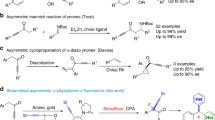
Abstract
An effective strategy for the chemoselective synthesis of β-enaminones is described by aza-Michael addition of aminoalkyl-, phenol- and thio-anilines to ynones under metal-free conditions. Diverse structural β-enaminones were obtained in up to 99% yield for 31 examples. The novel dual-1,5-disubstituted triazole scaffold was synthesized subsequently from β-enaminone. This strategy is highly efficient, highly chemoselective and metal-free.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
β-enaminones are unique scaffolds with nucleophilic and electrophilic motifs found in various N-containing heterocycles (Negri et al. 2004; Stanovnik et al. 2004; Elassar et al. 2003). Their specific structures and properties are present in pharmaceuticals and bioactive heterocycles such as anticonvulsant (Azzaro et al. 1981), anti-inflammatory (Dannhardt et al. 1998), antitumor agents (Boger et al. 1989), quinolone antibacterials (Wang et al. 1982) and quinoline antimalarials (Boger et al. 1989). Typical N-heterocyclic compounds based on β-enaminones include indole (Bernini et al. 2009; Liu et al. 2016, 2019; He et al. 2011; Sun et al. 2013; Tang et al. 2017), pyrrole (Yan et al. 2010; Cheng et al. 2018; Xu et al. 2020; Lei et al. 2016; Shen et al. 2013), pyridine (Cheng et al. 2017; Cheng et al. 2015; Yang et al. 2017; Shen et al. 2015; Kelgokmen et al. 2019), triazole (Cheng et al. 2013; Nino et al. 2019), quinolone (Xia et al. 2019, 2012), benzoxazole (Ge et al. 2020), thiazole (Wu et al. 2018), oxazole (Liu et al. 2020), quinolone (Zhang et al. 2019), quinoxaline (Ding et al. 2020) and triazine (Weng et al. 2018) (Fig. 1). Moreover, β-enaminones have also been employed as ligands for diastereoselective synthesis (Popov et al. 2003; Popov et al. 2001). Owing to their multifarious and prominent properties, the development of efficient methodologies to build such structures is still an area of very active research.
Conventionally, the most popular approaches to the construction of β-enaminones consist of: (a) the acid-catalyzed amination of 1,3-diketones (Scheme 1a) (Arcadi et al. 2003; Bartoli et al. 2004; Bhosale et al. 2006; Epifano et al. 2007; Xu et al. 2009); (b) the aza-Michael addition of amines to ynones (Scheme 1b) (Karpov et al. 2003a, b); or (c) the aza-Michael addition of ynone intermediates from α-keto acids and iodoalkynes (Scheme 1c) (Zeng et al. 2019). Nevertheless, methodologies based on the acid-catalyzed reactions limit their potential application in the pharmaceutical industry owing to low amination regioselectivity, while routes based on aza-Michael addition have higher regioselectivity (amination only on the β-site of ynones) and simple reaction conditions. However, if there are two nucleophilic sites in anilines (rout b and c), the desired product β-enaminones could be obtained as an isomeric mixture. Accordingly, the synthesis of single β-enaminones is still a challenging task and the development of more effective methods for such purpose is highly desirable. Herein, we designed an effective strategy for the synthesis of β-enaminones via the chemoselective aza-Michael addition of amines to ynones under metal-free conditions (Scheme 1d).
Results and discussion
At the outset of the study, the aza-Michael addition of 1,3-diphenylprop-2-yn-1-one 1a and 4-(aminomethyl)aniline 2a was chosen as a model reaction to screen the reaction parameters (Table 1). To our delight, the desired product 3aa was obtained in 40% yield when the reaction was conducted at room temperature in DCM for 5 h (entry 1). Simultaneously, the by-product 4aa was also provided in 15% yield. Further studies focused on screening of versatile solvent and indicated that this transformation was highly solvent dependent (entries 2–10). The yield of 3aa was improved to 90% by switching the solvent DCM to DMF, while no obvious deviations were observed between DMF, NMP and DMSO (entries 2–4). However, other solvents, such as EtOH, toluene, 1,4-dioxane, THF, CH3CN and H2O led to lower yield (entries 5–10). On the basis of the above results, the optimal reaction conditions were identified as follows: DMSO as the solvent at room temperature for 5 h to give the single product 3aa in 95% yield (Table 1, entry 4).
With the optimized conditions in hand, the scope and generality of the aza-Michael addition reactions were investigated (Table 2). Interestingly, a wide range of substituents was well tolerated under the reaction conditions, affording the corresponding β-enaminones (3aa–3af) in 80–99% yields. First, the substituents R1 of ynones 1 were examined, and the substituents with electron-donating groups (–Me, –nBu, –OMe) (3ba–3ea) provided the corresponding products in higher yields than those bearing electron-withdrawing substituents (–F, –Cl, –Br, –CN) (3fa–3 ka). Compared to the yield of 3fa with the yield of 3 ha (88% vs. 85%), we found that the steric hindrance did not influence this reaction obviously. Moreover, either electron-donating groups (such as –Me, –OMe) (3la–3oa and 3ta) or electron-withdrawing groups (such as –F, –Cl, –Br, –I) (3pa–3sa and 3ua) in the R2 substituent of ynones 1 could react smoothly with 4-(aminomethyl)aniline 2a, affording the corresponding β-enaminones in excellent yields. A significant influence of steric hindrance on this reaction was observed. For instance, the ortho-substituted substrate led to lower yield than the para-substituted substrate (3la vs. 3na). Other representative aromatic and aliphatic substrates, such as naphthyl, thienyl and aliphatic groups, were also tolerated (3va–3za). Additionally, 4-(2-aminoethyl)aniline 2b, 3-(aminomethyl)aniline 2c and 2-(aminomethyl)aniline 2d were also tested and furnished the desired products in 98%, 92% and 85% yields, respectively (3ab, 3ac, and 3ad). Finally, 4-(aminomethyl)phenol and 2-aminoethane-1-thiol were also investigated under the standard reaction conditions and good yields of the target products were obtained (3ae and 3af).
Yield of isolated product based on ynones 1 was reported.
To prove the practicality of this aza-Michael addition reaction, a gram-scale synthesis of β-enaminone 3aa was performed. When 2.06 g of 1,3-diphenylprop-2-yn-1-one 1a (10 mmol) and 1.47 g of 4-(aminomethyl)aniline 2a (12 mmol) were loaded, 3.05 g of β-enaminone 3aa was obtained in 93% yield (Eq. 1).

To further demonstrate the application of β-enaminones in the synthesis of complicated molecules, β-enaminone 3ac was employed with tosyl azide in the presence of LiOBut to furnish the 1,2,3-triazole 5ac in 85% yield (Cheng et al. 2013). Subsequently, the novel dual(triazole) structure 6ac containing two 1,5-disubstituted triazoles scaffold could be generated in 83% yield from 5ac and ynone 1a (Eq. 2).

