ (2020) A novel diarylethene-based fluorescence sensor with a benzohydrazide unit for the detection of Zn2+. J Phys Org Cheme e:4113. doi: https://doi.org/10.1002/poc.4113.
Mu HR, Yu M, Wang L, Zhang Y, Ding YJ (2020) Catching S2− and Cu2+ by a high sensitive and efficient salamo-like fluorescence-ultraviolet dual channel chemosensor. Phosphorus Sulfur 195:730–739. https://doi.org/10.1080/10426507.2020.1756807
Ozdemir M (2016) A selective fluorescent “turn-on” sensor for recognition of Zn2+ in aqueous media. Spectrochim Acta Part A 161:115–121. https://doi.org/10.1016/j.saa.2016.02.040
Pan YQ, Xu X, Zhang Y, Zhang Y, Dong W-K (2020) A high sensitive and selective bis(salamo)-type fluorescent chemosensor for identification of Cu2+ and the continuous recognition of S2−. Spectrochim Acta Part A 229:117917–117921. https://doi.org/10.1016/j.saa.2019.117927
Pan YQ, Zhang Y, Yu M, Zhang Y, Wang L (2020) Newly synthesized homomul trinuclear Co(II) and Cu(II) bissalamo-like complexes: Structural characterizations, Hirshfeld analyses, fluorescence and antibacterial properties. Appl Organomet Chem 34:e5441. https://orcid.org/0000-0002-4806-2708.
Pannipara MB, Sehemi AG, Irfan A, Assiri M, Kalam A, Yahya S (2018) AIE active multianalyte fluorescent probe for the detection of Cu2+, Ni2+ and Hg2+ ions. Spectrochim Acta Part A 201:54–60. https://doi.org/10.1016/j.saa.2018.04.052
Patil M, Keshav K, Kumawa M, Bothra S, Sahoo SK, Srivastava R, Rajput J, Bendre R, Kuwar A (2018) Monoterpenoid derivative based ratiometric fluorescent chemosensor for bioimaging and intracellular detection of Zn2+ and Mg2+ ions. J Photoch Photobio A 364:758–763. https://doi.org/10.1016/j.jphotochem.2018.07.015
Purkait R, Mahapatra AD, Chattopadhyay D, Sinha C (2019) An azine-based carbothioamide chemosensor for selective and sensitive turn-on-off sequential detection of Zn(II) and H2PO4−, live cell imaging and INHIBIT logic gate. Spectrochim Acta A 207:164–172. https://doi.org/10.1016/j.saa.2018.09.019
Rout K, Manna AK, Sahu M, Patra GK (2019) A guanidine based bis Schiff base chemosensor for colorimetric detection of Hg(II) and fluorescent detection of Zn(II) ions. Inorg Chim Acta 486:733–741. https://doi.org/10.1016/j.ica.2018.11.021
Sarkar A, Aratrika C, Tonmoy C, Suranjana P, Debabrata S, Suvendu M, Debasis D (2020) A chemodosimetric approach for fluorimetric detection of Hg2+ ions by trinuclear Zn(II)/Cd(II) schiff base complex: first case of intermediate trapping in a chemodosimetric approach. Inorg Chem 12:30–52. https://doi.org/10.1021/acs.inorgchem.0c00857
Sudipa M, Santi MM, Durbadal O, Debprasad C, Chittaranjan S (2019) Water soluble sulfaguanidine based Schiff base as a “Turn-on” fluorescent probe for intracellular recognition of Zn2+ in living cells and exploration for biological activities. Polyhedron 172:28–38. https://doi.org/10.1016/j.poly.2019.02.042
Sun YX, Lu RE, Li XR, Zhao YY, Li CY (2015) Synthesis and Supramolecular Structure of Ligands Containing Oxime Group Schiff Base and Cu(II) Complexes. Chinese J Inorg Chem 31:1055–1062. https://doi.org/10.11862/CJIC.2015.134
Sun YX, Pan YQ, Xu X, Zhang Y (2019) Unprecedented dinuclear Cu(II) N,O-donor complex: Synthesis, structural characterization, fluorescence property, and hirshfeld analysis. Crystals 9:607. https://doi.org/10.3390/cryst.9120607