Conclusions
In conclusion, an effective strategy for the chemoselective synthesis of β-enaminones was developed. This reaction tolerated a wide range of functional groups in moderate-to-excellent yields under metal-free conditions. Due to the importance of β-enaminones, this protocol could be further expanded to find wide applications in synthetic chemistry. This strategy is highly efficient, high chemoselective and metal-free.
Experimental section
General information
Unless otherwise stated, all reagents were used directly without further purification. Silica gel was purchased from Qing Dao Hai Yang Chemical Industry Co. All melting points were determined on a Beijing Science Instrument Dianguang Instrument Factory XT4B melting point apparatus and uncorrected. 1H and 13C NMR spectra were measured on a 400 MHz Bruker spectrometer (1H 400 MHz, 13C100 MHz), using CDCl3 as the solvent with tetramethylsilane (TMS) as the internal standard at room temperature. HRMS-ESI spectra were equipped with an ESI source and a TOF detector. PE is petroleum ether (60–90 °C).
Typical procedure for the preparation of (Z)-3-((4-aminobenzyl)amino)-1,3-diphenylprop-2-en-1-one (3aa)
A suspension of 1,3-diphenylprop-2-yn-1-one 1a (0.5 mmol, 103.0 mg) and 4-(aminomethyl)aniline 2a (0.6 mmol, 73.2 mg) in DMSO (2 mL) was stirred at rt for 5 h. After 1,3-diphenylprop-2-yn-1-one exhausted completely (monitored by TLC), saturated aqueous brine (20 mL) was added. The mixture was stirred for 10 min and then was extracted by EtOAc (3 × 10 mL). The combined organic layers were dried over Na2SO4. Removal of the solvent gave a residue, which was purified by a column chromatography (silica gel, PE/EtOAc/TEA = 100/15/1) to afford 3aa as light yellow solid; mp 95–98 °C. 1H NMR (400 MHz, CDCl3) δ 11.62 (s, 1H), 7.90 (d, J = 8.0 Hz, 2H), 7.44–7.39 (m, 8H), 7.00 (d, J = 8.0 Hz, 2H), 6.62 (d, J = 8.0 Hz, 2H), 5.81 (s, 1H), 4.30 (d, J = 4.0 Hz, 2H), 3.66 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 188.4, 166.6, 145.7, 140.2, 135.6, 130.6, 129.4, 128.5 (2C), 128.2 (2C), 128.1 (2C), 128.0, 127.7 (2C), 127.0 (2C), 115.2 (2C), 93.6, 48.1. HRMS m/z (ESI) calcd for C22H20N2O, (M + Na)+ 351.1468; found, 351.1469.
A similar procedure was used for the preparation of products 3ba–3af.
(Z)-3-((4-aminobenzyl)amino)-3-phenyl-1-(p-tolyl)prop-2-en-1-one (3ba) yellow solid, mp 93–96 °C. 1H NMR (400 MHz, CDCl3) δ 11.55 (s, 1H), 7.79 (d, J = 8.0 Hz, 2H), 7.44–7.40 (m, 5H), 7.19 (d, J = 8.0 Hz, 2H), 6.99 (d, J = 8.0 Hz, 2H), 6.61 (d, J = 8.0 Hz, 2H), 5.78 (s, 1H), 4.27 (d, J = 8.0 Hz, 2H), 3.62 (s, 2H), 2.37 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 188.3, 166.3, 145.7, 141.0, 137.5, 135.6, 129.4, 128.9 (2C), 128.4 (2C), 128.2 (3C), 127.8 (2C), 127.1 (2C), 115.3 (2C), 93.5, 48.1, 21.4. HRMS m/z (ESI) calcd for C23H22N2O, (M + Na)+ 365.1624; found, 365.1627.
(Z)-3-((4-aminobenzyl)amino)-1-(4-butylphenyl)-3-phenylprop-2-en-1-one (3ca) yellow solid, mp 79–82 °C. 1H NMR (400 MHz, CDCl3) δ 11.55 (t, J = 8.0 Hz, 1H), 7.81 (d, J = 8.0 Hz, 2H), 7.44–7.40 (m, 5H), 7.19 (d, J = 8.0 Hz, 2H), 6.99 (d, J = 8.0 Hz, 2H), 6.61 (d, J = 8.0 Hz, 2H), 5.78 (s, 1H), 4.28 (d, J = 4.0 Hz, 2H), 3.66 (s, 2H), 2.63 (t, J = 8.0 Hz, 2H), 1.60 (m, 2H), 1.34 (m, 2H), 0.91 (t, J = 8.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 188.4, 166.3, 146.0, 145.7, 137.7, 135.7, 129.4, 128.4 (2C), 128.24, 128.21 (3C), 128.18 (2C), 127.8 (2C), 127.1 (2C), 115.3 (2C), 93.6, 48.1, 35.5, 33.4, 22.3, 13.9. HRMS m/z (ESI) calcd for C26H28N2O, (M + Na)+ 407.2094; found, 407.2096.
(Z)-3-((4-aminobenzyl)amino)-3-phenyl-1-(m-tolyl)prop-2-en-1-one (3da) yellow solid, mp 87–90 °C. 1H NMR (400 MHz, CDCl3) δ 11.60 (t, J = 8.0 Hz, 1H), 7.71 (s, 1H), 7.68 (d, J = 4.0 Hz, 1H), 7.44–7.40 (m, 5H), 7.29–7.20 (m, 3H), 6.99 (d, J = 8.0 Hz, 2H), 6.61 (d, J = 8.0 Hz, 2H), 5.78 (s, 1H), 4.28 (d, J = 4.0 Hz, 2H), 3.60 (s, 2H), 2.37 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 188.6, 166.4, 145.7, 140.2, 137.8, 135.6, 131.4, 129.4, 128.4 (2C), 128.2 (2C), 128.1, 128.0, 127.73 (2C), 127.68, 124.2, 115.2 (2C), 93.7, 48.1, 21.4. HRMS m/z (ESI) calcd for C23H22N2O, (M + Na)+ 365.1624; found, 365.1625.
(Z)-3-((4-aminobenzyl)amino)-3-phenyl-1-(p-tolyl)prop-2-en-1-one (3ea) yellow solid, mp 103–106 °C. 1H NMR (400 MHz, CDCl3) δ 11.59 (t, J = 8.0 Hz, 1H), 7.47–7.40 (m, 7H), 7.28 (d, J = 8.0 Hz, 2H), 6.98 (m, 3H), 6.62 (d, J = 8.0 Hz, 2H), 5.78 (s, 1H), 4.29 (d, J = 4.0 Hz, 2H), 3.83 (s, 3H), 3.65 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 188.0, 166.6, 159.6, 145.7, 141.7, 135.5, 129.5, 129.1, 128.5 (2C), 128.2 (2C), 128.0, 127.7 (2C), 119.5, 117.2, 115.3 (2C), 111.5, 93.7, 55.3, 48.2. HRMS m/z (ESI) calcd for C23H22N2O2, (M + Na)+ 381.1573; found, 381.1575.
(Z)-3-((4-aminobenzyl)amino)-1-(4-fluorophenyl)-3-phenylprop-2-en-1-one (3fa) yellow solid, mp 113–115 °C. 1H NMR (400 MHz, CDCl3) δ 11.56 (s, 1H), 7.89 (t, J = 8.0 Hz, 2H), 7.46–7.36 (m, 5H), 7.05 (t, J = 8.0 Hz, 2H), 6.98 (d, J = 8.0 Hz, 2H), 6.61 (d, J = 8.0 Hz, 2H), 5.73 (s, 1H), 4.28 (d, J = 8.0 Hz, 2H), 3.62 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 186.9, 166.7, 164.4 (d, J = 249.0 Hz), 145.8, 136.4 (d, J = 3.0 Hz), 135.4, 129.5, 129.2 (d, J = 8.0 Hz, 2C), 128.5 (2C), 128.2 (2C), 127.9, 127.7 (2C), 115.3 (2C), 115.0 (d, J = 21.0 Hz, 2C), 93.2, 48.1. HRMS m/z (ESI) calcd for C22H19FN2O, (M + Na)+ 369.1374; found, 369.1378.
(Z)-3-((4-aminobenzyl)amino)-1-(3-fluorophenyl)-3-phenylprop-2-en-1-one (3ga) yellow solid, mp 98–101 °C. 1H NMR (400 MHz, CDCl3) δ 11.62 (s, 1H), 7.65 (d, J = 8.0 Hz, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.48–7.39 (m, 5H), 7.36–7.31 (m, 1H), 7.10 (t, J = 8.0 Hz, 1H), 6.99 (d, J = 8.0 Hz, 2H), 6.62 (d, J = 8.0 Hz, 2H), 5.74 (s, 1H), 4.30 (d, J = 4.0 Hz, 2H), 3.64 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 186.56 (d, J = 2.0 Hz), 167.1, 162.8 (d, J = 245.0 Hz), 145.8, 142.6 (d, J = 7.0 Hz), 135.3, 129.7, 129.6, 128.5 (2C), 128.2 (2C), 127.8, 127.7 (2C), 122.6 (d, J = 2.0 Hz), 117.4 (d, J = 21.0 Hz), 115.3 (2C), 113.9 (d, J = 22.0 Hz), 93.4, 48.2. HRMS m/z (ESI) calcd for C22H19FN2O, (M + Na)+ 369.1374; found, 369.1375.
(Z)-3-((4-aminobenzyl)amino)-1-(2-fluorophenyl)-3-phenylprop-2-en-1-one (3 ha) yellow solid, mp 115–118 °C. 1H NMR (400 MHz, CDCl3) δ 11.61 (s, 1H), 7.83 (t, J = 8.0 Hz, 1H), 7.44–7.32 (m, 6H), 7.17 (t, J = 8.0 Hz, 1H), 7.05 (d, J = 8.0 Hz, 1H), 7.00 (d, J = 8.0 Hz, 2H), 6.62 (d, J = 8.0 Hz, 2H), 5.76 (s, 1H), 4.30 (d, J = 8.0 Hz, 2H), 3.66 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 184.95 (d, J = 3.0 Hz), 166.7, 160.3 (d, J = 250.0 Hz), 145.8, 135.2, 131.7 (d, J = 9.0 Hz), 130.3 (d, J = 3.0 Hz), 129.5, 128.8 (d, J = 13.0 Hz), 128.5 (2C), 128.2 (2C), 127.83, 127.76 (2C), 124.0 (d, J = 4.0 Hz), 116.1 (d, J = 24.0 Hz), 115.3 (2C), 97.9 (d, J = 9.0 Hz), 48.2. HRMS m/z (ESI) calcd for C22H19FN2O, (M + Na)+ 369.1374; found, 369.1376.
(Z)-3-((4-aminobenzyl)amino)-1-(4-chlorophenyl)-3-phenylprop-2-en-1-one (3ia) yellow solid, mp 101–104 °C. 1H NMR (400 MHz, CDCl3) δ 11.60 (t, J = 4.0 Hz, 1H), 7.82 (d, J = 8.0 Hz, 2H), 7.45–7.33 (m, 7H), 6.98 (d, J = 8.0 Hz, 2H), 6.61 (d, J = 8.0 Hz, 2H), 5.73 (s, 1H), 4.28 (d, J = 8.0 Hz, 2H), 3.65 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 186.8, 166.9, 145.8, 138.6, 136.7, 135.3, 129.6, 128.5 (2C), 128.4 (2C), 128.3 (2C), 128.2 (2C), 127.8, 127.7 (2C), 115.3 (2C), 93.2, 48.2. HRMS m/z (ESI) calcd for C22H19ClN2O, (M + Na)+ 385.1078; found, 385.1080.
(Z)-3-((4-aminobenzyl)amino)-1-(4-bromophenyl)-3-phenylprop-2-en-1-one (3ja) yellow solid, mp 109–111 °C. 1H NMR (400 MHz, CDCl3) δ 11.61 (t, J = 4.0 Hz, 1H), 7.75 (d, J = 12.0 Hz, 2H), 7.51–7.38 (m, 7H), 6.98 (d, J = 12.0 Hz, 2H), 6.60 (d, J = 8.0 Hz, 2H), 5.73 (s, 1H), 4.28 (d, J = 4.0 Hz, 2H), 3.66 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 186.8, 166.9, 145.8, 139.0, 135.3, 131.3 (2C), 129.5, 128.6 (2C), 128.5 (2C), 128.2 (2C), 127.7, 127.6 (2C), 125.2, 115.2 (2C), 93.2, 48.2. HRMS m/z (ESI) calcd for C22H19BrN2O, (M + Na)+ 429.0573; found, 429.0576.
(Z)-4-(3-((4-aminobenzyl)amino)-3-phenylacryloyl)benzonitrile (3 ka) yellow solid, mp 78–80 °C. 1H NMR (400 MHz, CDCl3) δ 11.74 (t, J = 4.0 Hz, 1H), 7.95 (d, J = 12.0 Hz, 2H), 7.66 (d, J = 8.0 Hz, 2H), 7.49–7.39 (m, 5H), 6.99 (d, J = 12.0 Hz, 2H), 6.63 (d, J = 8.0 Hz, 2H), 5.75 (s, 1H), 4.32 (d, J = 4.0 Hz, 2H), 3.28 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 185.5, 167.6, 145.9, 144.0, 134.9, 132.0 (2C), 129.8, 128.6 (2C), 128.2 (2C), 127.5 (2C), 127.4, 127.3 (2C), 118.6, 115.2 (2C), 113.6, 93.5, 48.3. HRMS m/z (ESI) calcd for C23H19N3O, (M + Na)+ 354.1601; found, 354.1600.