Upadhyay Y, Anand T, Babu LT, Paira P, Crisponi C, Ashok Kumar SK, Kumar R, Sahoo SK (2018) Three-in-one type fluorescent sensor based on a pyrene pyridoxal cascade for the selective detection of Zn(II), hydrogen phosphate and cysteine. Dalton T 47:742. https://doi.org/10.1039/C7DT04234E
Vetriarasu V, Selva Kumar R, Ashok Kumar SK, Sahoo SK (2019) Highly selective turn-on fluorogenic chemosensor for Zn2+ based on chelation enhanced fluorescence. Inorg Chem Commun 102:171–179. https://doi.org/10.1016/j.inoche.2019.02.020
Wang JL, Hao YF, Wang H, Yang SX, Tian HY, Sun BG, Liu YG (2017) Rapidly responsive and high selective fluorescent probe for bisulfite detection in food. J Agric Food Chem 65:2883–2887. https://doi.org/10.1021/acs.jafc.7b00353
Wang L, Wei ZL, Liu C, Dong WK, Ru JX (2020) Synthesis and characterization for a high selective bis(salamo)-based chemical sensor and imaging in living cell. Spectrochim Acta A 239:118496. https://doi.org/10.1016/j.saa.2020.118496
Wang L, Wei ZL, Chen ZZ, Liu C, Dong WK, Ding YJ (2020) A chemical probe capable for fluorescent and colorimetric detection to Cu2+ and CNˉ based on coordination and nucleophilic addition mechanism. Microchem J 155:104801. https://doi.org/10.1016/j.microc.2020.104801
Wang B, Liu X, Duan W, Dai S, Lu H (2020) Visual and ratiometric fluorescent determination of Al3+ by a red-emission carbon dot-quercetin system. Microchem J 156:104807. https://doi.org/10.1016/j.microc.2020.104807
Wang L, Pan YQ, Wang JF, Zhang Y, Ding YJ (2020a) A high selective and sensitive half-salamo-based fluorescent chemosensor for sequential detection of Pb(II) ion and Cys. J Photochem Photobio A 400:112719. https://doi.org/10.1016/j.jphotochem.2020.112719
Wang JF, Feng T, Li YJ, Sun YX, Dong WK, Ding YJ (2021) Novel structurally characterized Co(II) metal-organic framework and Cd(II) coordination polymer self-assembled from a pyridine-terminal salamo-like ligand bearing various coordination modes. J Mol Struct 1231:129950. https://doi.org/10.1016/j.molstruc.2021.129950
Wang JF, Bian RN, Feng T, Xie KF, Wang L, Ding YJ (2021) A highly sensitive dual-channel chemical sensor for selective identification of B4O72-. Microchem J 160:105676. https://doi.org/10.1016/j.microc.2020.105676
Wei ZL, Wang L, Wang JF, Guo WT, Zhang Y, Dong WK (2020) Two high sensitive and efficient salamo-like copper (II) complex probes for recognition of CNˉ. Spectrochim Acta Part A 228:1386–1425. https://doi.org/10.1016/j.saa.2019.117775
Wu Y, Ding WM, Li J, Guo G, Zhang SZ, Jia HR, Sun YX (2021) A Highly selective turn-on fluorescent and naked-eye colorimetric dual-channel probe for cyanide anions detection in water samples. J Fluoresc 31:437–446. https://doi.org/10.1007/s10895-020-02677-x
Xu X, Bian RN, Guo SZ, Dong WK, Ding YJ (2020) A new asymmetric salamo-based chemical sensor for dual channel detection of Cu2+ and B4O72−. Inorg Chim Acta 513:119945. https://doi.org/10.1016/j.ica.2020.119945
Xu X, Li YJ, Feng T, Dong WK, Ding YJ (2021) Highly efficient detection of Cu2+ and B4O72− based on a recyclable asymmetric salamo-based probe in aqueous medium. Luminescence 36:169–179. https://doi.org/10.1002/bio.3932