(Z)-3-((4-aminobenzyl)amino)-1-phenyl-3-(p-tolyl)prop-2-en-1-one (3la) yellow solid, mp 108–111 °C. 1H NMR (400 MHz, CDCl3) δ 11.61 (s, 1H), 7.89 (d, J = 8.0 Hz, 2H), 7.44–7.36 (m, 3H), 7.31 (d, J = 8.0 Hz, 2H), 7.23 (d, J = 8.0 Hz, 2H), 7.01 (d, J = 8.0 Hz, 2H), 6.62 (d, J = 8.0 Hz, 2H), 5.79 (s, 1H), 4.30 (d, J = 4.0 Hz, 2H), 3.58 (s, 2H), 2.40 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 188.2, 166.8, 145.7, 140.3, 139.6, 132.7, 130.6, 129.1 (2C), 128.20, 128.18 (2C), 128.13 (2C), 127.7 (2C), 127.0 (2C), 115.3 (2C), 93.6, 48.1, 21.3. HRMS m/z (ESI) calcd for C23H22N2O, (M + Na)+ 365.1624; found, 365.1627.
(Z)-3-((4-aminobenzyl)amino)-1-phenyl-3-(m-tolyl)prop-2-en-1-one (3ma) yellow oil. 1H NMR (400 MHz, CDCl3) δ 11.53 (t, J = 8.0 Hz, 1H), 7.81 (d, J = 8.0 Hz, 2H), 7.36–7.12 (m, 7H), 6.92 (d, J = 8.0 Hz, 2H), 6.54 (d, J = 8.0 Hz, 2H), 5.71 (s, 1H), 4.21 (d, J = 4.0 Hz, 2H), 3.33 (s, 2H), 2.31 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 188.3, 166.8, 145.7, 140.3, 138.3, 135.5, 130.6, 130.2, 128.3 (2C), 128.2 (2C), 128.14, 128.12 (2C), 127.0 (2C), 124.8, 115.2 (2C), 93.5, 48.2, 21.4. HRMS m/z (ESI) calcd for C23H22N2O, (M + Na)+ 365.1624; found, 365.1626.
(Z)-3-((4-aminobenzyl)amino)-1-phenyl-3-(o-tolyl)prop-2-en-1-one (3na) yellow oil. 1H NMR (400 MHz, CDCl3) δ 11.64 (t, J = 8.0 Hz, 1H), 7.87 (d, J = 8.0 Hz, 2H), 7.42–7.19 (m, 7H), 6.93 (d, J = 8.0 Hz, 2H), 6.59 (d, J = 8.0 Hz, 2H), 5.70 (s, 1H), 4.08 (d, J = 4.0 Hz, 2H), 3.65 (s, 2H), 2.30 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 188.3, 166.0, 145.8, 140.1, 135.2, 135.1, 130.6, 130.2, 129.0, 128.5 (2C), 128.1 (2C), 127.7, 127.6, 127.0 (2C), 125.8, 115.2 (2C), 92.8, 47.7, 19.3. HRMS m/z (ESI) calcd for C23H22N2O, (M + Na)+ 365.1624; found, 365.1624.
(Z)-3-((4-aminobenzyl)amino)-3-(4-methoxyphenyl)-1-phenylprop-2-en-1-one (3oa) yellow solid, mp 125–128 °C. 1H NMR (400 MHz, CDCl3) δ 11.63 (t, J = 8.0 Hz, 1H), 7.89 (d, J = 8.0 Hz, 2H), 7.43–7.35 (m, 5H), 7.01 (d, J = 8.0 Hz, 2H), 6.94 (d, J = 8.0 Hz, 2H), 6.61 (d, J = 8.0 Hz, 2H), 5.79 (s, 1H), 4.32 (d, J = 4.0 Hz, 2H), 3.83 (s, 3H), 3.48 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 188.1, 166.6, 160.5, 145.7, 140.3, 130.5, 129.3 (2C), 128.2, 128.1 (3C), 127.8, 127.0 (2C), 115.2 (2C), 113.8 (2C), 93.6, 55.3, 48.1. HRMS m/z (ESI) calcd for C23H22N2O2, (M + Na)+ 381.1573; found, 381.1574.
(Z)-3-((4-aminobenzyl)amino)-3-(4-fluorophenyl)-1-phenylprop-2-en-1-one (3pa) yellow solid, mp 135–138 °C. 1H NMR (400 MHz, CDCl3) δ 11.58 (t, J = 8.0 Hz, 1H), 7.88 (t, J = 8.0 Hz, 2H), 7.44–7.37 (m, 6H), 7.11 (t, J = 8.0 Hz, 2H), 6.98 (d, J = 8.0 Hz, 2H), 6.62 (d, J = 8.0 Hz, 2H), 5.77 (s, 1H), 4.27 (d, J = 8.0 Hz, 2H), 3.65 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 188.5, 165.5, 163.3 (d, J = 248.0 Hz), 145.8, 140.1, 131.6 (d, J = 4.0 Hz), 130.8, 129.8 (d, J = 8.0 Hz, 2C), 128.2 (2C), 128.1 (2C), 128.0, 127.0 (2C), 115.6 (d, J = 21.0 Hz, 2C), 115.3 (2C), 93.8, 48.1. HRMS m/z (ESI) calcd for C22H19FN2O, (M + Na)+ 369.1374; found, 369.1375.
(Z)-3-((4-aminobenzyl)amino)-3-(4-chlorophenyl)-1-phenylprop-2-en-1-one (3qa) yellow solid, mp 150–153 °C. 1H NMR (400 MHz, CDCl3) δ 11.54 (t, J = 4.0 Hz, 1H), 7.88 (d, J = 8.0 Hz, 2H), 7.45–7.33 (m, 7H), 6.98 (d, J = 8.0 Hz, 2H), 6.62 (d, J = 8.0 Hz, 2H), 5.76 (s, 1H), 4.26 (d, J = 8.0 Hz, 2H), 3.67 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 188.6, 165.3, 145.8, 140.0, 135.6, 134.0, 130.8, 129.2 (2C), 128.8 (2C), 128.2 (2C), 128.1 (2C), 128.0, 127.1 (2C), 115.3 (2C), 93.7, 48.1. HRMS m/z (ESI) calcd for C22H19ClN2O, (M + Na)+ 385.1078; found, 385.1079.
(Z)-3-((4-aminobenzyl)amino)-3-(4-bromophenyl)-1-phenylprop-2-en-1-one (3ra) yellow solid, mp 145–148 °C. 1H NMR (400 MHz, CDCl3) δ 11.54 (t, J = 8.0 Hz, 1H), 7.88 (d, J = 8.0 Hz, 2H), 7.56 (d, J = 8.0 Hz, 2H), 7.45–7.37 (m, 3H), 7.27 (d, J = 8.0 Hz, 2H), 6.97 (d, J = 8.0 Hz, 2H), 6.62 (d, J = 8.0 Hz, 2H), 5.75 (s, 1H), 4.25 (d, J = 8.0 Hz, 2H), 3.65 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 188.6, 165.2, 145.8, 140.0, 134.4, 131.7 (2C), 130.8, 129.4 (2C), 128.2 (2C), 128.1 (2C), 127.9, 127.0 (2C), 123.8, 115.3 (2C), 93.7, 48.1. HRMS m/z (ESI) calcd for C22H19BrN2O, (M + Na)+ 429.0573; found, 429.0574.
(Z)-3-((4-aminobenzyl)amino)-3-(4-iodophenyl)-1-phenylprop-2-en-1-one (3sa) yellow solid, mp 140–142 °C. 1H NMR (400 MHz, CDCl3) δ 11.52 (t, J = 8.0 Hz, 1H), 7.87 (d, J = 8.0 Hz, 2H), 7.77 (d, J = 8.0 Hz, 2H), 7.45–7.37 (m, 3H), 7.14 (d, J = 8.0 Hz, 2H), 6.98 (d, J = 8.0 Hz, 2H), 6.62 (d, J = 8.0 Hz, 2H), 5.75 (s, 1H), 4.26 (d, J = 8.0 Hz, 2H), 3.66 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 188.6, 165.3, 145.8, 140.0, 137.7 (2C), 135.0, 130.9, 129.5 (2C), 128.2 (2C), 128.1 (2C), 127.9, 127.0 (2C), 115.3 (2C), 95.6, 93.6, 48.1. HRMS m/z (ESI) calcd for C22H19IN2O, (M + Na)+ 477.0434; found, 477.0435.
(Z)-3-((4-aminobenzyl)amino)-3-(benzo[d][1,3]dioxol-5-yl)-1-phenylprop-2-en-1-one (3ta) yellow solid, mp 116–119 °C. 1H NMR (400 MHz, CDCl3) δ 11.56 (t, J = 8.0 Hz, 1H), 7.88 (d, J = 8.0 Hz, 2H), 7.44–7.36 (m, 3H), 7.02 (d, J = 8.0 Hz, 2H), 6.93–6.84 (m, 3H), 6.63 (d, J = 8.0 Hz, 2H), 6.02 (s, 2H), 5.78 (s, 1H), 4.33 (d, J = 4.0 Hz, 2H), 3.64 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 188.3, 166.2, 148.6, 147.7, 145.7, 140.3, 130.7, 129.3, 128.17 (2C), 128.16 (3C), 127.0 (2C), 121.9, 115.3 (2C), 108.38, 108.35, 101.5, 93.6, 48.2. HRMS m/z (ESI) calcd for C23H20N2O3, (M + Na)+ 395.1366; found, 395.1369.
(Z)-3-((4-aminobenzyl)amino)-3-(3,4-dichlorophenyl)-1-phenylprop-2-en-1-one (3ua) yellow solid, mp 118–120 °C. 1H NMR (400 MHz, CDCl3) δ 11.47 (t, J = 4.0 Hz, 1H), 7.88 (d, J = 4.0 Hz, 2H), 7.51–7.38 (m, 5H), 7.23 (d, J = 8.0 Hz, 2H), 6.97 (d, J = 8.0 Hz, 2H), 6.63 (d, J = 8.0 Hz, 2H), 5.75 (s, 1H), 4.25 (d, J = 8.0 Hz, 2H), 3.47 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 188.9, 163.7, 145.9, 139.8, 135.4, 133.8, 132.9, 131.0, 130.6, 129.8, 128.3 (2C), 128.1 (2C), 127.8, 127.12, 127.08 (2C), 115.3 (2C), 93.8, 48.2. HRMS m/z (ESI) calcd for C22H18Cl2N2O, (M + Na)+ 419.0688; found, 419.0690.
(Z)-3-((4-aminobenzyl)amino)-3-(naphthalen-2-yl)-1-phenylprop-2-en-1-one (3va) yellow solid, mp 123–125 °C. 1H NMR (400 MHz, CDCl3) δ 11.69 (t, J = 8.0 Hz, 1H), 7.95–7.87 (m, 6H), 7.59–7.38 (m, 6H), 7.02 (d, J = 8.0 Hz, 2H), 6.63 (d, J = 8.0 Hz, 2H), 5.92 (s, 1H), 4.35 (d, J = 4.0 Hz, 2H), 3.66 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 188.4, 166.5, 145.7, 140.2, 133.5 (2C), 133.0, 132.8, 130.7, 128.3, 128.19 (2C), 128.16 (2C), 128.0, 127.8, 127.4, 127.0 (3C), 126.7, 125.1, 115.3 (2C), 93.9, 48.3. HRMS m/z (ESI) calcd for C26H22N2O, (M + Na)+ 401.1624; found, 401.1626.
(Z)-3-((4-aminobenzyl)amino)-1-phenyl-3-(thiophen-3-yl)prop-2-en-1-one (3wa) yellow solid, mp 170–174 °C. 1H NMR (400 MHz, CDCl3) δ 11.67 (t, J = 8.0 Hz, 1H), 7.91 (d, J = 12.0 Hz, 2H), 7.48–7.37 (m, 5H), 7.18 (d, J = 4.0 Hz, 1H), 7.03 (d, J = 12.0 Hz, 2H), 6.64 (d, J = 8.0 Hz, 2H), 5.90 (s, 1H), 4.39 (d, J = 8.0 Hz, 2H), 3.68 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 188.3, 161.4, 145.7, 140.2, 136.1, 130.7, 128.1 (2C), 128.04, 127.97 (2C), 127.3, 127.0 (2C), 126.2, 125.8, 115.3 (2C), 93.3, 48.1. HRMS m/z (ESI) calcd for C20H18N2OS, (M + Na)+ 357.1032; found, 357.1033.
(Z)-3-((4-aminobenzyl)amino)-3-phenyl-1-(thiophen-2-yl)prop-2-en-1-one (3xa) yellow solid, mp 136–140 °C. 1H NMR (400 MHz, CDCl3) δ 11.27 (t, J = 8.0 Hz, 1H), 7.54 (d, J = 4.0 Hz, 1H), 7.45–7.39 (m, 6H), 7.04 (t, J = 4.0 Hz, 1H), 6.98 (d, J = 12.0 Hz, 2H), 6.60 (d, J = 8.0 Hz, 2H), 5.69 (s, 1H), 4.26 (d, J = 4.0 Hz, 2H), 3.69 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 181.2, 166.2, 147.0, 145.8, 135.2, 129.9, 129.4, 128.4 (2C), 128.1 (2C), 127.63, 127.60 (3C), 127.5, 115.1 (2C), 93.1, 48.1. HRMS m/z (ESI) calcd for C20H18N2OS, (M + Na)+ 357.1032; found, 357.1035.