Xu X, Feng T, Feng SS, Dong WK (2021) Influence of structural variation of salamo-based ligand on supramolecular architectures, Hirshfeld analyses, and fluorescence properties of new tetranuclear NiII complexes. Appl Organomet Chem 35:e6057. https://doi.org/10.1002/aoc.6057
Xue J, Tian LM, Yang ZY (2019) A novel rhodamine-chromone Schiff-base as turn-on fluorescent probe for the detection of Zn(II) and Fe(III) in different solutions. J Photochem Photobio A 369:77–84. https://doi.org/10.1016/j.jphotochem
Yu B, Sun YX, Yang CJ, Guo JQ, Li J (2017) Synthesis and crystal structures of an unexpected tetranuclear Zinc(II) complex and a benzoquinone compound derived from Zn2+ and Cd(II)-promoted reactivity of schiff base ligands. Z Anorg Allg Chem 643:689–698. https://doi.org/10.1002/zaac.201700034
Yu B, Li CY, Sun YX, Jia HR, Guo JQ, Li J (2017) A new azine derivative colourimetric and fluorescent dual-channel probe for cyanide detection. Spectrochim Acta Part A 184:249–254. https://doi.org/10.1016/j.saa.2017.05.012
Zhang HJ, Chang J, Jia HR, Sun YX (2018) Syntheses, supramolecular structures and spectroscopic properties of Cu(II) and Ni(II) complexes with Schiff base containing oxime group. Chinese J Inorg Chem 34:2261–2270. https://doi.org/10.11862/CJIC.2018.261
Zhang JZ, Zhao Z, Shang H, Liu QS, Liu F (2019) An easy-to-synthesize multi-photoresponse smart sensor for fast detecting Zn2+ and quantifying Fe3+ based on the enol/keto binding mode. New J Chem 11(58):54. https://doi.org/10.1039/C9NJ03635K
Zhang SZ, Chang J, Zhang HJ, Sun YX, Wu Y, Wang Y-B (2020) Synthesis, crystal structure and spectral properties of binuclear Ni(II) and cubane-like Cu4(μ3-O)4 Cored Tetranuclear Cu(II) complexes based on coumarin schiff base. Chinese J Inorg Chem 36:503–514. https://doi.org/10.11862/CJIC.2020.056
Zhang SZ, Guo G, Ding WM, Li J, Wu Y, Sun YX (2021) Synthesis and spectroscopic properties of two different structural Schiff base Zn(II) complexes constructed with/without auxiliary ligands. J Mol Struct 1230:129627. https://doi.org/10.1016/j.molstruc.2020.129627
Zhao Q, An XX, Liu LZ, Dong WK (2019) Syntheses, luminescences and Hirshfeld surfaces analyses of structurally characterized homo-trinuclear Zn2+ and hetero-pentanuclear Zn2+-LnIII (Ln=Eu, Nd) bis(salamo)-like complexes. Inorg Chim Acta 490:6–15. https://doi.org/10.1016/j.ica.2019.02.040.38
Acknowledgements
This work was supported by the Science and Technology Program of Gansu Province (18YF1GA054) and the Program for Excellent Team of Scientific Research in Lanzhou Jiaotong University (201706), both of which are gratefully acknowledged.
Funding
This work was supported by the Science and Technology Program of Gansu Province (18YF1GA054) and the Program for the Excellent Team of Scientific Research in the Lanzhou Jiaotong University (201706).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by all authors. The first draft of the manuscript was written by Juan Li and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, J., Zhang, SZ., Guo, G. et al. A high selective “turn-on” fluorescent chemosensor for detection of Zn2+ in aqueous media. Chem. Pap. 75, 4697–4706 (2021). https://doi.org/10.1007/s11696-021-01684-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01684-x