(Z)-3-((4-aminobenzyl)amino)-1-phenylnon-2-en-1-one (3ya) yellow solid, mp 78–83 °C. 1H NMR (400 MHz, CDCl3) δ 11.74 (t, J = 8.0 Hz, 1H), 7.88 (d, J = 8.0 Hz, 2H), 7.42–7.37 (m, 3H), 7.08 (d, J = 8.0 Hz, 2H), 6.63 (d, J = 12.0 Hz, 2H), 5.73 (s, 1H), 4.41 (d, J = 8.0 Hz, 2H), 3.72 (s, 2H), 2.34 (t, J = 8.0 Hz, 2H), 1.61 (m, 2H), 1.43–1.26 (m, 6H), 0.91 (t, J = 8.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 187.6, 168.8, 145.9, 140.5, 130.2, 128.1 (2C), 128.0 (2C), 127.1, 126.7 (2C), 115.2 (2C), 91.1, 46.3, 32.3, 31.4, 29.0, 28.0, 22.4, 13.9. HRMS m/z (ESI) calcd for C22H28N2O, (M + Na)+ 359.2094; found, 359.2096.
(Z)-3-((4-aminobenzyl)amino)-1-cyclopropyl-3-phenylprop-2-en-1-one (3za) yellow solid, mp 128–132 °C. 1H NMR (400 MHz, CDCl3) δ 10.92 (t, J = 8.0 Hz, 1H), 7.41–7.35 (m, 5H), 6.93 (d, J = 8.0 Hz, 2H), 6.57 (d, J = 8.0 Hz, 2H), 5.25 (s, 1H), 4.18 (d, 2H), 3.66 (s, 2H), 1.72 (m, 1H), 1.01 (m, 2H), 0.75 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 197.5, 163.9, 145.6, 135.3, 129.1 (2C), 128.3 (2C), 128.04 (2C), 128.01, 127.6 (2C), 115.1 (2C), 96.5, 47.8, 20.2, 9.0 (2C). HRMS m/z (ESI) calcd for C19H20N2O, (M + Na)+ 315.1468; found, 315.1470.
(Z)-3-((4-aminophenethyl)amino)-1,3-diphenylprop-2-en-1-one (3ab) yellow oil. 1H NMR (400 MHz, CDCl3) δ 11.42 (t, J = 8.0 Hz, 1H), 7.88 (d, J = 8.0 Hz, 2H), 7.42–7.34 (m, 6H), 7.25–7.22 (m, 2H), 6.85 (d, J = 8.0 Hz, 2H), 6.56 (d, J = 8.0 Hz, 2H), 5.71 (s, 1H), 3.51 (s, 2H), 3.34 (q, J = 4.0 Hz, J = 4.0 Hz, 2H), 2.72 (t, J = 8.0 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 188.1, 166.7, 144.9, 140.2, 135.5, 130.5, 129.5 (2C), 129.2, 128.3 (2C), 128.1 (2C), 127.9, 127.5 (2C), 126.9 (2C), 115.1 (2C), 93.3, 48.6, 36.5. HRMS m/z (ESI) calcd for C23H22N2O, (M + Na)+ 365.1624; found, 365.1626.
(Z)-3-((3-aminobenzyl)amino)-1,3-diphenylprop-2-en-1-one (3ac) yellow solid, mp 117–120 °C. 1H NMR (400 MHz, CDCl3) δ 11.69 (s, 1H), 7.90 (d, J = 8.0 Hz, 2H), 7.46–7.37 (m, 8H), 7.08 (t, J = 8.0 Hz, 1H), 6.57 (t, J = 8.0 Hz, 3H), 5.83 (s, 1H), 4.32 (d, J = 8.0 Hz, 2H), 3.69 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 188.6, 166.8, 146.8, 140.1, 139.6, 135.4, 130.7, 129.6, 129.5, 128.5 (2C), 128.2 (2C), 127.7 (2C), 127.1 (2C), 116.9, 114.1, 113.2, 93.8, 48.3. HRMS m/z (ESI) calcd for C22H20N2O, (M + Na)+ 351.1468; found, 351.1470.
(Z)-3-((2-aminobenzyl)amino)-1,3-diphenylprop-2-en-1-one (3ad) yellow solid, mp 145–148 °C. 1H NMR (400 MHz, CDCl3) δ 11.50 (s, 1H), 7.89 (d, J = 8.0 Hz, 2H), 7.47–7.36 (m, 8H), 7.09 (m, 2H), 6.76–6.66 (m, 2H),, 5.85 (s, 1H), 4.29 (d, J = 4.0 Hz, 2H), 3.61 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 188.7, 166.8, 144.1, 140.0, 135.4, 130.8, 129.7, 128.9, 128.8, 128.7 (2C), 128.2 (2C), 127.7 (2C), 127.1 (2C), 122.6, 119.1, 116.4, 94.1, 45.8. HRMS m/z (ESI) calcd for C22H20N2O, (M + Na)+ 351.1468; found, 351.1471.
(Z)-3-((4-hydroxybenzyl)amino)-1,3-diphenylprop-2-en-1-one (3ae) yellow solid, mp 167–172 °C. 1H NMR (400 MHz, CDCl3) δ 11.73 (t, J = 8.0 Hz, 1H), 9.19 (s, 1H), 7.91 (d, J = 8.0 Hz, 2H), 7.50–7.39 (m, 8H), 7.05 (d, J = 8.0 Hz, 2H), 6.84 (d, J = 8.0 Hz, 2H), 5.86 (s, 1H), 4.36 (d, J = 4.0 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 188.8, 167.6, 156.8, 139.7, 135.0, 131.0, 129.8, 128.6 (2C), 128.4 (2C), 128.3 (2C), 128.0, 127.6 (2C), 127.1 (2C), 115.8 (2C), 94.1, 48.5. HRMS m/z (ESI) calcd for C22H19NO2, (M + Na)+ 352.1308; found, 352.1309.
(Z)-3-((2-mercaptoethyl)amino)-1,3-diphenylprop-2-en-1-one (3af) (Štefane et al. 2002) yellow solid, mp 138–143 °C. 1H NMR (400 MHz, CDCl3) δ 11.43 (t, J = 4.0 Hz, 1H), 7.89 (d, J = 8.0 Hz, 2H), 7.46–7.37 (m, 8H), 5.80 (s, 1H), 3.49 (q, J = 8.0 Hz, J = 8.0 Hz, 2H), 2.66 (t, J = 8.0 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 188.6, 166.3, 139.9, 135.2, 130.8, 129.5, 128.6 (2C), 128.1 (2C), 127.7 (2C), 127.0 (2C), 94.0, 43.2, 38.5. HRMS m/z (ESI) calcd for C17H17NOS, (M + Na)+ 306.0923; found, 306.0925.
3-((5-phenyl-1H-1,2,3-triazol-1-yl)methyl)aniline (5ac) yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.72 (s, 1H), 7.43–7.37 (m, 3H), 7.28–7.26 (m, 2H), 7.02 (t, J = 8.0 Hz, 1H), 6.55 (d, J = 8.0 Hz, 1H), 6.39 (t, J = 4.0 Hz, 2H), 5.42 (s, 2H), 3.54 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 146.9, 138.0, 136.5, 132.9, 129.5, 129.3, 128.7 (2C), 128.6 (2C), 126.6, 116.5, 114.5, 113.1, 51.5. HRMS m/z (ESI) calcd for C15H14N4, (M + Na)+ 273.1111; found, 273.1114.
5-phenyl-1-(3-(5-phenyl-1H-1,2,3-triazol-1-yl)benzyl)-1H-1,2,3-triazole (6ac) white solid, mp 159–162 °C. 1H NMR (400 MHz, CDCl3) δ 7.82 (s, 1H), 7.70 (s, 1H), 7.44–7.27 (m, 8H), 7.21–7.09 (m, 6H), 5.55 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 138.0, 137.6, 137.1, 136.8, 133.3, 133.1, 129.8, 129.6, 129.2, 128.9 (2C), 128.8 (2C), 128.6 (2C), 128.3 (2C), 127.7, 126.3, 126.2, 124.7, 123.7, 50.9. HRMS m/z (ESI) calcd for C23H18N6, (M + Na)+ 401.1485; found, 401.1488.
Supporting information
Full experimental details, 1H and 13C NMR spectra. This material can be found via the Supporting information.
References
Arcadi A, Bianchi G, Giuseppe SD et al (2003) Gold catalysis in the reactions of 1,3-dicarbonyls with nucleophiles. Green Chem 5(1):64–67. https://doi.org/10.1039/B210165C
Azzaro M, Geribaldi S, Videau B (1981) Use of boron trifluoride etherate in the preparation of 2-amino-1-alkenyl ketones from β-diketones and low-boiling amines. Synthesis 11:880–881. https://doi.org/10.1055/s-1981-29629
Bartoli G, Bosco M, Locatelli M et al (2004) Zn(ClO4)2·6H2O as a powerful catalyst for the conversion of β-ketoesters into β-enamino esters. Synlett 2:0239–0242. https://doi.org/10.1055/s-2003-44974
Bernini R, Fabrizi G, Sferrazza A et al (2009) Copper-catalyzed C–C bond formation through C–H functionalization: synthesis of multisubstituted indoles from N-aryl enaminones. Angew Chem Int Ed 48(43):8078–8081. https://doi.org/10.1002/anie.200902440
Bhosale RS, Suryawanshi PA, Ingle SA et al (2006) Ionic liquid promoted synthesis of β-enamino ketones at room temperature. Synlett 06:933–935. https://doi.org/10.1055/s-2006-939042
Boger DL, Ishizaki T, Wysocki JRJ et al (1989) Total synthesis and evaluation of (+-)-N-(tert-butoxycarbonyl)-CBI, (+-)-CBI-CDPI1, and (+-)-CBI-CDPI2: CC-1065 functional agents incorporating the equivalent 1,2,9,9a-tetrahydrocyclopropa[1,2-c]benz[1,2-e]indol-4-one (CBI) left-hand subunit. J Am Chem Soc 111(16):6461–6463. https://doi.org/10.1021/ja00198a089
Cheng G, Zeng X, Shen J et al (2013) A metal-free multicomponent cascade reaction for the regiospecific synthesis of 1,5-disubstituted 1,2,3-triazoles. Angew Chem Int Ed 52(50):13265–13268. https://doi.org/10.1002/anie.201307499
Cheng G, Weng Y, Yang X et al (2015) Base-promoted N-pyridylation of heteroarenes using N-propargyl enaminones as equivalents of pyridine scaffolds. Org Lett 17(15):3790–3793. https://doi.org/10.1021/acs.orglett.5b01733
Cheng G, Xue L, Weng Y et al (2017) Transition-metal-free cascade approach toward 2-Alkoxy/2-Sulfenylpyridines and Dihydrofuro [2,3-b] pyridines by trapping in situ generated 1,4-Oxazepine. J Org Chem 82(18):9515–9524. https://doi.org/10.1021/acs.joc.7b01541
Cheng G, Lv W, Xue L (2018) Base-promoted ring-closing carbonyl–allene metathesis for the synthesis of 2,4-disubstituted pyrroles. Green Chem 20(19):4414–4417. https://doi.org/10.1039/C8GC01675E
Dannhardt G, Bauer A, Nowe U (1998) Non-steroidal anti-inflammatory agents. Part 23. Synthesis and pharmacological activity of enaminones which inhibit both bovine cyclooxygenase and 5-lipoxygenase. J Prakt Chem 340(3):256–263. https://doi.org/10.1002/prac.19983400309
Ding Q, Li M, Sun Y et al (2020) Copper-catalyzed [4+2] annulation reaction of β-enaminones and aryl diazonium salts without external oxidant: synthesis of highly functionalized 3H–1,2,4-triazines via homogeneous or heterogeneous strategy. Org Chem Front 7(3):457–463. https://doi.org/10.1039/C9QO01413F
Elassar AZA, El-Khair AA (2003) Recent developments in the chemistry of enaminones. Tetrahedron 59(43):8463–8480. https://doi.org/10.1016/S0040-4020(03)01201-8
Epifano F, Genovese S, Curini M (2007) Ytterbium triflate catalyzed synthesis of β-enaminones. Tetrahedron Lett 48(15):2717–2720. https://doi.org/10.1016/j.tetlet.2007.02.064
Ge B, Peng Y, Liu J et al (2020) Acid-promoted cleavage of the C–C double bond of N-(2-Hydroxylphenyl) enaminones for the synthesis of benzoxazoles. Tetrahedron 76(2):130818. https://doi.org/10.1016/j.tet.2019.130818
He Z, Liu W, Li Z (2011) I2-catalyzed indole formation via oxidative cyclization of N-aryl enamines. Chem Asian J 6(6):1340–1343. https://doi.org/10.1002/asia.201100045
Karpov AS, Müller TJJ (2003a) New entry to a three-component pyrimidine synthesis by TMS−Ynones via Sonogashira coupling. Org Lett 5(19):3451–3454. https://doi.org/10.1021/ol035212q
Karpov AS, Müller TJJ (2003b) Straightforward novel one-pot enaminone and pyrimidine syntheses by coupling-addition-cyclocondensation sequences. Synthesis 18:2815–2826. https://doi.org/10.1055/s-2003-42480
Kelgokmen Y, Zora M (2019) A new strategy for the synthesis of pyridines from N-propargylic β-enaminothiones. Org Biomol Chem 17(9):2529–2541. https://doi.org/10.1039/C8OB03180K
Lei T, Liu WQ, Li J et al (2016) Visible light initiated hantzsch synthesis of 2,5-diaryl-substituted pyrroles at ambient conditions. Org Lett 18(10):2479–2482. https://doi.org/10.1021/acs.orglett.6b01059
Liu J, Wei W, Zhao T et al (2016) Iodine/copper iodide-mediated C-H functionalization: synthesis of imidazo [1,2-a] pyridines and indoles from N-aryl enamines. J Org Chem 81(19):9326–9336. https://doi.org/10.1021/acs.joc.6b01960
Liu P, Yang M, Gong Y et al (2019) Copper-catalyzed cascade cyclization reaction of enamines and electron-deficient terminal alkynes: synthesis of polysubstituted pyrido[1,2-a]indoles. Org Lett 22(1):36–40. https://doi.org/10.1021/acs.orglett.9b03739
Liu J, Ba D, Lv W et al (2020) Base-promoted michael addition/smiles rearrangement/N-arylation cascade: one-step synthesis of 1,2,3-trisubstituted 4-quinolones from ynones and sulfonamides. Adv Synth Catal 362(1):213–223. https://doi.org/10.1002/adsc.201900960
Negri G, Kascheres C, Kascheres AJ (2004) Recent development in preparation reactivity and biological activity of enaminoketones and enaminothiones and their utilization to prepare heterocyclic compounds. J Heterocycl Chem 41(4):461–491. https://doi.org/10.1002/jhet.5570410402
Nino AD, Algieri V, Tallarida MA et al (2019) (2019) Regioselective synthesis of 1,4,5-trisubstituted-1,2,3-triazoles from aryl azides and enaminones. Eur J Org Chem 33:5725–5731. https://doi.org/10.1002/ejoc.201900889
Popov SA, Tkachev AV (2001) Synthesis of 2-alkyl and 2-aryl pyrimidines from β-chlorovinyl ketones of cyclopentanone type. Synth Commun 31(2):233–243. https://doi.org/10.1081/SCC-100000204
Popov SA, Gatilov YV, Rybalova TV et al (2003) Study of chiral β-enaminones prepared from pyrrolidine cytosine, salsoline and 2-amino-1-(4-nitrophenyl) propane-1,3-diol: resolution of salsoline via diastereomeric modified carane-type β-enaminones. Tetrahedron: Asymmetry 14(2):233–238. https://doi.org/10.1016/S0957-4166(02)00781-4
Shen J, Cheng G, Cui X (2013) “One pot” regiospecific synthesis of polysubstituted pyrroles from benzylamines and ynones under metal free conditions. Chem Commun 49(90):10641–10643. https://doi.org/10.1039/C3CC43844A
Shen J, Cai D, Kuai C et al (2015) Base-promoted β-C(sp3)–H functionalization of enaminones: an approach to polysubstituted pyridines. J Org Chem 80(13):6584–6589. https://doi.org/10.1021/acs.joc.5b00635
Stanovnik B, Svete J (2004) Synthesis of heterocycles from alkyl 3-(dimethylamino) propenoates and related enaminones. Chem Rev 104(5):2433–2480. https://doi.org/10.1021/cr020093y
Štefane B, Polanc S (2002) A new and a convenient route to enaminones and pyrazoles. New J Chem 26(1):28–32. https://doi.org/10.1039/B108524G
Sun JW, Wang XS, Liu Y (2013) Copper(II)-catalyzed sequential C, N-difunctionalization of 1, 4-naphthoquinone for the synthesis of benzo[f]indole-4,9-diones under base-free condition. J Org Chem 78(20):10560–10566. https://doi.org/10.1021/jo401842d
Tang S, Gao X, Lei A (2017) Electrocatalytic intramolecular oxidative annulation of N-aryl enamines into substituted indoles mediated by iodides. Chem Commun 53(23):3354–3356. https://doi.org/10.1039/C7CC00410A
Wang YF, Izawa T, Kobayashi S et al (1982) Stereocontrolled synthesis of (+)-negamycin from an acyclic homoallylamine by 1, 3-asymmetric induction. J Am Chem Soc 104(23):6465–6466. https://doi.org/10.1021/ja00387a060
Weng Y, Lv W, Yu J et al (2018) Preparation of 2,4,5-trisubstituted oxazoles through iodine-mediated aerobic oxidative cyclization of enaminones. Org Lett 20(7):1853–1856. https://doi.org/10.1021/acs.orglett.8b00376
Wu M, Jiang Y, An Z et al (2018) Iron-catalyzed synthesis of substituted thiazoles from enamines and elemental sulfur through C−S bond formation. Adv Synth Catal 360(21):4236–4240. https://doi.org/10.1002/adsc.201800693
Xia XF, Zhang LL, Song XR et al (2012) Copper-catalyzed oxidative cyclization of enynes for the synthesis of 4-carbonyl-quinolines with O2. Org Lett 14(10):2480–2483. https://doi.org/10.1021/ol300896h
Xia XF, He W, Wang D (2019) Metal-free oxidative annulation/cyclization of 1,6-enynes for the synthesis of 4-carbonylquinolines. Adv Synth Catal 361(12):2959–2964. https://doi.org/10.1002/adsc.201900013
Xu SL, Li CP, Li JH (2009) Solid-state synthesis of β-enamino ketones from solid 1,3-dicarbonyl compounds and ammonium salts or amines. Synlett 05:818–822. https://doi.org/10.1055/s-0028-1087914
Xu L, Wu L, Chen T et al (2020) Superbase-promoted N-α-sp3C-H functionalization of tertiary enaminones: synthesis of polysubstituted pyrroles. ChemistrySelect 5(2):655–659. https://doi.org/10.1002/slct.201903792
Yan RL, Luo J, Wang CX et al (2010) Cu(I)-catalyzed synthesis of polysubstituted pyrroles from dialkyl ethylenedicarboxylates and β-enamino ketones or esters in the presence of O2. J Org Chem 75(15):5395–5397. https://doi.org/10.1021/jo101022k
Yang X, Hu F, Wang Y et al (2017) Base-catalyzed cascade synthesis of 2,3-dihydrofuro[2,3-b] pyridines and 2,3-dihydro-1H-pyrrolo[2,3-b] pyridines from N-propargylic β-enaminones. Chem Commun 53(54):7497–7500. https://doi.org/10.1039/C7CC03308G
Zeng X, Liu C, Yang W et al (2019) Metal-free method for direct synthesis of functionalized β-Ketoenamines. J Org Chem 84(6):3656–3661. https://doi.org/10.1021/acs.joc.8b03247
Zhang H, Shen J, Yang Z et al (2019) PIDA-mediated intramolecular oxidative C–N bond formation for the direct synthesis of quinoxalines from enaminones. RSC Adv 9(14):7718–7722. https://doi.org/10.1039/C9RA01200A
Acknowledgements
Project supported by Hainan Provincial Nature Science Foundation of China (Nos. 2019RC214 & 820QN264), Fundamental Research Funds of Hainan Medical University (No. XRC180009) and the Program of Hainan Provincial Key Laboratory for Research and Development of Tropical Herbs (No. KF201804).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cui, X., Chen, Y., Wang, W. et al. Chemoselective synthesis of β-enaminones from ynones and aminoalkyl-, phenol- and thioanilines under metal-free conditions. Chem. Pap. 75, 3625–3634 (2021). https://doi.org/10.1007/s11696-021-01599-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01599-7